Research Article
Volume 2 Issue 1 - 2018
Isolation of New Aromatic Ester from Plumeria acutifolia bark
Department of Pharmaceutical Sciences, Faculty of Medical Sciences. Guru Jambheshwar University of Science and Technology,
Hisar-125001
*Corresponding Author: Naresh Kumar, Department of Pharmaceutical Sciences, Faculty of Medical Sciences. Guru Jambheshwar
University of Science and Technology, Hisar-125001.
Received: March 10, 2018; Published: March 19, 2018
Abstract
It is a small deciduous tree, contains copious milky juice. It is native of Mexico and cultivated in Indian gardens. The root bark is bitter and useful in leprosy, pains, fever. The infrequent fatty acids, glycosides sugar derivatives and new aromatic ester were isolated form methanol extract of stem bark of plant Plumeria acutifolia. The structures of these three compounds were recognized on the basis of chemical reactions and spectral analysis as n-octyl stearate, heptoglucosyl rhamoside and a new aromatic ester a vanillic acid-O-tetraarabinosyl stearate. This study advocates that plant contain a large number of bioactive compounds.
Keywords: Plumeria acutifolia; Apocynaceae; Fatty acids and Aromatic ester
Introduction
It is a small deciduous tree with, contains copious milky juice. It is native of Mexico and cultivated in Indian gardens. The root bark is bitter, pungent, acrid and stimulant. It is used in leprosy, pains, fever (Ayurveda) treatment [1] and in rheumatic pains (Yunani). In Mumbai, it is used in intermittent fever like Cinchona. In this paper, the isolation, characterization and structure elucidation of new aromatic ester along with fatty acids and glycosides sugar derivatives are described.
Experimental
The bark of Plumeria acutifolia were collected from campus of Guru Jambheshwar University of Sciences and Technology, Hisar in June 2010 and authenticated by Dr H.B. Singh, Head Raw Material Herbarium & Museum, and Ref. NISCAIR/RHMD/Consult-2010-11/11/1413/11. A voucher specimen has been retained in Department of Pharmaceutical Science, Guru Jambheshwar University of Science & Technology, Hisar. The plant material was air-dried at room temperature and then powdered. The chemicals and reagents used were of Qualigens and SD Fine, Mumbai, LR grade. All other chemicals used were of analytical grade [2,3]. Melting points were determined in centigrade scale in one end open capillary and uncorrected. UV spectra were recorded in methanol on Elmer EZ-301 spectrophotometer and λ max values are in nm.
The bark of Plumeria acutifolia were collected from campus of Guru Jambheshwar University of Sciences and Technology, Hisar in June 2010 and authenticated by Dr H.B. Singh, Head Raw Material Herbarium & Museum, and Ref. NISCAIR/RHMD/Consult-2010-11/11/1413/11. A voucher specimen has been retained in Department of Pharmaceutical Science, Guru Jambheshwar University of Science & Technology, Hisar. The plant material was air-dried at room temperature and then powdered. The chemicals and reagents used were of Qualigens and SD Fine, Mumbai, LR grade. All other chemicals used were of analytical grade [2,3]. Melting points were determined in centigrade scale in one end open capillary and uncorrected. UV spectra were recorded in methanol on Elmer EZ-301 spectrophotometer and λ max values are in nm.
IR spectra were recorded on Shimadzu FTIR- 8201 spectrophotometer using KBr pellets and Vmax values are in cm-1. IHNMR and 13CNMR were recorded on Bruker Anance 400 spectrometer using deuterated dimethylsuifoxide (DMSO d6), deuterated benzene (C6D6) and deuterated chloroform (CDCl3) as solvents with trimethyl silane (TMS). Fast atomic bombardment mass spectra (FABMS) data were recorded on JEOL SX 102/DA-6000 mass spectrometer. Silica gel (60-120 mesh) was used for column chromatography.
Extraction and Isolation
The bark of Plumeria acutifolia (PA) (5.0 kg) was collected and air dried. The air dried barks cut into small pieces and pulverized made into a coarsely powdered. The powdered were subjected to hot extraction process with methanol for 72 hrs. The liquids were concentrated by distillation followed by drying and kept in desiccators [4]. The extract obtained was dissolved in minimum amount of methanol and adsorbed on silica gel to form slurry. The air dried slurry was chromatograph on silica gel loaded in petroleum ether (b.p. 60-80°C). The column was eluted with petroleum ether, ethyl acetate, chloroform and methanol to search out compounds on the basis of chemical reaction and spectral analysis. The following compounds were isolated on basis.
The bark of Plumeria acutifolia (PA) (5.0 kg) was collected and air dried. The air dried barks cut into small pieces and pulverized made into a coarsely powdered. The powdered were subjected to hot extraction process with methanol for 72 hrs. The liquids were concentrated by distillation followed by drying and kept in desiccators [4]. The extract obtained was dissolved in minimum amount of methanol and adsorbed on silica gel to form slurry. The air dried slurry was chromatograph on silica gel loaded in petroleum ether (b.p. 60-80°C). The column was eluted with petroleum ether, ethyl acetate, chloroform and methanol to search out compounds on the basis of chemical reaction and spectral analysis. The following compounds were isolated on basis.
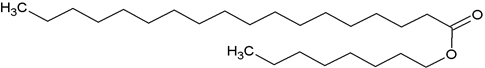
n-octyl stearate (NPA-1): Elution of the column with chloroform furnished light yellow crystals of compound NPA-1 it recrystallised
from Chloroform: Methanol (1:1), 244 mg (0.062%), RF 0.56, m.p. 85-87°C. IR νmax (KBr): 2921, 2850, 1723, 1637, 1439, 1266, 1026, 881,
720 cm-1. 1H-NMR (CDCl3) : δ 3.82 (2 H, d, J = 8.8 Hz, H2-1ʹ), 2.32 (2H, t, J =7.2 Hz, H2-2),1.82 (2H, m, CH2), 1.63 (2H, m, CH2), 1.55 (2H,
m, CH2),1.47 (2H, m, CH2), 1.23 (34 H, brs, 17 x CH2), 0.87 (3H, t, J = 6.5 Hz, Me - 8ʹ), 0.83 (3H, t, J = 6.2 Hz, Me - 18ʹ), 13C-NMR (CDCl3)
: δ 171.36 (C-1), 65.25 (C-1ʹ), 36.35 (CH2), 33.61 (CH2), 29.61 (CH2), 29.09 (CH2), 29.01(CH2), 28.69 (CH2), 28.52 (CH2), 26.57 (CH2),
24.45 (CH2), 22.07 (CH2), 13.91 (Me-8), 13.85 (Me-18). ESI MS m/z (rel. int.): 396 [M] + C26H52O2 (4.3), 283 (6.1).
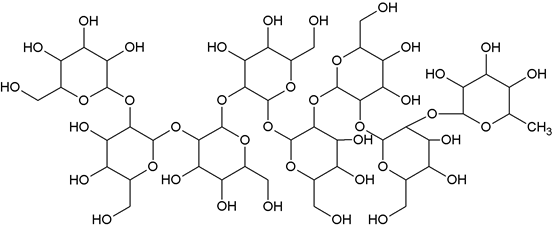
Heptoglucosyl rhamoside (NPA-2): Elution of the column with chloroform: methanol (19:1) furnished yellow brown colors crystals of compound NPA-2 recrystallised from chloroform: methanol (1:1), 238 mg (0.050%), Rf 0.56, m.p.160-162°C. IR νmax (KBr): 3561, 3455, 3376, 3285, 2919, 2851, 1633, 1436, 1309, 1289, 1163, 1038, 1004, 865, cm-1. 1H-NMR (CDCl3) : δ 5.54 (1 H, d, J = 8.4 Hz, H-1a), 5.18 (2H, brs, H-1b, H-1c), 5.13 (1H, d, m, J = 7.4 Hz, H-1d), 4.90 (3H, brs, H-1e, H-1f, H-1g ), 4.52 (1 H, d, J = 8.0 Hz, H-1h) 4.39 (2H, m, H-5a, H-5b), 4.27 (5H, m, H-5c, H-5d, H-5e, H-5f, H-5g), 4.51 (1H, m, H-5h), 3.92 (1H, m, H-2a), 3.88 (1H, m, H-2b), 3.82 (1H, m, H-2d), 3.80 (1H, m, H-2e), 3.76 (1H, m, H-2f), 3.74 (1H, m, H-2h), 3.69 (1H, m, H-2g), 3.67 (1H, m, H-3a), 3.64 (3H, m, H-3b, H-3c, H-3d), 3.60 (1H, m, H-3e), 3.57 (2H, m, H-3f, H-3h), 3.50 (1H, m, H-4a), 3.41 (1H, m, H-4b), 3.34 (1H, m, H-4c), 3.29 (2H, m, H-4d, H-4e), 3.24 3H, m, H-4f, H-4g, H-4h), 3.11(2H, d, J = 6.8 Hz, H2 – 6a), 3.08 (2H, d, J = 10.8 Hz, H2 – 6b), 3.06 (2H, d, J = 6.2 Hz, H2 – 6c), 3.06 (2H, d, J = 6.2 Hz, H2 – 6d), 3.04(4H, d, J = 9.2 Hz, H2 – 6e, H2-6f), 3.01(2H, d, J = 8.4 Hz, H2 – 6g), 1,27 (3H, d, J = 5.2 Hz, Me – 6h). 13C-NMR (CDCl3) : δ 104.59 (1a), 83.36 (2a), 74.72 (3a), 70.73 (4a), 77.45 (5a), 60.92 (6a), 104.47 (1b), 83.11 (2b), 73.53 (3b), 68.22 (4b), 77.19 (5b), 61.52 (6b), 98.51 (1c), 82.33 (2c), 72.91 (3c), 70.34 (4c), 76.24 (5c), 61.66 (6c), 97.34 (1d), 82.29 (2d), 72.81 (3d), 69.67 (4d), 76.22 (5d), 62.58 (6d), 96.46 (1e), 81.31 (2e), 72.42 (3e), 70.27 (4e), 76.11 (5e), 63.35 (6e), 92.67 (1f), 79.06 (2f), 72.08 (3f), 69.65 (4f), 77.19 (5f), 63.42 (6f), 92.20 (1g), 77.70 (2g), 71.02 (3g), 68.70 (4g), 75.76 (5g), 63.51 (6g), 102.44 (1h), 75.27 (2h), 73.26 (3h), 73.26 (4h), 64.80 (5h), 21.25 (6h). ESI MS m/z (rel. int.): 1298 [M]+ C48H82O40 (1.3).
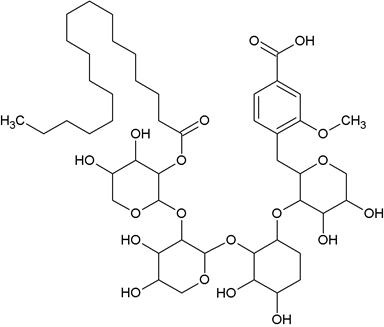
Vanillic acid-O-tetraarabinosyl stearate (NPA-3): Elution of the column with chloroform: methanol (9: 1) furnished pale yellow crystals of compound NPA-3 recrystallized from chloroform: methanol (1:1), 234 mg (0.047%), RF 0.42, m.p. 116-117°C. IR νmax (KBr): 3410, 3383, 3250, 2920, 2851, 1755, 169o, 1635, 1525, 1437, 1289, 1037 cm-1. 1H-NMR (CDCl3) : δ 7.26 (1 H, d, J = 1.8 Hz, H-2), 7.01 (1H, dd, m, H-6), 6.14 (1H, d, J = 7.8, H-5), 5.32 (1H, d, J = 7.2 Hz, H-1a), 5.07 (1H, d, J = 7.1 Hz, H-1b) 4.93 (1H, brs, H-1c), 4.86 (1H, d, J = 7.2 Hz, H-1d), 4.28 (2H, d, J = 7.2 Hz, H-2a, H-2d), 4.14 (2H, m, H-2b, H-2c), 3.59 (1H, m, H-3a, H-3b), 3.33 (1H, m, H-3c), 3.28 (1H, m, H-3d), 3.25 (2H, m, H-4a, H-4b), 3.21 (2H, m, H-4c, H-4d), 3.18 (2H, brs, H2-5a), 3.15 (2H, brs, H2-5b), 3.10 (2H, brs, H-5c), 3.05 (2H, brs, H2-5d), 3.50 (3H, brs, OMe), 2.02 (2H, t, J = 7.2 Hz, H-2), 1.73 (2H, m, CH2), 1.52 (2H, m, CH2), 1.28 (8 H, brs, 4 x CH2), 1.25 (18 H, brs, 9 x CH2), 0.85 (3H, t, J = 6.5 HZ, Me-18'). 13C-NMR (DMSO-d6): δ 148.31 (C-1), 139.54 (C-2), 150.98 (C-3), 166.17 (C-4), 137.30 (C-5), 129.27 (C-6), 181.26 (C-7), 109.01 (C-1a) 88.13 (C-2b), 77.23 (C-3a), 69.76 (C-4a), 63.02 (C-5a), 98.20 (C-1b), 84.76 (C-2b), 76.37 (C-3b), 67.55 (C-4b), 61.13 (C-5b), 98.02 (C-1b), 84.76 (C-2b), 76,37 (C-3b), 67.55 (C-4b), 61.13 (C-5b), 95.77 (C-2c), 84.12 (C-1c), 72.96 (C-3c), 67.31 (C-4c), 60.90 (C-5c), 91.90 (C-1d), 88.75 (C-2b), 72.44 (C-3d), 67.29 (C-4b), 63.02 (C-5d), 170.47 (C-1ʹ), 48.52 (C-2ʹ), 32.47 (CH2), 31.51 (CH2), 28.0 (8 H, CH2), 28.67 (3CH2), 24.45 (CH2), 22.06 (CH2), 13.91 (Me-18), 51.29 (OMe). ESI MS m/z (rel. int) : 962 [M]+ (C46 H74 O21) (1.1), 283 (5.3), 267 (2.5).
Result and Discussion [5-7]
The Compound NPA-1, named n-octyl stearate, was obtained as a yellow crystalline product from chloroform (100%) eluant. It exhibite distinct IR absorption bands for ester function (1723 cm-1) and aliphatic chain (720 cm-1). On the basis of Mass and 13CNMR spectra. The molecular ions peaks of NPA-1 were determined at m/z 396 consistent to the molecular formula of a fatty acid substituted aliphatic ester (C26H52O2). The ions peaks arising at m/z 283 indicating that stearic acid and esterifies with n-octanol.
The 1HNMR spectrum of compound NPA-1 exhibited signals as two-one proton triplets at δ 3.82 (J= 8.8 Hz), 2.32 (J= 7.2 Hz) and three-proton triplet at δ 0.87 (J= 6.5 Hz) and 0.83 (J= 6.2 Hz) assigned to oxygenated methylene H2-1', methylene near by the ester group and methylene Me-8' and Me-18' H2-2 respectively. The methylene protons appeared from δ 1.82 to 1.23. The 13CNMR spectrum of compound NPA-1 displayed signals for ester carbon at δ 171.36 (C-1), oxygenated methylene carbon at δ 65.27, methylene carbons between δ 36.35-22.07 and methyl groups at δ13.91 and 13.85. On the basis of these evidences the structure of NPA-1 has been formulated as n- octyl stearate (n-octyl n-octadecanoate).
The compound NPA-2, named heptoglucosyl rhamoside was obtained as a colourless crystalline product from chloroform: methanol (19:1) eluant. It gave positive tests for glycosides and showed IR absorption bands for hydroxyl groups at (3561, 3455, 3376, 3285 cm-1). On the basis of mass and 13CNMR spectra, the molecular ions peaks of NPA-2 were determined at m/z 1298 consistent to the molecular formula of an octaglycoside (C48H82O40). The 1HNMR spectrum of compound NPA-2 exhibited anomeric signals as one-proton doublets at δ 5.54 (J= 8.4 Hz), 5.13 (J= 7.4 Hz), 4.52 (J= 8.0 Hz), as a two-proton broad singlet at δ 5.18 and as a three-proton broad singlet at δ 4.90. The other sugar protons appeared between δ 4.39- 3.01.
A three-proton doublet at δ1.27 was due to C-6h secondary methyl protons. The 13CNMR spectrum of compound NPA-2 displayed a signals for the anomeric carbon from δ 104.59 (1a) to 92.20 and other sugar carbon from δ 83.36 to 60.92. The presence of sugar C-2 carbons in the deshielded region from δ 83.36 to 77.70 indicated (1→2) linkage of the sugar units. On the basis of these evidences the structure of NPA-2 has been formulated as β-D-glucoopyranosyl-(2a→ 1b)- β-D-glucopyranosyl- (2b→ 1c)- β-D-glucopyranosyl-(2c→ 1d)-β-D-glucopyranosyl-(2d→ 1e)-β-D- glucopyranosyl-(2e→ 1f)- β-D-glucopyranosyl-(2f→ 1g)- β-D-glucopyranosyl-(2g→ 1h)- β-D-rhamnopyranoside.
The compound NPA-3, named a vanillic acid-O-tetraarabinosyl stearate was obtained as a pale yellow crystalline product from chloroform: methanol (9:1) eluant. It gave positive tests for glycosides and showed IR absorption bands for hydroxyl groups at peaks (3410, 3383, 3250 cm-1), carboxylic function (1690 cm-1), ester group (1755 cm-1) and aromatic ring (1635, 1525, 1037 cm-1). On the basis of mass and 13CNMR spectra, the molecular ions peaks of NPA-3 was determined at m/z 962 consistent to the molecular formula of a vanillic acid substituted tetra glycoside ester, (C46H74O21). The ions peaks arising at m/z 283 indicated stearic acid was esterified with the sugar chain.
The 1HNMR spectrum of compound NPA-3 exhibited aromatic signals as one-proton doublets at δ 7.26 (J= 1.8 Hz), 6.14 (J= 7.8 Hz), 5.32 (J =7.2 Hz) and a one-proton multiplet at δ 7.01 assigned to H-2, H-5 and H-6 respectively. Four one-proton signals as doublets at δ 5.32 (J =7.2 Hz), 5.07 (J =7.1 Hz), 4.86 (J =7.2 Hz) and a broad singlet at 4.93 anomeric H-1a, H-1b, H-1d and H-1c respectively. The other sugar protons resonated between δ 4.28-3.05. The methoxy protons appeared as a three-proton broad singlets at δ 3.50.
The methylene protons were observed from δ 2.30 to 1.25. A Three one-proton triplet at δ 0.85 (J =6.5 Hz) was accounted to C-18' primary methyl protons. The 13CNMR spectrum of compound NPA-3 displayed signals for carboxylic carbon at δ 181.26 (C-7), aromatic carbons between δ 150.98-129.27, methoxy carbons at δ 51.29, sugar carbons between δ 109.01-60.90, methylene carbons at δ 32.47 to 22.06 and methyl carbon at δ 13.91. On the basis of these evidences the structure of NPA-3 has been formulated as Vanillic acid-O-β-D-arabinopyranosyl-(2a→ 1b)-β-D-arabinopyranosyl (2b→ 1c) -β-D-arabinopyranosyl-(2c→ 1d)-β-D- Arabinopyranosyl-2-d-n-octadecanoate.
Financial support and sponsorship
The author wishes to thanks the University Grants Commission, New Delhi, for providing a financial assistance in the form Senior Research Fellowship (SRF).
The author wishes to thanks the University Grants Commission, New Delhi, for providing a financial assistance in the form Senior Research Fellowship (SRF).
Conflicts of interest
There are no conflicts of interest.
There are no conflicts of interest.
References
- Rastogi RP and Merhotra BN. “Compendium of Indian Medicinal Plants”. Central Drug Research Institute and Publication & Information Directorate 2 (1991): 536-548.
- Fried B and Sherma J. “Thin layer chromatography”. Edn 4.7 (1986): 83-94.
- Harbone JB. “Phytochemical methods: A guide to modern techniques of plant analysis”. Chapmann and Hall, Landon (1988): 1-226.
- Schimidt PC. “Technological aspects of the development and production of plant extracts”. Pharmaceutical Industry 59 (1997): 59-69.
- Sharma SK and Ali M. “Isolation of some novel phytoconstituents from Anaphalis araneosa roots”. Indian Journal of Chemistry 42.11 (2003): 2858-2862.
- Sharma SK and Ali M. “A new stgmastane derivative from roots of Malva perviflora”. Indian Journal of Chemistry 38.6 (1999): 747-748.
- Sharma SK and Ali M. “New 9 β-linostane-type triterpenoids acid and 13, 14 secosteroidal esters from Artemesia scopana”. Journal of Natural Products 59.2 (1996): 181-184.
Citation:
Naresh Kumar and Surendra Kr Sharma. “Isolation of New Aromatic Ester from Plumeria acutifolia bark”. Chronicles of
Pharmaceutical Science 2.1 (2018): 469-473.
Copyright: © 2018 Naresh Kumar and Surendra Kr Sharma. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.