Review Article
Volume 2 Issue 3 - 2018
Block Copolymer Micelles in Drug Delivery and Cancer Therapy
1Pharmaceutical Sciences Laboratory, Faculty of Science and Engineering, Abo Akademi University, 20520 Turku, Finland
2Vedica College of Pharmacy, R.K.D.F University, Bhopal, 462033 (M.P), India
2Vedica College of Pharmacy, R.K.D.F University, Bhopal, 462033 (M.P), India
*Corresponding Author: Kuldeep K Bansal, Pharmaceutical Sciences Laboratory, Faculty of Science and Engineering, Abo Akademi University, 20520 Turku, Finland.
Received: April 16, 2018; Published: April 24, 2018
Abstract
Block copolymer micelles has been emerged as a better and safer alternatives to small surfactant molecules for drug delivery applications. This review focused on the applications of amphiphilic blocks copolymer in drug delivery. The design of block copolymers along with the possible mechanism of drug loading in the hydrophobic core of the micelles has been presented. Further, fabrication methods of micelles were discussed along with shortcomings of individual procedure. The advantages of polymeric micelles in targeted cancer therapy have been stated giving emphasis on active and passive targeting. Finally, the prospective and improvement possibility in selection of block copolymers is being argued.
Keywords: Micelles; Targeted delivery; Amphiphilic block copolymer; Drug delivery; Active targeting; Passive targeting
Introduction
A surfactant is a molecule, which comprises both a water soluble and insoluble portion. Surfactants can form micelles after being dispersed in aqueous solutions by self-assembly above their critical micelle concentrations (CMC). The CMC is defined as the concentration of surfactant molecules above which they start forming micelles. However, small surfactant molecules such as sodium lauryl sulphate, polysorbates etc. usually have a very high CMC value thus can dissociate upon dilution in the bloodstream or other biological fluids in vivo. Due to this limitation, the use of these surfactants as drug delivery vehicles have been limited and therefore alternative amphiphilic block copolymers surfactants have been developed to address this problem [1]. Polymeric micelles prepared from amphiphilic block copolymers have recently attracted more attention due to their unique structure with low CMC values.
Amphiphilic block copolymers can form micelles in aqueous solvent with a hydrophobic core sterically stabilized by a hydrophilic shell (figure 1). The hydrophobic core serves as a reservoir for drugs with low aqueous solubility while the hydrophilic shell prevents the adsorption of opsonise on the surface. Additionally the nano-scopic sized polymeric micelles (10–200 nm in diameter) are sufficiently large to avoid renal excretion (>50 kDa) as well as small enough to bypass the filtration of inter-endothelial cells in the spleen. All these factors contribute towards the longer blood circulation time of micelles, which leads to improved accumulation at tissue sites with vascular abnormalities [2-5]. PEG is the polymer of choice to be used as the hydrophilic block whereas the hydrophobic block can be chosen based on the required application including but not limited to poly (lactic acid), poly (caprolactone), poly (aspartic acid), poly(decalactone), poly(carbonate), polyion complex etc. [2,3, 6-8].
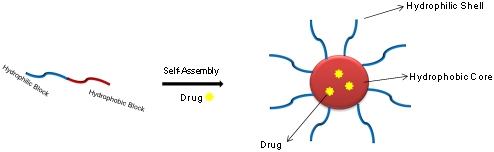
Figure 1: Pictorial presentation of self-assembly of an amphiphilic
block copolymer into micelles when dispersed in water [9].
Some of the reasons, which makes PEG consistently a polymer of choice for fabricating amphiphilic block copolymers are related to its inexpensive, non-toxic nature, and is a FDA approved polymer for the use in drug products [3]. Additionally, in micelles structure, PEG forms a dense, brush-like shell which imparts steric stability to the formulation [4]. Further, PEG is known to increase the circulatory time of carriers by impeding their uptake by the cells of the Reticuloendothelial System (RES) [10]. Moreover, PEG can be easily functionalised to attach the targeting ligands for targeted drug delivery applications [11-13].
Polymeric micelles have been widely utilised as solubilising tool for hydrophobic drugs [3]. The micelle structures are known to have an anisotropic distribution of water and therefore the core of the micelles is usually water free [14]. During the drug loading procedure, the hydrophobic drugs migrate towards the hydrophobic block (core) due to the hydrophobic interaction. Hydrophobic interaction is defined as the interaction between the non-polar substances in water. This interaction brings the non-polar (hydrophobic) molecules together in order to have minimal contact with water. This is a spontaneous process and is reasonably stronger than other weak intermolecular forces such as hydrogen bonding [15,16]. Therefore, during drug encapsulation procedure, hydrophobic core and drug come together to obtain drug loaded micelles. Furthermore, hydrophilic block provides the steric stability to micelles due to which they remain well dispersed in aqueous solution without aggregation [2,4]. In terms of thermodynamics, the drug solubilisation in micelles core can be considered as a partitioning of the drug between polar and non-polar phases [14]. In addition to the solubilisation tool, micelles have also known to increase the bioavailability, reduce the toxicity and offer the control release of loaded drugs leading to patient compliance [4,17].
As shown in figure 1, drug molecules are generally localised within the hydrophobic core separated from the outside environment by hydrophilic shell. This unique feature prevents the direct interaction of encapsulated drugs with the physiological environment such as cells or body fluids. This in turn, prevents any undesirable pharmacodynamics and pharmacokinetics reactions, which leads in improved bioavailability and reduction in toxicity of a drug. Using polymeric micelles as a drug delivery carrier is certainly beneficial because of various advantages it holds over other carrier systems like easier preparation method with tunable property, good loading capacity and better formulation stability [3,4,23,24]. All these advantages are due to the unique structure (core-shell) of polymeric micelles as discussed above. A number of micelles formulations are already in the clinical trials such as NK012, SP1049C, NC-6004, NK911 etc., (figure 2) while FDA has approved Genexol-PM for the treatment of breast cancer [3,25].
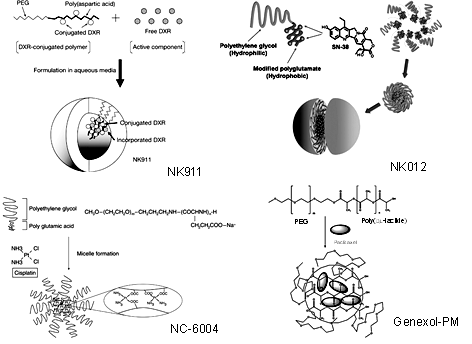
Figure 2: Schematic presentation of NK911 [18], NK012 [19],
NC-6004 [20] and Genexol-PM [21] micelle formulation.
Methods of Fabrication of Drug Loaded Polymeric Micelles
The four frequently used methods for the preparation of micelles and drug encapsulation are described below:
The four frequently used methods for the preparation of micelles and drug encapsulation are described below:
Dialysis Method
In this method, block copolymer and drug are dissolved in a water miscible non-volatile organic solvent (such as dimethyl sulfoxide and N, N-dimethyl form amide) followed by dialysis of the obtained solution against water. During dialysis, water will gradually replace the organic solvent from the dialysis bag leading to the self-assembly of amphiphilic polymer in micelles with encapsulated hydrophobic drug. It was suggested that during dialysis any unencapsulated drug will be removed from micellar solution leaving behind the drug loaded micelles only [22]. However, it should be noted that the replacement of organic solvent with water is a slow process. Hence, diffusion of some amount of drug into external media (water) might be possible before self-assembly. To avoid this problem Allen., et al. prepared the drug loaded micelles by adding the water directly to the drug-polymer solution (in DMSO) followed by dialysis in order to remove the solvent [23].
In this method, block copolymer and drug are dissolved in a water miscible non-volatile organic solvent (such as dimethyl sulfoxide and N, N-dimethyl form amide) followed by dialysis of the obtained solution against water. During dialysis, water will gradually replace the organic solvent from the dialysis bag leading to the self-assembly of amphiphilic polymer in micelles with encapsulated hydrophobic drug. It was suggested that during dialysis any unencapsulated drug will be removed from micellar solution leaving behind the drug loaded micelles only [22]. However, it should be noted that the replacement of organic solvent with water is a slow process. Hence, diffusion of some amount of drug into external media (water) might be possible before self-assembly. To avoid this problem Allen., et al. prepared the drug loaded micelles by adding the water directly to the drug-polymer solution (in DMSO) followed by dialysis in order to remove the solvent [23].
Oil-in-Water Emulsion Method
In this method, block copolymer and drug are dissolved in a water immiscible volatile organic solvent such as chloroform, ethyl acetate and methylene chloride. The solution is then slowly added to the aqueous phase under stirring to make an oil-in-water emulsion. In some cases, additional surfactants are also used to make a stable emulsion. The organic solvent is then evaporated at room temperature to yield the drug loaded micelles [22,24]. However, the use of chlorinated solvents are not usually recommended in drug delivery applications.
In this method, block copolymer and drug are dissolved in a water immiscible volatile organic solvent such as chloroform, ethyl acetate and methylene chloride. The solution is then slowly added to the aqueous phase under stirring to make an oil-in-water emulsion. In some cases, additional surfactants are also used to make a stable emulsion. The organic solvent is then evaporated at room temperature to yield the drug loaded micelles [22,24]. However, the use of chlorinated solvents are not usually recommended in drug delivery applications.
Solvent Evaporation/Film Method
In this method, block copolymer and drug are dissolved in a suitable volatile organic solvent and then the solvent is evaporated to make a thin polymer-drug film on the wall of a flask. The film is then reconstituted with the aid of aqueous solvent by vigorous shaking to produce the drug loaded polymeric micelles [24,25]. Large scale production is possible with the solvent evaporation method. However, the use of this method is not preferred to make micelles from block copolymers with high hydrophobic to hydrophilic ratio. Due to the high hydrophobicity, the complete reconstitution of such polymers by simple mixing is difficult [22].
In this method, block copolymer and drug are dissolved in a suitable volatile organic solvent and then the solvent is evaporated to make a thin polymer-drug film on the wall of a flask. The film is then reconstituted with the aid of aqueous solvent by vigorous shaking to produce the drug loaded polymeric micelles [24,25]. Large scale production is possible with the solvent evaporation method. However, the use of this method is not preferred to make micelles from block copolymers with high hydrophobic to hydrophilic ratio. Due to the high hydrophobicity, the complete reconstitution of such polymers by simple mixing is difficult [22].
Co-solvent evaporation/Nanoprecipitation Method
In this method, block copolymer and drug are dissolved in a water miscible volatile organic solvent (such as acetone, tetrahydrofuran) and then added drop wise to water under stirring. The diffusion of solvent in water with simultaneous evaporation triggered the self-assembly of copolymer, yielding the drug loaded polymeric micelles [26,27]. In this method, solubility of copolymer and drug is must in the chosen solvent, which makes this procedure troublesome on few occasion.
In this method, block copolymer and drug are dissolved in a water miscible volatile organic solvent (such as acetone, tetrahydrofuran) and then added drop wise to water under stirring. The diffusion of solvent in water with simultaneous evaporation triggered the self-assembly of copolymer, yielding the drug loaded polymeric micelles [26,27]. In this method, solubility of copolymer and drug is must in the chosen solvent, which makes this procedure troublesome on few occasion.
Polymeric Micelles in Cancer Therapy
Cancer therapy (chemotherapy) needs targeted delivery of cytotoxic drugs to tumours to avoid unwanted side-effects, which are attributed to the distribution of drugs in normal tissues. Targeted delivery of drugs to the tumors (cancer cells) can be achieved with the aid of suitable drug delivery carriers based on active and passive targeting strategies (figure 3) [28-32].
Cancer therapy (chemotherapy) needs targeted delivery of cytotoxic drugs to tumours to avoid unwanted side-effects, which are attributed to the distribution of drugs in normal tissues. Targeted delivery of drugs to the tumors (cancer cells) can be achieved with the aid of suitable drug delivery carriers based on active and passive targeting strategies (figure 3) [28-32].
Indeed, polymeric micelles as a drug delivery carrier for cytotoxic drugs offer numerous advantages in chemotherapy [24, 33-35]. For instance, the incorporation of cytotoxic drugs into micelles has been reported to increase the half-life of drug by circumventing its elimination by the liver and/or kidneys thus increasing the bioavailability [34]. Additionally, small size micelles have been reported to passively target the tumors by the Enhanced Permeability and Retention (EPR) effect [36,37]. Moreover, many anticancer drugs are hydrophobic in nature and hence encapsulating them within polymeric micelles can enhance their aqueous solubility (thus they can be more easily administrable in the body) and consequently bioavailability [24,34,35]. Furthermore, the controlled release of bio-actives for a longer duration at a tumor site can also increase the effectiveness of treatment [24,34,35]. In addition to that, the shell of polymeric micelles can be modified for active targeting by attaching specific ligands. This modification enhanced the selectivity of polymeric micelles for tumor cells and consequently improved the intracellular drug delivery [34,38]. Thus, the use of micelles for cancer therapy can be beneficial in order to improve the bioavailability and to reduce the side effects of anticancer drugs [36,37].
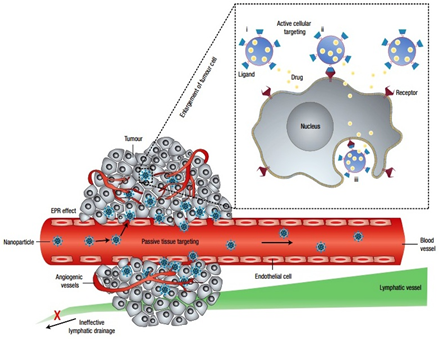
Figure 3: Schematic presentation of targeted therapy to tumors with the aid of nanoparticles (micelles) by active and passive mechanism [32]. (Reproduced with permission).
Passively targeted micelles for Cancer Therapy
Targeting solid tumors using long circulatory drug delivery carriers via the Enhanced Permeability and Retention (EPR) effect is considered as passive targeting. The EPR effect was first described by Maeda and co-worker [39]. Physiological and pathological studies of solid tumours suggested that the tumor vasculature possessed some unique characteristics such as incomplete architecture and immature lymphatic capillaries. Tumor vasculature generally has poorly aligned and defective endothelial cells with broad fenestrations (up to 4 μm) and lacking smooth muscle layer (or innervations and functional lymphatics). Additionally, impaired receptor function for vasoactive mediators especially angiotensin II in tumor vascular has been observed (figure 3) [28,30].
Targeting solid tumors using long circulatory drug delivery carriers via the Enhanced Permeability and Retention (EPR) effect is considered as passive targeting. The EPR effect was first described by Maeda and co-worker [39]. Physiological and pathological studies of solid tumours suggested that the tumor vasculature possessed some unique characteristics such as incomplete architecture and immature lymphatic capillaries. Tumor vasculature generally has poorly aligned and defective endothelial cells with broad fenestrations (up to 4 μm) and lacking smooth muscle layer (or innervations and functional lymphatics). Additionally, impaired receptor function for vasoactive mediators especially angiotensin II in tumor vascular has been observed (figure 3) [28,30].
The excessive production of vascular mediators, such as vascular endothelial growth factor (VEGF), bradykinin, fibroblast growth factor (bFGF), nitric oxide, peroxynitrite, prostaglandins, and matrix metalloproteinase, are responsible for the hyper-permeability in tumor tissues [40,41]. VEGF, a protein excessively secreted by tumors, plays an important role in the angiogenesis process which includes degradation of vascular basement membrane and surrounding extracellular matrix, as well as vascular endothelial cell division and migration [5]. This enhanced vascular permeability ensures the adequate supply of oxygen and nutrients for rapid growth of tumor tissues [41,42]. Recently, reduction in vascular permeability in colon carcinomas when treated with anti-VEGF antibody confirmed the role of VEGF in enhanced permeability of tumor vasculature [43]. Furthermore, due to the defective lymphatic function in tumors, continuous draining and renewal of interstitial fluid is minimal [44]. As a result, high retention time of a macromolecule has been observed in tumor tissues compared to normal tissues [32,45]. These two factors (i.e. Enhanced Permeation and Retention) comprise the EPR effect, due to which selective extravasation and accumulation of macromolecules in tumor tissues were observed [37,39,41].
Indeed several polymeric micelle formulations have been reported which accumulate at the tumor sites via the EPR effect [37]. For instance, PEG-poly (g-benzyl L-glutamate) block copolymer micelles loaded with cisplatin, demonstrated high accumulation in solid tumor in Lewis lung carcinoma bearing mice, compared to free drug. The high accumulation at the tumor site was suggested to occur via the EPR effect due to the prolonged blood circulation and small size ( approx. 30 nm in diameter) of micelles [46]. This formulation is now in Phase II clinical trials with the trade name “NC-6004”(20). NK105, PEG-poly (aspartic acid) micelles loaded with paclitaxel is another formulation which is in clinical trials. Approximately 50% of carboxylic acid groups of poly (aspartic acid) have been modified with 4-phenyl-1-butanol in the NK105 formulation, which increased the hydrophobicity of polymer and eventually paclitaxel loading (23% w/w approx.). The average size of 85 nm was observed with this formulation after redispersion in aqueous solvent. Approximately, 90-fold increase in the plasma area under curve (AUC), 25-fold increase in tumor AUC in Colon-26 tumors bearing CDF1 mice was observed, when compared with free drug. This high tumor uptake efficiency was attributed to the EPR effect of long circulatory NK105 micelles. Phase II clinical trials of NK105 were conducted in Japan, which was successfully completed in 2010 with positive results. Phase III Studies are on-going on patients with breast cancer and due to end by September 2016 [47,48]. Some more examples of polymeric micelles studied for tumor targeting via EPR effect are listed in table 1.
| Polymer | Drug | Size of micelles |
| PEG2000-PE/Vitamin E[49] | Paclitaxel, Curcumin |
15-20 nm |
| Pluronic® L61 and F127 (SP1049C)[50] | Doxorubicin | 30 nm |
| mPEG-b-poly(D,L-lactide)[51] | Docetexal | 16.62 ± 0.31 nm |
| mPEG-b-poly(D,L-lactide) (Genexol-PM)[52] | Paclitaxel | < 50 nm |
Table 1: Examples of micelles formulations, which demonstrated enhanced tumor uptake by EPR effect. (mPEG- monomethoxyl PEG).
Actively Targeted Micelles for Cancer Therapy
Tumor targeting potential of polymeric micelles can be further enhanced by attaching the targeting ligands on to the micelle surface (actively targeted micelles). The concept of active targeting is based on the ligand–receptor interactions at the target site i. e. tumor. After reaching the target site, ligand decorated micelles should interact with certain specific receptors present on the tumor cell and then be internalised by receptor-mediated endocytosis (figure 3 and 4) [30,34,38].
Tumor targeting potential of polymeric micelles can be further enhanced by attaching the targeting ligands on to the micelle surface (actively targeted micelles). The concept of active targeting is based on the ligand–receptor interactions at the target site i. e. tumor. After reaching the target site, ligand decorated micelles should interact with certain specific receptors present on the tumor cell and then be internalised by receptor-mediated endocytosis (figure 3 and 4) [30,34,38].
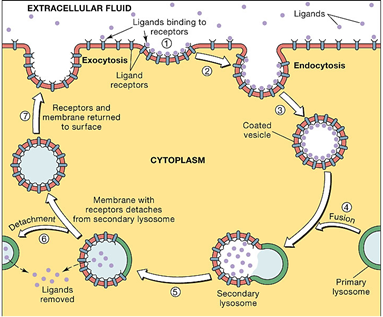
Figure 4: Receptor mediated endocytosis mechanism of a ligand after being attached to the specific receptor (source - http://droualb.faculty.mjc.edu).
Increase in the cellular concentration of anticancer agents via receptor mediated endocytosis leads to superior therapeutic efficacy of the drugs. This in turn reduces the dose size and side effects of cytotoxic drugs [53,54]. The selection of ligands is usually based on any receptor, which is overexpressed by tumor cells or tumor vasculature but have minimal or no expression by normal cells. Commonly used targeting ligands include antibodies, peptides, proteins, carbohydrates, small organic molecules and aptamers. The attachment of a ligand on to the surface of micelles is generally achieved either by the post-modification of a block copolymer with bifunctional spacer molecules or by the direct synthesis of hetero-bifunctional blocks [2]. Several polymeric micellar formulations based on ligand mediated targeting have been reported in literatures and were reviewed recently [24,34,55].
For instance, monoclonal antinucleosomal antibody (2C5) conjugated poly (ethylene glycol)-block-phosphatidyl ethanolamine (PEG-b-PE) micelles loaded with Doxorubicin (DOX) have been tested in a DOX-resistant ovarian cancer cell spheroid model. The 2C5 conjugated micelles demonstrated higher uptake (two fold) and penetration with greater cell death in spheroids compared to free DOX and non-targeted DOX micelles. The mean size observed for PEG–PE targeted micelles was 15 nm [56]. In another study Herceptin conjugated to d-α-tocopheryl polyethylene glycol succinate (vitamin E TPGS) micelles have been developed for targeted co-delivery of docetaxel and siRNA [57]. Antibodies are very popular as targeting ligands, but only limited conjugation of these moieties on micelle surface is possible due to their large size (~150 kDa). Furthermore, rapid clearance of antibody conjugated micelles might be observed due to their potential immunogenicity [58,59].
Transferrin (Tf) (protein) conjugation is another widely studied approach to fabricate targeted carriers for the specific delivery of cytotoxic drugs to the cancer cells [60,61]. For instance, Yue., et al. developed the transferrin conjugated mPEG-b-PLA polymeric micelles for their enhanced uptake in cancer cells [62]. The size range of the micelles was between 85-110 nm. They were tested on three human cell lines, SGC-7901 (gastric carcinoma), SKOV-3 (ovarian carcinoma), and MCF-7 (breast carcinoma) for uptake studies. Higher uptake of Tf- conjugated micelles (TfM-RhB) was evident by confocal laser scanning microscopy (CLSM) (using Rhodamine as marker) on MCF-7 and SGC-7901 cell lines compared to Tf-free micelles (M-RhB). SKOV-3 cells expressed a low level of transferrin and hence little difference in uptake was observed between TfM-RhB and M-RhB (figure 5). This study suggested that the high uptake was due to transferrin receptor mediated endocytosis [62]. High cellular uptake and effective tumor growth inhibition have been also demonstrated by using arginylglycylaspartic acid (RGD) (peptide) [63,64], lactose [65] and galactose [66] (carbohydrates) and A10-aptamer [67] as targeting ligand.
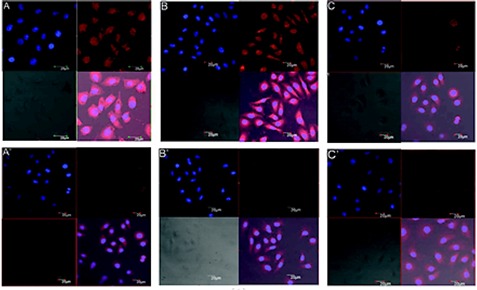
Figure 5: CLSM images of human MCF-7 (A and A′), SGC-7901 (B and B′), and SKOV3 (C and C′) cells incubated with TfM-RhB (A, B, and C) or M-RhB (A′, B′, and C′)[62] (reproduced with permission).
Due to the higher expression level of folate receptors in tumors (100 to 300 times) compared to normal tissue, folic acid (FA) as targeting ligand has been widely studied for cancer chemotherapy [68-70]. Folic acid is a commercially available small molecule that can be easily conjugated on to micelles surfaces [71]. Qiu., et al. reported the fabrication of targeted micelles using folate-modified poly (2-ethyl-2-oxazoline)-b-poly (ε-caprolactone) (FA-PEOz-PCL) block copolymer [72]. DOX loaded FA-PEOz-PCL micelles with the size range of 157-191 nm were tested for cellular uptake using folate receptor positive (FR+) Human HeLa cervical carcinoma cell lines (HeLa), human KB nasopharyngeal epidermal carcinoma cell lines (KB), Multidrug-resistant human breast cancer MCF-7/ADR cell lines and folate receptor negative (FR-) human A549 lung adenocarcinoma cell lines.
A higher cellular uptake of folate conjugated micelles (FA4) was observed with FR+ cell lines compared to non-folate micelles (FA0) and DOX. Further, folate receptor mediated endocytosis was confirmed by addition of free folic acid in cell culture media (FA4 + Folate). Addition of free folic acid competes with folate receptors for binding and thus reduced uptake of FA4 as evident by CLSM images. Moreover FA4 demonstrated lower IC50 values in FR+ cell lines compared to FA0 [72]. Recently, folic acid conjugate redox-responsive cross-linked block copolymer loaded with doxorubicin has been reported [73]. Microscopy images demonstrate that the conjugation of FA enhanced the cellular uptake efficiency whereas sustained release of drug was observed in environment mimicking tumor.
Conclusion
Polymeric drug-delivery systems have been investigated to address the problems associated with drugs such as poor aqueous solubility, stability and significant side effects. Indeed, polymeric micelles as a drug delivery carrier have demonstrated their potential to address some of the above mentioned problems as discussed earlier. Polymeric micelles can be easily prepared by conjugating a hydrophilic and hydrophobic polymer followed by its dispersion in aqueous solvent. Further, the unique core-corona structure of polymeric micelles provides satisfactory stability to this formulation. Due to these advantages, several polymeric micelles have been studied for the effective treatment of cancer and some of them are in clinical trials.
Apparently, biodegradable polymers because of their low toxicity and biodegradability are the polymers of choice to fabricate micelles for in vivo applications. Undoubtedly, polyesters are the front-runner biodegradable polymers used to generate the micelles. Polyesters derived from renewable feedstocks recently have attracted more attention due to the depletion of fossil fuel reserves and their increased prices. However, new sustainable materials are produced frequently; their applications in drug delivery have been rather less investigated.
Conflict of interest
Author declares no conflict of interest.
Author declares no conflict of interest.
References
- Trivedi R and Kompella UB. “Nanomicellar formulations for sustained drug delivery: strategies and underlying principles”. Nanomedicine 5.3 (2010): 485-505.
- Gaucher G., et al. “Block copolymer micelles: preparation, characterization and application in drug delivery”. Journal of Controlled Release 109.1.3 (2005): 169-188.
- Lu Y and Park K. “Polymeric micelles and alternative nanonized delivery vehicles for poorly soluble drugs”. International Journal of Pharmaceutics 453.1 (2013): 198-214.
- Torchilin VP. “Micellar nanocarriers: Pharmaceutical perspectives”. Pharmaceutical Research 24.1 (2007): 1-16.
- Nishiyama N and Kataoka K. “Current state, achievements, and future prospects of polymeric micelles as nanocarriers for drug and gene delivery”. Pharmacology & Therapeutics 112.3 (2006): 630-648.
- Harada A and Kataoka K. “Polyion complex micelle formation from double-hydrophilic block copolymers composed of charged and non-charged segments in aqueous media”. Polymer Journal 50 (2017): 95-100.
- Gao Y., et al. “Supramolecular assembly of poly (β-cyclodextrin) block copolymer and benzimidazole-poly (ε-caprolactone) based on host-guest recognition for drug delivery”. Colloids and Surfaces B: Biointerfaces 160 (2017): 364-371.
- Kakde D., et al. “Amphiphilic block copolymers from a renewable?-decalactone monomer: prediction and characterization of micellar core effects on drug encapsulation and release”. Journal of Materials Chemistry B 4.44 (2016): 7119-7129.
- Bansal KK., et al. “Renewable poly(δ-decalactone) based block copolymer micelles as drug delivery vehicle: in vitro and in vivo evaluation”. Saudi Pharmaceutical Journal 26.3 (2018): 358-368.
- Gref R., et al. “'Stealth' corona-core nanoparticles surface modified by polyethylene glycol (PEG): influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption”. Colloids and Surfaces B-Bio interfaces 18.3.4 (2000): 301-313.
- Di Y., et al. “pH-sensitive and folic acid-targeted MPEG-PHIS/FA-PEG-VE mixed micelles for the delivery of PTX-VE and their antitumor activity”. International journal of nanomedicine 12 (2017): 5863-5877.
- Fang Y., et al. “Targeted glioma chemotherapy by cyclic RGD peptide-functionalized reversibly core-crosslinked multifunctional poly (ethylene glycol)-b-poly (ε-caprolactone) micelles”. Acta Biomaterialia 50 (2017): 396-406.
- Hu J-B., et al. “E-selectin-targeted Sialic Acid-PEG-dexamethasone Micelles for Enhanced Anti-Inflammatory Efficacy for Acute Kidney Injury”. Theranostics 7.8 (2017): 2204-2219.
- Torchilin VP. “Structure and design of polymeric surfactant-based drug delivery systems”. Journal of Controlled Release 73.2.3 (2001): 137-172.
- Kauzmann W. “Some Factors in the Interpretation of Protein Denaturation”. Advances in Protein Chemistry 14 (1959): 1-63.
- Schellman JA. “In Memoriam: Walter Kauzmann (1916-2009)”. Protein Science 19.3 (2010): 363-371.
- Kataoka K., et al. “Block copolymer micelles for drug delivery: design, characterization and biological significance”. Advanced Drug Delivery Reviews 47.1 (2001): 113-131.
- Matsumura Y., et al. “Phase I clinical trial and pharmacokinetic evaluation of NK911, a micelle-encapsulated doxorubicin”. British Journal of Cancer 91.10 (2004): 1775-1781.
- Matsumura Y. “Polymeric Micellar Delivery Systems in Oncology”. Japanese Journal of Clinical Oncology 38.12 (2008): 793-802.
- Uchino H., et al. “Cisplatin-incorporating polymeric micelles (NC-6004) can reduce nephrotoxicity and neurotoxicity of cisplatin in rats”. British Journal of Cancer 93.6 (2005): 678-687.
- Kim SC., et al. “In vivo evaluation of polymeric micellar paclitaxel formulation: toxicity and efficacy”. Journal of Controlled Release 72.1.3 (2001): 191-202.
- Aliabadi HM and Lavasanifar A. “Polymeric micelles for drug delivery”. Expert opinion on drug delivery 3.1 (2006): 139-162.
- Allen C., et al. “|Polycaprolactone-b-poly (ethylene oxide) copolymer micelles as a delivery vehicle for dihydrotestosterone”. Journal of Controlled Release 63.3 (2000): 275-286.
- Kedar U., et al. “Advances in polymeric micelles for drug delivery and tumor targeting”. Nanomedicine-Nanotechnology Biology and Medicine 6.6 (2010): 714-729.
- Jee J-P., et al. “Encapsulation and Release of Amphotericin B from an ABC Triblock Fluorous Copolymer”. Pharmaceutical Research 29.1 (2012): 69-82.
- Gou M., et al. “Curcumin-loaded biodegradable polymeric micelles for colon cancer therapy in vitro and in vivo”. Nanoscale 3.4 (2011): 1558-1567.
- Bansal KK., et al. “New biomaterials from renewable resources - amphiphilic block copolymers from [small delta]-decalactone”. Polymer Chemistry (2015): 7196-7210.
- Cancer Nanotechnology: Methods and Protocols. Cancer Nanotechnology: Methods and Protocols (2010): 624.
- Brannon-Peppas L and Blanchette JO. “Nanoparticle and targeted systems for cancer therapy”. Advanced Drug Delivery Reviews 56.11 (2004): 1649-1659.
- Danhier F., et al. “To exploit the tumor microenvironment: Passive and active tumor targeting of nanocarriers for anti-cancer drug delivery”. Journal of Controlled Release 148.2 (2010):135-146.
- Bae YH and Park K. “Targeted drug delivery to tumours: Myths, reality and possibility”. Journal of Controlled Release 153.3 (2011): 198-205.
- Peer D., et al. “Nanocarriers as an emerging platform for cancer therapy”. Nature Nanotechnology 2.12 (2007): 751-760.
- Kwon GS and Kataoka K. “BLOCK-COPOLYMER MICELLES AS LONG-CIRCULATING DRUG VEHICLES”. Advanced Drug Delivery Reviews 16.2.3 (1995): 295-309.
- Oerlemans C., et al. “Polymeric Micelles in Anticancer Therapy: Targeting, Imaging and Triggered Release”. Pharmaceutical Research 27.12 (2010): 2569-2589.
- Torchilin VP. “Targeted polymeric micelles for delivery of poorly soluble drugs”. Cellular and Molecular Life Sciences 61.19.20 (2004): 2549-2559.
- Maeda H., et al. “Polymeric drugs for efficient tumor-targeted drug delivery based on EPR-effect”. European Journal of Pharmaceutics and Bio pharmaceutics 71.3 (2009): 409-419.
- Torchilin V. “Tumor delivery of macromolecular drugs based on the EPR effect”. Advanced Drug Delivery Reviews 63.3 (2011): 131-135.
- Sutton D., et al. “Functionalized micellar systems for cancer targeted drug delivery”. Pharmaceutical Research 24.6 (2007): 1029-1046.
- Maeda H., et al. “Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review”. Journal of Controlled Release 65.1.2 (2000): 271-284.
- Jain RK. “Delivery of molecular and cellular medicine to solid tumours”. Advanced Drug Delivery Reviews 46.1.3 (2012): 353-365.
- Maeda H. “Tumor-Selective Delivery of Macromolecular Drugs via the EPR Effect: Background and Future Prospects”. Bio conjugate Chemistry 21.5 (2010): 797-802.
- Fang J., et al. “The EPR effect: Unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect”. Advanced Drug Delivery Reviews 63.3 (2011): 136-151.
- Yuan F., et al. “Time-dependent vascular regression and permeability changes in established human tumor xenografts induced by an anti-vascular endothelial growth factor vascular permeability factor antibody”. Proceedings of the National Academy of Sciences of the United States of America 93.25 (1996): 14765-14770.
- Padera TP., et al. “Cancer cells compress intratumour vessels”. Nature 427 (2004): 695.
- Noguchi Y., et al. “Early phase tumor accumulation of macromolecules: a great difference in clearance rate between tumor and normal tissues”. Japanese Journal of Cancer Research 89.3 (1998): 307-314.
- Nishiyama N., et al. “Novel cisplatin-incorporated polymeric micelles can eradicate solid tumors in mice”. Cancer Research 63.24 (2003: 8977-8983.
- Hamaguchi T., et al. “NK105, a paclitaxel-incorporating micellar nanoparticle formulation, can extend in vivo antitumour activity and reduce the neurotoxicity of paclitaxel”. British Journal of Cancer 92.7 (2005): 1240-1246.
- Kato K., et al. “Phase II study of NK105, a paclitaxel-incorporating micellar nanoparticle, for previously treated advanced or recurrent gastric cancer”. Investigational New Drugs 30.4 (2012): 1621-1627.
- Abouzeid AH., et al. “Polyethylene glycol-phosphatidylethanolamine (PEG-PE)/vitamin E micelles for co-delivery of paclitaxel and curcumin to overcome multi-drug resistance in ovarian cancer”. International Journal of Pharmaceutics 64.1.2 (2014): 178-184.
- Danson S., et al. “Phase I dose escalation and pharmacokinetic study of pluronic polymer-bound doxorubicin (SP 1049C) in patients with advanced cancer”. British Journal of Cancer 90.11 (2004): 2085-2091.
- Li Y., et al. “A Novel Monomethoxy Polyethylene Glycol-Polylactic Acid Polymeric Micelles with Higher Loading Capacity for Docetaxel and Well-Reconstitution Characteristics and Its Anti-metastasis Study”. Chemical & Pharmaceutical Bulletin 60.9 (2012): 1146-1154.
- Kim TY., et al. “Phase I and pharmacokinetic study of Genexol-PM, a cremophor-free, polymeric micelle-formulated paclitaxel, in patients with advanced malignancies”. Clinical Cancer Research 10.11 (2004): 3708-3716.
- Bertrand N., et al. “Cancer nanotechnology: The impact of passive and active targeting in the era of modern cancer biology”. Advanced Drug Delivery Reviews 66 (2014): 2-25.
- Byrne JD., et al. “Active targeting schemes for nanoparticle systems in cancer therapeutics”. Advanced Drug Delivery Reviews 60.15 (2008): 1615-1626.
- Jhaveri AM and Torchilin VP. “Multifunctional polymeric micelles for delivery of drugs and siRNA”. Frontiers in pharmacology 5 (2014): 77.
- Perche F., et al. “Accumulation and toxicity of antibody-targeted doxorubicin-loaded PEG-PE micelles in ovarian cancer cell spheroid model”. Journal of Controlled Release 164.1 (2012): 95-102.
- Zhao J., et al. “Targeted co-delivery of docetaxel and siPlk1 by herceptin-conjugated vitamin E TPGS based immunomicelles”. Biomaterials 34.13 (2013): 3411-3421.
- Goldenberg DM and Sharkey RM. “Using antibodies to target cancer therapeutics”. Expert Opinion on Biological Therapy 12.9 (2012): 1173-1190.
- Kamaly N., et al. “Targeted polymeric therapeutic nanoparticles: design, development and clinical translation”. Chemical Society Reviews 41.7 (2012): 2971-3010.
- Vinogradov S., et al. “Polyion complex micelles with protein-modified corona for receptor-mediated delivery of oligonucleotides into cells”. Bioconjugate Chemistry 10.5 (1999): 851-860.
- Sawant RR., et al. “Targeted Transferrin-Modified Polymeric Micelles: Enhanced Efficacy in Vitro and in Vivo in Ovarian Carcinoma”. Molecular Pharmaceutics 11.2 (2014): 375-381.
- Yue J., et al. “Transferrin-Conjugated Micelles: Enhanced Accumulation and Antitumor Effect for Transferrin-Receptor-Overexpressing Cancer Models”. Molecular Pharmaceutics 9.7 (2012): 1919-1931.
- Miura Y., et al. “Cyclic RGD-Linked Polymeric Micelles for Targeted Delivery of Platinum Anticancer Drugs to Glioblastoma through the Blood-Brain Tumor Barrier”. Acs Nano 7.10 (2013): 8583-8592.
- Nasongkla N., et al. “cRGD-functionalized polymer micelles for targeted doxorubicin delivery”. Angewandte Chemie-International Edition 43.46 (2004): 6323-6327.
- Ma Pa., et al. “Lactose mediated liver-targeting effect observed by ex vivo imaging technology”. Biomaterials 31.9 (2010): 2646-2654.
- Yang R., et al. “Galactose-Decorated Cross-Linked Biodegradable Poly(ethylene glycol)-b-poly(epsilon-caprolactone) Block Copolymer Micelles for Enhanced Hepatoma-Targeting Delivery of Paclitaxel”. Biomacromolecules 12.8 (2011): 3047-3055.
- Xu W., et al. “Aptamer-conjugated and doxorubicin-loaded unimolecular micelles for targeted therapy of prostate cancer”. Biomaterials 34.21 (2013): 5244-5253.
- Ross JF., et al. “Differential regulation of folate receptor isoforms in normal and malignant tissues in vivo and in established cell lines”. Physiologic and clinical implications.” Cancer 73.9 (1994): 2432-2443.
- Hilgenbrink AR and Low PS. “Folate receptor-mediated drug targeting: From therapeutics to diagnostics”. Journal of Pharmaceutical Sciences 94.10 (2005): 2135-2146.
- Sudimack J and Lee RJ. “Targeted drug delivery via the folate receptor”. Advanced Drug Delivery Reviews 41.2 (2000): 147-162.
- Lu Y and Low PS. “Folate-mediated delivery of macromolecular anticancer therapeutic agents”. Advanced Drug Delivery Reviews 64 (2012): 342-352.
- Qiu L-Y., et al. “Folate-modified poly (2-ethyl-2-oxazoline) as hydrophilic corona in polymeric micelles for enhanced intracellular doxorubicin delivery”. International Journal of Pharmaceutics 456.2 (2013): 315-324.
- Lv Y., et al. “Folate-conjugated amphiphilic block copolymer micelle for targeted and redox-responsive delivery of doxorubicin”. Journal of Biomaterials Science Polymer Edition 29.1 (2018): 92-106.
Citation:
Kuldeep Kumar Bansal and Narendra Lariya. “Block Copolymer Micelles in Drug Delivery and Cancer Therapy”. Chronicles of
Pharmaceutical Science 2.3 (2018): 534-544.
Copyright: © 2018 Kuldeep Kumar Bansal and Narendra Lariya. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.