Research Article
Volume 2 Issue 3 - 2018
Brain Depression – Unifying Mechanism Involving Antioxidant Therapy: Reactive Oxygen Species, Oxidative Stress, and Phenolics.
1Department of Chemistry and Biochemistry, San Diego State University, San Diego, CA, USA
2Library and Information Access, San Diego State University, San Diego, CA, USA
2Library and Information Access, San Diego State University, San Diego, CA, USA
*Corresponding Author: Peter Kovacic, Department of Chemistry and Biochemistry, San Diego State University, San Diego, CA, USA.
Received: April 26, 2018; Published: May 05, 2018
Abstract
Reactive oxygen species (ROS) and oxidative stress (OS) play roles in depression, as also in Alzheimer’s (AD), Parkinson’s (PD) disease
and Schizophrenia (SCZ). Various sources, including oxidases, serve as generators of ROS-OS, such as mitochondria, NADPH,
cytochromes P450, monoamines, ET metal complexes, G72 gene, and microglia. Diverse types of antioxidants (AOs) exert a positive
influence on the harmful effects. However, there are fewer drugs in the phenols and phenolic ethers category, in comparison with AD
and PD. A unifying mechanism based on ET-ROS-OS-AO is involved. Other possible influential aspects are discussed in a multifaceted
approach.
Keywords: Depression; Radicals; Oxidative stress; Reactive oxygen species; Antioxidants
Abbreviations: ET: Electron transfer; ROS: Reactive oxygen species; OS: Oxidative stress; AO: Antioxidant; SCZ: Schizophrenia; AD:
Alzheimer’s disease; PD: Parkinson’s disease
Introduction
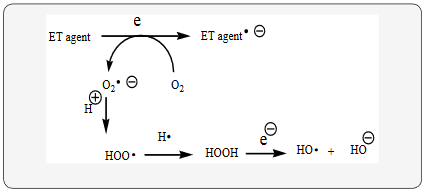
Depression fits into the unifying mechanism which has been widely applied, previously in an article involving electron transfer (ET), reactive oxygen species (ROS) and oxidative stress (OS) Kovacic, & Somanathan, 2017a) This unifying mechanism argues that the preponderance of bioactive substances, usually as the metabolites, incorporate ET functionalities. We believe these ET-metabolites play an important role in physiological responses. The main group includes quinones (or phenolic precursors), metal complexes (or complexors), aromatic nitro compounds (or reduced hydroxylamine and nitroso derviatives), and conjugated imines (or iminium species). Resultant redox cycling is illustrated in Scheme 1. In vivo redox cycling with oxygen can occur, giving rise to OS through generation of ROS, such as hydrogen peroxide, hydroperoxides, alkyl peroxides, and diverse radicals (hydroxyl, alkoxyl, hydroperoxyl, and superoxide) (Scheme 1). Cellular and mitochondrial enzymes can also perform cataytically in the reduction of O2.
In some cases, ET results in involvement with normal electrical effects (e.g. neurochemistry). Generally, active entities possessing ET groups display reduction potentials in the physiologically responsive range. Hence, ET in vivo can occur resulting in production of ROS which can be beneficial in cell signaling at low concentrations, but produce toxic results at high levels. Electron donors consist of phenols, N-heterocycles or disulfides in proteins which produce relatively stable radical cations. ET, ROS and OS have been increasingly implicated in the mode of action of drugs and toxins, e.g. anticancer drugs (Kovacic & Osuna, 2000), carcinogens (Kovacic & Jacintho, 2001), cardiovascular toxins (Kovacic & Thurn, 2005), toxins (Kovacic, Pozos, Somanathan, Shangari, & O’Brien, 2005), ototoxins (Kovacic & Somanathan, 2008) and various other categories (Hallowell & Gutteridge, 1999).
In addition to the above, there is a plethora of experimental evidence supporting the theoretical framework. This evidence includes generation of the common ROS, lipid peroxidation, degradation products of oxidation, depletion of AOs, effect of exogenous AOs, and DNA oxidation and cleavage products, as well as electrochemical data (Kovacic & Somanathan, 2017b). This comprehensive, unifying mechanism is consistent with the frequent observation that many ET substances display a variety of activities (e.g. multiple-drug properties), as well as toxic effects.
It is important to recognize that mode of action in the bio domain is often involved with many physiological actions and is multifaceted. In addition to ET-ROS-OS in relation to mechanism, much attention in the literature is paid to AO action entailing physiological action. Phenolic or their derivative play important roles in depression. However, the numbers are less than for AD and PD (Kovacic & Weston, 2017c) The AO thiols are present in greater numbers as drugs in our relevant reviews (Kovacic & Weston, 2017a; Kovacic & Weston, 2017b).
Various symptoms of depression exist with the most common one being low mood and an inability to enjoy activities. Other symptoms include difficulty concentrating, trouble sleeping or sleeping more than usual, feeling tired, small tasks appear impossible to manage, and agitation. However, with major depression there is a profound sadness or a sense of despair. A particularly painful symptom of this illness is an unshakable felling of worthlessness and guilt. The symptoms of major depression are defined as lasting a minimum of two weeks, but typically last much longer (Depression overview, 2017).
Many people with depression also have anxiety along with an increased risk for abusing alcohol and other substances. Furthermore, depression can be lethal since it clearly increases the risk for suicide (Abramson, Alloy, & Panzarella, 2005) Most likely, depression involves changes in the areas of the brain that control mood, in which these nerve cells may be functioning poorly in certain regions of the brain. This altered communication between nerve cells or nerve circuits can make it harder for a person to regulate mood (Depression overview, 2017).
Depression can occur at any age. However, the rates of depression surge during middle to late adolescence. In many western countries, about 20 percent of the population will experience a clinically significant episode of depression at some point in their lives with over 80 percent of depressed patients having more than one depressive episode (Abramson, Alloy, & Panzarella, 2005). In many cases, depression often does not appear to be related to a specific event; however, an episode of depression can be triggered by a stressful event (significant vocational, physical, or interpersonal impairment or event) or even a hormonal change. A major depressive episode may occur within the first two to three months after giving birth to a baby. Additionally, depression can be seasonal; depression that occurs mainly during the winter months is referred to as a seasonal affective disorder (SAD) (Depression overview, 2017). Depression is viewed as heterogeneous with multiple possible causes (Abramson, Alloy, & Panzarella, 2005).
Discussion
Phenols and Phenolic Ethers
Sedatives are frequently used for the treatment of depression and anxiety. Depression has been associated with OS in patients. Fisetin (Figure 1) produced a marked reduction in ROS levels on treatment of patients (Angelini., et al. 2010). The drug, a polyphenolic flavonoid, can function as an AO as reported for other brain illnesses (Kovacic & Weston, 2017a; Kovacic & Weston, 2017b).
Sedatives are frequently used for the treatment of depression and anxiety. Depression has been associated with OS in patients. Fisetin (Figure 1) produced a marked reduction in ROS levels on treatment of patients (Angelini., et al. 2010). The drug, a polyphenolic flavonoid, can function as an AO as reported for other brain illnesses (Kovacic & Weston, 2017a; Kovacic & Weston, 2017b).
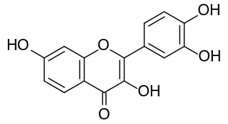
Olfactory bulbectomy (OB) in an animal model of depression can emulate the symptoms. Treatment of patients with quercetin (Figure 2) prevented depressant effects and hyperactivity. Quercetin, a polyphenolic flavonoid, belongs to the phenolic class of related AD drugs (Kovacic & Weston, 2017b) and PD (Kovacic & Weston, 2017a) drugs. In relation to OS, quercetin treatment reversed hyperactivity induced by OB, prevented depressant effects, and reversed lipid hydroperoxides content. The AO effects of the drug also contribute to other positive aspects, such as reversed behavioral hyperactivity. There is involvement of NO and receptors in depression (Holzmann., et al. 2015).
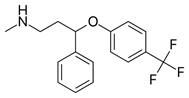
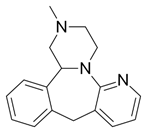
Fluoxetine (Prozac) (Figure 3), a drug used for treatment of depression, inhibits serotonin reuptake. Research indicates mediation of free radical reactions and production of liver damage. Evidence shows that the drug does not release toxic fluoride during metabolism (Inkielewicz-Stępniak, 2011). Evidence points to induction of AOs by use of brain drugs. Application of fluoxetine and atorvastatin caused an increase in AO enzyme activity and total AO status (Herbet., et al. 2016). The changes indicate an increase in AO levels on application of atorvastatin with serotonin reuptake inhibitors, suggesting an effect on redox status. Fluoxetine used in major depressive disorder, belongs to the phenolic ether class. Ether cleavage results in formation of the corresponding phenol (Kovacic & Weston, 2017a; Kovacic & Weston, 2017b). AO behavior is a common property of phenols. The phenol fits into the structure-activity relationships proposed for phenolic PD and AD drugs (Kovacic & Weston, 2017c).
In a 2017 article, mechanisms that link depression and diabetes are discussed, including increase in lipid peroxidation and decreased AO activity (Rebai, Jasmin, & Boudah, 2017). The tests included measures of OS and activity of AO enzymes, namely catalase, glutathione peroxidase and glutathione – S – transferase. Melatonin (Figure 4) and fluoxetine decreased the signs of depression and anxiety. Melatonin had a favorable influence on the AOs. Antidepressants and AOs exert a positive effect on mood and oxidative illnesses. A possible factor is decrease in generation of ROS. Melatonin is a phenolic ether that can be dealkylated to the corresponding phenolic serotonin which may have an influence as an AO (Kovacic & Weston, 2017a).
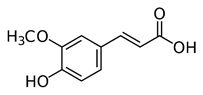
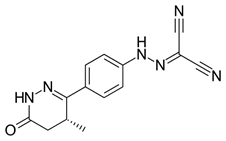
Ferulic acid (FA) (Figure 5), a natural phenolic substance, lessoned the OS and changes in behavior (Zeni, Camargo, & Dalmagro, 2017). FA may possibly be an agent for control of depression. Much evidence supports the AO action of the phenolic class. Depression entails activation of neuro-oxidative and neuro-nitrosative pathways which are drug targets in depression. Studies showed that rosiglitazone (Figure 6), a phenolic ether, had a favorable effect on depression. The drug attenuates ROS generated from microchondria (Pipatpiboon., et al. 2012).
Recently, the potential role of OS has been emphasized in the pathology of brain disorders, such as depression. There is the associated aspect of decreased levels of AOs (Smaga., et al. 2015). Smaga’s 2015 study addressed the effects of drug induced modulation of redox balance and measures for reduction of OS.
Thiols and disulfides
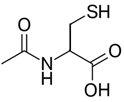
Stressful events in early life can produce increased OS in the CNS, with the risk of developing brain diseases, such as depression. Research on the sources of OS from stress could lead to new therapeutic measures (Schiavone, Colaianna, & Curtis, 2015). Increased evidence links OS to the pathology of depression. N-Acetylcysteine (Figure 7) demonstrates antidepressant action via decrease in OS. The substance enhanced cell AO action. The effect is associated with increase in SOD.
Stressful events in early life can produce increased OS in the CNS, with the risk of developing brain diseases, such as depression. Research on the sources of OS from stress could lead to new therapeutic measures (Schiavone, Colaianna, & Curtis, 2015). Increased evidence links OS to the pathology of depression. N-Acetylcysteine (Figure 7) demonstrates antidepressant action via decrease in OS. The substance enhanced cell AO action. The effect is associated with increase in SOD.
Research on depression often places focus on OS and inflammation. A study deals with the effects of the AO N-acetylcysteine and JAK/STAT signaling pathway (Al-Samhari., et al. 2016). Modulation of the signaling mode may be involved in the antidepressant influence of the AO thiol. Future research should target signaling as a means for improved therapy.
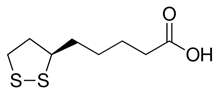
α- Lipoic acid (LA) (Figure 8) is a potent AO, which has been reported to alleviate a wide variety of illnesses involving ROS-OS. Depression is linked to the pro-inflammatory state, involving stress, which can also generate ROS. Prior evidence (Kovacic & Weston, 2018) and the present report support the AO as a promising agent for augmentation therapy in depression. LA was shown to exert protective effects including decrease in OS and reduction of redox signaling (Suh., et al. 2015). Reductive change yields the more potent AO dithiol.
Other drugs
Corticosterone (CORT) is known to produce a depression like state resulting in an increase in oxidative stress markers in the brain. In Silva., et al.’s 2016 study, CORT produced anxiety and depressive like behavior, accompanied by lipid peroxidation which were reversed by mirtazapine (Figure 9) or the combination with LA. The reversal is likely due to AO action. OS is known to be involved in the neurobiology of depression. Nitrate levels were increased by CORT and reversed by the AO combination. The effects of the AO Lipoic acid were examined in this study as well (Silva., et al. 2016).
Corticosterone (CORT) is known to produce a depression like state resulting in an increase in oxidative stress markers in the brain. In Silva., et al.’s 2016 study, CORT produced anxiety and depressive like behavior, accompanied by lipid peroxidation which were reversed by mirtazapine (Figure 9) or the combination with LA. The reversal is likely due to AO action. OS is known to be involved in the neurobiology of depression. Nitrate levels were increased by CORT and reversed by the AO combination. The effects of the AO Lipoic acid were examined in this study as well (Silva., et al. 2016).
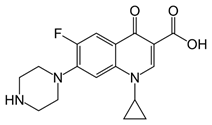
An investigation was made of depression induced by ciprofloxacin (CPX) (Figure 10), a quinolone antibiotic (Ilgin., et al. 2015). The drug increased MDA and decreased GSH and catalase activity indicating decreased AO defenses and OS. CPX produced neurological toxicity. Increased OS is believed to be an important underlying factor.
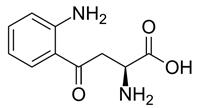
Neurotoxic and neuroprotective effects induced by kynurenine (Figure 11) are demonstrated in depression. The role of kynurenine metabolism involving neurochemical interactions is addressed as a contributing factor in depression (Myint., et al. 2012).
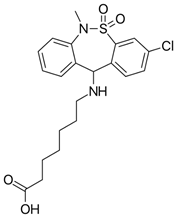
Evidence indicates that microglia are importantly involved in depression. Microglia are glia cells that exert various functions, including maintaining homeostasis. Activation can lead to various factors that may be involved in brain disorders, such as depression. A recent 2017 study was made of inhibition of microglial activation by tianeptime (Figure 12), an antidepressant drug (Slusarczyk., et al. 2017). Slusarczyk found an anti-inflammatory effect of tianeptime in lipopolysaccharide-stimulated microglial cells by decreasing the expression of proinflammatory cytokines, including the release of nitric oxide (NO), reactive oxygen species (ROS), and the expression of inducible NO synthase.
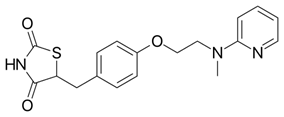
Levosimendan (Figure 13) is another drug with anti-inflammatory responses in addition to immunomodulatory effects by decreasing ROS-OS. The effects involved leucocytes, which are an important source of ROS in patients with depression (Hasslacher, 2011).
Depression, a frequent illness, does not possess a reliable biomarker. Characteristics include increase in OS and decrease in AO levels. SOD, which eliminates superoxide ROS, was markedly decreased in depressed patients (Camkurt., et al. 2016). Results point to enhanced OS. Malondialdehyde (MDA), an oxidation product, was commonly increased in serious depression. The conclusion suggested MDA as a preferred biomarker for major depression. Chronic mild stress (CMS) is commonly used as a model for depression. OS, induced by CMS, was generated by increase in ROS (Duda., et al. 2016). The extract of Cocoas nucifera husk (HECN) exhibits antidepressant effects. HECN generally decreased MDA levels, as well as nitrate concentrations (Lima., et al. 2016). The antidepressant properties appear to reflect AO and neurotropic effects.
Extract of the banana plant is known to exhibit AO activity (Reddy., et al. 2016). Antidepressant type activity is displayed by the plant extract which may reflect an AO presence which combats OS. Various reports demonstrate that different psychiatric illnesses are associated with enhanced levels of oxidative DNA damage, involving neurotoxic effects. In Raza., et al.’s 2016 study, they revealed that damaged genomic DNA may contribute to the pathophysiology of depression (Raza et al., 2016). This finding may be significant in relation to antipsychiatric drug design.
The extract of the Albizia adianthifolia (a tree in the Fabaceae family, also commonly known as Flat-crown) herb displays various medicinal properties, including AO and antidepressant. A 2015 study, suggested that the aqueous extract of this herb could lessen induced anxiety and depression (Beppe., et al. 2015). Statistically AO potential was also exhibited; suggesting a connection involving behavioral measures, AO potential and lipid peroxidation.
Maca (Lepidium meyenii, a cruciferous vegetable), considered a medicinal food in Peru, possesses antidepressant and anxiolytic effects. In Ai., et al.’s 2014 study, Maca increased dopamine levels and ROS was markedly inhibited, accompanied by attenuation of OS (Ai., et al. 2014). Fluoxetine, also discussed in this study, was used as a positive control drug.
References
- Abramson LY., et al. “Encyclopedia of cognitive science”. (2005).
- Ai Z., et al. “Antidepressant-like behavioral, anatomical, and biochemical effects of petroleum ether extract from maca (Lepidium meyenii) in mice exposed to chronic unpredictable mild stress”. Journal of Medicinal Food 17.5 (2014): 535-542.
- Al-Samhari MM., et al. “Possible involvement of the JAK/STAT signaling pathway in N-acetylcysteine-mediated antidepressant-like effects”. Experimental Biology and Medicine 241.5 (2016): 509-518.
- Angelini A., et al. “Modulation of multidrug resistance p-glycoprotein activity by flavonoids and honokiol in human doxorubicin- resistant sarcoma cells (MES-SA/DX-5): implications for natural sedatives as chemosensitizing agents in cancer therapy”. Journal of BIOLOGICAL REGULATORS & Homeostatic Agents 24.2 (2010): 197-205.
- Beppe GJ., et al. “The aqueous extract of Albizia adianthifolia leaves attenuates 6-hydroxydopamine-induced anxiety, depression and oxidative stress in rat amygdala”. BMC Complementary and Alternative Medicine 15 (2015: 374.
- Camkurt MA., et al. “Evaluation of Malondialdehyde, superoxide dismutase and catalase activity and their diagnostic value in drug naïve, first episode, non-smoker major depression patients and healthy controls”. Psychiatry Research 238 (2016): 81-85.
- Depression overview. In Harvard Medical School Health A-Z, editied by Harvard Medical School Health Publications (2017).
- Duda W., et al. “The Effect of Chronic Mild Stress and Imipramine on the Markers of Oxidative Stress and Antioxidant System in Rat Liver”. Neurotoxicity Research 30.2 (2016): 173-184.
- Halliwell B and Gutteridge JMC. Free Radicals in Biology and Medicine. New York: Oxford University Press (1999): 1-897.
- Hasslacher J., et al. “Levosimendan inhibits release of reactive oxygen species in polymorphonuclear leukocytes in vitro and in patients with acute heart failure and septic shock: a prospective observational study”. Critical Care 15.4 (2011): 166.
- Herbet M., et al. “Effect of the interaction between atorvastatin and selective serotonin reuptake inhibitors on the blood redox equilibrium”. Experimental and Therapeutic Medicine 12.5 (2016): 3440-3444.
- Holzmann I., et al. “Antidepressant-like effect of quercetin in bulbectomized mice and involvement of the antioxidant defenses, and the glutamatergic and oxidonitrergic pathways”. Pharmacology Biochemistry and Behavior 136 (2015): 55-63.
- Ilgin S., et al. “Ciprofloxacin-induced neurotoxicity: evaluation of possible underlying mechanisms”. Toxicology Mechanisms and Methods 25.5 (2015): 374-381.
- Inkielewicz-Stępniak I. “Impact of fluoxetine on liver damage in rats”. Pharmacological Reports 63.2 (2011): 441-447.
- Kovacic P and Jacintho JD. “Reproductive toxins. Pervasive theme of oxidative stress and electron transfer”. Current Medicinal Chemistry 8.7 (2001): 863-892.
- Kovacic P and Osuna JA. “Mechanisms of anticance agents: Emphasis on oxidative stress and electron transfer”. Current Pharmaceutical Design 6.3 (2000): 277-309.
- Kovacic P., et al. “Mechanism of mitochondrial uncouples, inhibitors, and toxins: Focus on electron transfer, free radicals, and structure-activity relationships”. Current Medicinal Chemistry 12.22 (2005): 2601-2623.
- Kovacic P and Somanathan R. “Ototoxicity and noise trauma: electron transfer, reactive oxygen species, cell signaling, electrical effects, and protection by antioxidants: practical medical aspects”. Medical Hypotheses 70.5 (2008): 914-923.
- Kovacic P and Somanathan R. “Unifying mechanism for nutrients as anticancer agents: Electron transfer, reactive oxygen species and oxidative stress”. Global Journal of Health Science 9.8 (2017): 66-83.
- Kovacic P and Somanathan R. “Various factors involving aging: Electron transfer, reactive oxygen species and oxidative stress”. International Journal of Current Research 9.7 (2017): 53518-53528.
- Kovacic P and Thurn LA. “Cardiovascular toxicity from the perspective of oxidative stress, electron transfer, and prevention by antioxidants”. Current Vascular Pharmacology3.2 (2005): 107-117.
- Kovacic P and Weston W. “Treatment of Parkinson’s disease with phenolic antioxidant drugs: Oxidative stress, reactive oxygen species and selectivity”. Chronicles of Pharmaceutical Science 1.4 (2017): 193-198.
- Kovacic P and Weston W. “Phenolic antioxidants as drugs for Alzheimer’s disease: oxidative stress and selectivity”. Novel Approaches in Drug Designing and Development 3.20 (2017): 555606.
- Kovacic P and Weston W. “Novel Structure-Activity Relationship and Quantitative Data for Phenolic Drugs Involving Alzheimer’s and Parkinson’s disease: Antioxidants, Oxidative Stress, and Selectivity”. Chronicles of Pharmaceutical Science 1.5 (2017): 299-306.
- Kovacic P and Weston W. “Antioxidant Therapy by Lipoic Acid in Various Illnesses: Reactive Oxygen Species and Oxidative Stress”. Novel Approaches in Drug Designing & Development 3.3 (2018): 555612.
- Lima EB., et al. “Antidepressant, antioxidant and neurotrophic properties of the standardized extract of Cocos nucifera husk fiber in mice”. Journal of Natural Medicines 70.3 (2016): 510-521.
- Myint AM., et al. “The role of the kynurenine metabolism in major depression”. Journal of Neural Transmission 119.2 (2012): 245-251.
- Pipatpiboon N., et al. “PPARγ agonist improves neuronal insulin receptor function in hippocampus and brain mitochondria function in rats with insulin resistance induced by long term high-fat diets”. Endocrinology 153.1 (2012): 329-338.
- Raza MU., et al. “DNA Damage in Major Psychiatric Diseases”. Neurotoxicity Research 30.2 (2016): 251-267.
- Rebai R., et al. “The antidepressant effect of melatonin and fluoxetine in diabetic rats is associated with a reduction of the oxidative stress in the prefrontal and hippocampal cortices”. Brain Research Bulletin 134 (2017): 142-150.
- Reddy AJ., et al. “Effect of Musa sapientum Stem Extract on Animal Models of Depression”. Pharmacognosy Research 8.4 (2016): 249-252.
- Schiavone, S., et al. “Impact of early life stress on the pathogenesis of mental disorders: relation to brain oxidative stress”. Current Pharmaceutical Design 21.11 (2015): 1404-1412.
- Silva MC., et al. “Evidence for protective effect of lipoic acid and desvenlafaxine on oxidative stress in a model depression in mice”. Progress in Neuro-Psychopharmacology & Biological Psychiatry 64 (2016): 142-148.
- Slusarczyk J., et al. “Anti-inflammatory properties of tianeptine on lipopolysaccharide-induced changes in microglial cells involve toll-like receptor-related pathways”. Journal of Neurochemistry 136.5 (2016): 958-970.
- Smaga I., et al. “Oxidative stress as an etiological factor and a potential treatment target of psychiatric disorders. Part 2. Depression, anxiety, schizophrenia and autism”. Pharmacological Reports 67.3 (2015): 569-580.
- Suh SH., et al. “Alpha-lipoic acid attenuates lipopolysaccharide-induced kidney injury”. Clinical and Experimental Nephrology19.1 (2015): 82-91.
- Zeni ALB., et al. “Ferulic acid reverses depression-like behavior and oxidative stress induced by chronic corticosterone treatment in mice”. Steroids 125 (2017): 131-136.
Citation:
Peter Kovacic and Wil Weston. “Brain Depression – Unifying Mechanism Involving Antioxidant Therapy: Reactive Oxygen
Species, Oxidative Stress, and Phenolics.” Chronicles of Pharmaceutical Science 2.3 (2018): 545-553.
Copyright: © 2018 Peter Kovacic and Wil Weston. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.