Research Article
Volume 2 Issue 3 - 2018
Bioequivalence Study of Dolutegravir, Emtricitabine and Tenofovir Alafenamide Tablets 50mg/200mg/25mg in Healthy Volunteers under Fasting Conditions
1Clinical Pharmacology Department, APL Research Centre, Aurobindo Pharma Ltd, Survey no.313, Bachupally, Hyderabad, Telangana, India
2Clinical Operations, Axis Clinicals Latina, Mexico
2Clinical Operations, Axis Clinicals Latina, Mexico
*Corresponding Author: Akula Thukaram Bapuji, Sr. Vice President, Aurobindo Pharma Ltd, Hyderabad, Telangana, India.
Received: April 17, 2018; Published: May 05, 2018
Abstract
Background: Dolutegravir inhibits HIV integrase by binding to the integrase active site and blocking the strand transfer step of retroviral deoxyribonucleic acid (DNA) integration which is essential for the HIV replication cycle. Tenofovir Alafenamide is indicated for the treatment of chronic hepatitis B virus (HBV) infection in adults with compensated liver disease. Emtricitabine, a synthetic nucleoside analog of cytidine, is phosphorylated by cellular enzymes to form Emtricitabine 5'-triphosphate. Emtricitabine 5'-triphosphate inhibits the activity of the HIV-1 reverse transcriptase by competing with the natural substrate deoxycytidine 5'-triphosphate and by being incorporated into nascent viral DNA which results in chain termination. Combination of Emtricitabine and Tenofovir Alafenamide is indicated in in combination with other antiretroviral agents, for the treatment of HIV-1 infection in adults and pediatric patients 12 years of age and older. Aurobindo Pharma Ltd, India developed a fixed dose combination of Dolutegravir, Emtricitabine and Tenofovir Alafenamide Tablets. Pharmacokinetic and bioequivalence data of fixed dose combination of Dolutegravir, Emtricitabine and Tenofovir Alafenamide Tablets in healthy volunteers is not available.
Objective: This study was designed to evaluate the bioequivalence of fixed dose combination of Dolutegravir, Emtricitabine and Tenofovir Alafenamide Tablets 50mg/200mg/25mg (Test formulation) manufactured by Aurobindo Pharma Ltd, India with Tivicay (Dolutegravir) Tablets 50mg of Viiv Healthcare, USA and Descovy (Emtricitabine and Tenofovir Alafenamide Tablets) 200mg/25mg of Gilead Sciences, USA (Reference formulation) in healthy volunteers. This study compared dissolution profiles, relative bioavailability, pharmacokinetics, safety and tolerability of fixed dose combination of Dolutegravir, Emtricitabine and Tenofovir Alafenamide tablets 50mg/200mg/25mg (Test formulation) and Descovy (Emtricitabine and Tenofovir Alafenamide Tablets) 200mg/25mg Gilead Sciences, USA (Reference formulations).
Methods: To evaluate the pharmaceutical equivalence of the test and reference products, in vitro dissolution test was performed using the US Pharmacopeia (USP) dissolution apparatus-II, paddle method and a simple model independent approach using the difference factor (f1) and the similarity factor (f2) was adopted to compare dissolution profiles. After an overnight fasting of at least 10.0 hours, a single oral 50mg/200mg/25mg dose of the test/reference formulations were administered to 12 healthy volunteers under fasting conditions in a randomized, open-label, 2-treatment, 2-sequence, 2-period crossover study with washout period of 7 days. Dolutegravir, Emtricitabine and Tenofovir Alafenamide plasma concentrations were quantified using a validated LC-MS/MS detection method and were used to determine the pharmacokinetic parameters. As mandated by the US Food and Drug Administration, the test and reference formulations were considered bioequivalent if the T/R ratios and90% CI’s of the geometric mean ratios for the log-transformed values of pharmacokinetic parameters were within the predetermined range of 80.00 to 125.00.
Results: When subjected to a simple model independent approach of dissolution profile comparison, f1 (difference) and f2 (similarity factor) for Dolutegravir were found to be 11.40 and 53.52 respectively.
When subjected to a simple model independent approach of dissolution profile comparison, f1 (difference) and f2 (similarity factor) for Emtricitabine were found to be 5.81 and 56.10 respectively When subjected to a simple model independent approach of dissolution profile comparison, f1 (difference) and f2 (similarity factor) for Tenofovir Alafenamide were found to be 5.11 and 58.51 respectively. Similarly, the test & reference formulations of Dolutegravir, Emtricitabine and Tenofovir Alafenamide Tablets were safe and well tolerated by all the volunteers. The T/R ratios (90% CI’s) for the ratios of Cmax, AUC0-t and AUC0-inf respectively for Dolutegravir were 126.51 (105.85-151.20), 121.51 (103.17-143.11) and 120.45 (102.16-142.02). The T/R ratios (90% CI’s) for the ratios of Cmax, AUC0-t and AUC0-inf respectively for Emtricitabine were 104.19 (89.84-120.82), 105.18 (99.95-110.68) and 102.65 (98.48-107.01). The T/R ratios (90% CI’s) for the ratios of Cmax, AUC0-t and AUC0-inf respectively for Tenofovir Alafenamide were 101.77 (79.86-129.70), 89.92 (83.66-107.42) and 94.72 (83.58-107.34).
Conclusion: The results of this single-dose fasting study showed that test formulation developed as fixed dose combination of Dolutegravir, Emtricitabine and Tenofovir Alafenamide tablets 50mg/200mg/25mg form was found to be not bioequivalent to reference formulation w.r.t Dolutegravir and bioequivalent to reference formulation w.r.t Emtricitabine and Tenofovir Alafenamide in healthy volunteers. Both test and reference formulations were safe and well tolerated.
Keywords: Dolutegravir; Emtricitabine; Tenofovir Alafenamide; LC-MS/MS detection method; Human plasma; Pharmacokinetics; Safety
Abbreviations: DNA: Deoxyribonucleic acid; HIV: Human Immunodeficiency virus; BMI: Body mass index; CI: Confidence Intervals; AUC: Area under the curve
Introduction
Dolutegravir sodium an HIV INSTI is Sodium (4R,12aS)-9-{[(2,4-difluorophenyl)methyl]carbamoyl}-4-methyl6, 8-dioxo-3,4,6,8,12,12a-hexahydro-2H-pyrido [1',2':4,5] pyrazino [2,1-b] [1,3] oxazin-7-olate [1]. Dolutegravir (DTG; S/GSK1349572) is an integrase inhibitor for the treatment of HIV infection, does not require boosting with ritonavir, and possesses activity against raltegravir-resistant strains [2].
Emtricitabine is 5-fluoro-1-(2R, 5S)-[2-(hydroxymethyl)-1, 3-oxathiolan-5-yl] cytosine [3]. Emtricitabine is a once-daily nucleoside reverse transcriptase inhibitor (NRTI) that selectively and potently inhibits human immunodeficiency virus type 1 (HIV-1) replication. Emtricitabine is used in combination with other antiviral agents for the treatment of HIV-1 and is currently under investigation for the treatment of hepatitis B virus (HBV) infection. Like other NRTIs, Emtricitabine is activated to a triphosphate derivative, which mediates the antiviral effect. Emtricitabine triphosphate is incorporated into a primer DNA strand resulting in chain termination and blockade of DNA- or RNA-directed DNA synthesis [4].
Tenofovir Alafenamide fumarate drug substance is L-alanine, N [(S)-[[(1R)-2-(6-amino-9H-purin-9-yl)-1-methylethoxy] methyl] phenoxyphosphinyl]-, 1methylethyl ester, (2E)-2-butenedioate [5]. Tenofovir alafenamide (TAF) is oral pro-drug of Tenofovir. TAF is more stable in plasma than TDF, provides higher intracellular levels of the active phosphorylated metabolite Tenofovir diphosphate (TFV DP), and approximately 90% lower circulating levels of TFV relative to TDF. TAF is proposed to provide similar efficacy as TDF but with significantly less proteinuria, less need for renal monitoring and less impact on bone mineralisation. As TAF is more stable in plasma than TDF, higher intracellular levels are achieved, providing enhanced delivery of TFV and 90% lower circulating levels of TFV relative to TDF [6].
Bioequivalencemeans comparison of pharmacokinetics of two different formulations which are expected to show similar in vivo response i.e. similar in terms of safety and efficacy. Two formulations are said to be bioequivalent if they exhibit similar rate and extent of absorption assessed by means of pharmacokinetic parameters Cmax & AUC derived from plasma concentration time curve when administered in same molar dose.
Bioequivalence can be established through various methods, including in vivo and in vitro methods; however, the pharmacokinetic approach is the most commonly used method. The reason might be that the primary aim of bioequivalence studies is to assess the rate and extent of drug absorption, which can be readily assessed by key pharmacokinetic parameters such as Cmax, Tmax, AUC, mean residence time (MRT), area under moment curve (AUMC), and t1/2[7,8].
Currently pharmacokinetic and bioequivalence data on fixed dose combination of Dolutegravir, Emtricitabine and Tenofovir Alafenamide Tablets 50 mg/200 mg/25 mg in healthy volunteers is not available.
This study compared the relative bioavailability and pharmacokinetics of test formulation (fixed dose combination of Dolutegravir, Emtricitabine and Tenofovir Alafenamide Tablets 50mg/200mg/25mg) developed by Aurobindo Pharma Ltd, India with that of reference formulations - Tivicay (Dolutegravir) Tablets 50mg of Viiv Healthcare, USA and Descovy (Emtricitabine and Tenofovir Alafenamide Tablets) 200mg/25mg of Gilead Sciences, USA in healthy mexican volunteers under fasting conditions.
Methods
Formulations: The reference formulation and test formulation of Dolutegravir, Emtricitabine and Tenofovir Alafenamide Tablets were 50mg/200mg/25mg Tablets. The test formulation is fixed dose combination of Dolutegravir, Emtricitabine and Tenofovir Alafenamide Tablets 50mg/200mg/25mg manufactured by Aurobindo Pharma Ltd, India. The reference formulation is Tivicay (Dolutegravir) Tablets 50mg of Viiv Healthcare, USA and Descovy (Emtricitabine and Tenofovir Alafenamide Tablets) 200mg/25mg of Gilead Sciences, USA. Each tablet of test formulation contains Dolutegravir sodium equivalent to 50 mg of dolutegravir, Emtricitabine 200 mg and Tenofovir Alafenamide 25 mg. Each tablet of reference formulation (Tivicay) contains dolutegravir sodium equivalent to 50 mg of Dolutegravir and each tablet of reference formulation (Descovy) contains Emtricitabine 200 mg and Tenofovir Alafenamide 25 mg.
Assay and In Vitro Dissolution Studies: To evaluate the pharmaceutical equivalence between the test and reference products, dissolution test was performed using the US Pharmacopeia (USP) dissolution apparatus-II, paddle method according to the official monograph. Samples collected at various time intervals were analyzed using the RP-HPLC/UV detection method, and a simple model independent approach using the difference factor (f1) and the similarity factor (f2) was adopted to compare dissolution profiles. The f1 calculates the percent difference between the 2 curves at each time point and is the measure of the relative error between the 2 curves, whereas the f2 is a logarithmic reciprocal square root transformation of the sum of squared error and is the measure of the similarity in the percent of dissolution between the 2 curves. These factors were calculated using the following equations, respectively:
f1 = {[St = 1n |Rt-Tt|]/[St = 1 nRt]} x 100.
f2 = 50xlog {[1+ (1/n) St = 1 n(Rt-Tt)2]-0.5 x 100}
f2 = 50xlog {[1+ (1/n) St = 1 n(Rt-Tt)2]-0.5 x 100}
Where n is the number of time points, Rt is the dissolution value of the reference product at time t, and Tt is the dissolution value of the test product at time t.
Using the mean dissolution values from both curves at each time interval, f1 and f2 were calculated using the aforementioned equations. For curves to be considered similar, f1 values should be close to 0, and f2 values should be close to 100. Generally, f1 values up to 15 (0–5) and f2 values ≥ 50 (50–100) ensure sameness or equivalence of the 2 curves and, thus, of the performance of the 2 products.
Volunteers: This study was conducted according to the principles of the Declaration of Helsinki and its amendments. The study protocol was approved by the Ethical Committee of the AXIS CLINICALS LATINA S.A. de C.V, Ing. Basiliso Romo Anguiano No. 225Col. Guadalupe Insurgentes, Ciudad de México, C.P. 07870.The study objectives and the effects of drugs used in the study were explained to volunteers at the start of the study, and informed consent was obtained. Study volunteers were also compensated financially.
Healthy adult male and female Mexican volunteers aged between 18 and 55 years were recruited for this study. A detailed medical history was obtained and a clinical examination was performed for all volunteers at the beginning of the study under the supervision of a qualified physician. In addition, 12-lead electrocardiography, complete blood count, blood pressure, blood sugar level, liver function tests, lipid profile, and renal function tests were also carried out in all study volunteers. Volunteers were confirmed with negative test result of alcoholometry, respiratory income of each period prior to the administration of Darunavir and Ritonavir formulations. All the Subjects were within the BMI ranging from 18 to 25 kg/m2. Participating women should not be pregnant or lactating. They must sign a letter of commitment of being not pregnant (since the signing of informed consent).
| No. Vol. | Gender | Age (years) | Weight (kg) | Height (m) | BMI (kg/m2) |
| 01 | Male | 19 | 62.3 | 1.67 | 22.3 |
| 02 | Male | 22 | 54.1 | 1.64 | 20.1 |
| 03 | Female | 38 | 59.0 | 1.57 | 23.9 |
| 04 | Male | 23 | 63.0 | 1.68 | 22.3 |
| 05 | Male | 35 | 71.9 | 1.71 | 24.6 |
| 06 | Female | 26 | 50.0 | 1.50 | 22.2 |
| 07 | Male | 28 | 63.6 | 1.74 | 21.0 |
| 08 | Male | 38 | 56.7 | 1.60 | 22.2 |
| 09 | Male | 19 | 64.9 | 1.77 | 20.7 |
| 10 | Female | 31 | 53.4 | 1.57 | 21.7 |
| 11 | Male | 44 | 61.2 | 1.63 | 23.0 |
| 12 | Male | 27 | 70.4 | 1.73 | 23.5 |
| Average | 29.17 | 60.88 | 1.65 | 22.29 | |
| Standard Deviation | 8.12 | 6.63 | 0.08 | 1.32 | |
| Minimum | 19.00 | 50.00 | 1.50 | 20.10 | |
| Maximum | 44.00 | 71.90 | 1.77 | 24.60 | |
| % CV | 27.85 | 10.89 | 4.90 | 5.93 | |
| Volunteers per gender: | Male | 09 | |||
| Female | 03 | ||||
Table 1: Demographical data of the volunteers who participated in the clinical study.
Volunteers with a history of hypersensitivity to study drug or any other drugs belonging to the same therapeutic group and history of bronchial asthma were excluded. Volunteers with recent history of drug abuse, including alcohol and with a history or physical examination evidence of gastrointestinal disease, kidney, liver, endocrine, respiratory, cardiovascular, dermatological or hematological clinically significant were also excluded from the study. Volunteers who had taken potentially toxic drugs within 30 days before the start of the study and who have taken any medication within 14 days or 7 half-lives thereof, prior to the start of the study were also excluded. Volunteers who have donated or lost 450 mL or more of blood within 60 days prior to study and who consumed grapefruit juice or drinks, or spicy foods in the 10 hours prior to admission of the study drug were also excluded from the study.
Study Design and Drug Administration: Various bioequivalence parameters of the 2 products were assessed under fasting conditions in a randomized, open-labeled, balanced, 2-treatment, 2-sequence, 2-period, single-dose, crossover study with a 1-week washout period at the AXIS CLINICALS LATINA S.A. de C.V, Ing. Basiliso Romo Anguiano No. 225Col. Guadalupe Insurgentes, Ciudad de México, C.P. 07870after obtaining approval from REC (Research and Ethics Committee), Mexico & COFEPRIS (Federal Commission for the Protection Against Sanitary Risks) with authorization number 173300410B0472/2017.
Using SAS software generated randomization schedule, volunteers were randomly divided into 2 groups (group 1 and group 2), each group consisting of 6 healthy volunteers. During the first study period, healthy volunteers from group 1 received a single oral 50mg/200mg/25mg dose of the reference formulation, whereas healthy volunteers from group 2 received the test formulation. In the second study period, the order was reversed. A non-blind approach was applied; both volunteers and investigators were aware of the formulations given to each group.
| Study Period | Group | Dolutegravir, Emtricitabine and Tenofovir Alafenamide Formulation Administered |
| First Period | 1 | One tablet of Tivicay (Dolutegravir) Tablets 50mg and One Tablet of Descovy (Emtricitabine and Tenofovir Alafenamide Tablets) 200mg/25mg (Reference formulation) |
| 2 | One tablet of Dolutegravir, Emtricitabine and Tenofovir Alafenamide Tablets 50mg/200mg/25mg (Test formulation) | |
| 1 week washout period | ||
| Second Period | 1 | One tablet of Dolutegravir, Emtricitabine and Tenofovir Alafenamide Tablets 50mg/200mg/25mg (Test formulation) |
| 2 | One tablet of Tivicay (Dolutegravir) Tablets 50mg and One Tablet of Descovy (Emtricitabine and Tenofovir Alafenamide Tablets) 200mg/25mg (Reference formulation) | |
Table 2: Study Design for Bioequivalence Evaluation of Test and Reference Formulations of Dolutegravir, Emtricitabine and Tenofovir Alafenamide Tablets 50mg/200mg/25mg.
In each period, a single oral dose of either one Reference tablet or Test Tablet of Dolutegravir, Emtricitabine and Tenofovir Alafenamide 50mg/200mg/25mg was administered with 240 mL of drinking water at room temperature under fasting conditions each period by study personnel under the supervision of Investigator.
Tolerability Assessment: Tolerability in volunteers was assessed before medication administration and at 0.00, 3.00, 8.00, and 16.00 hours during the study through physical examination, monitoring vital signs (temperature, blood pressure, heart rate, and respiratory rate) and interviewing them about adverse events that may be associated with the use of Dolutegravir (eg, insomnia, fatigue, and headache), Emtricitabine and Tenofovir Alafenamide (eg, headache, stomach pain, tiredness, cough, nausea, back pain, headache, diarrhoea and rash) under the supervision of a qualified physician.
One (01) adverse event was reported in 01 volunteer during the study and according to medical criterion it was of moderate severity. The adverse event reported was headache. There were no serious adverse events reported during the entire study. Both test (fixed dose combination of Dolutegravir, Emtricitabine and Tenofovir Alafenamide Tablets 50mg/200mg/25mg) and reference (Tivicay (Dolutegravir) Tablets 50mg of Viiv Healthcare, USA and Descovy (Emtricitabine and Tenofovir Alafenamide Tablets) 200mg/25mg) formulations were safe and well tolerated by all the healthy volunteers in this study.
Sample Collection and Processing: Venous blood samples (6 mL) were collected at 0.00 (pre-dose in duplicate) and at 0.17, 0.33, 0.67, 0.83, 1.00, 1.33, 1.50, 1.67, 2.00, 2.50, 3.00, 3.50, 4.00, 5.00, 6.00, 8.00, 10.00, 12.00, 18.00, 24.00, 36.00, 48.00 and 72.00 hours post dose, drawn by venipuncture, by placing a catheter fixed, preferably in the anterior aspect of the forearm.
Blood samples were collected in pre-labeled vacationer tubes containing K2EDTA as anticoagulant; they were centrifuged to separate plasma immediately at 3500 rpm for 10 minutes, at a temperature between 2°C and 15°C. Each plasma sample was divided in to two aliquots. Aliquot-1 was transferred with 950µL of plasma and 50µL of 10% citric acid (w/v) (briefly mix), which was used for the analysis of Emtricitabine and Tenofovir alafenamide. Remaining volume of plasma sample was transferred to aliquot-2 which was used for the analysis of Dolutegravir. Cryogenic tubes were frozen at-70 ± 15°C until analysis.
Analysis of Samples
Two different analytical methods were used for measurement of Dolutegravir, Emtricitabine and Tenofovir Alafenamide in study samples. One method was used for measurement of Dolutegravir in plasma; and second method was used for simultaneous measurement of Emtricitabine and Tenofovir Alafenamide in plasma.
Two different analytical methods were used for measurement of Dolutegravir, Emtricitabine and Tenofovir Alafenamide in study samples. One method was used for measurement of Dolutegravir in plasma; and second method was used for simultaneous measurement of Emtricitabine and Tenofovir Alafenamide in plasma.
Analysis of Samples for Dolutegravir: Samples were analyzed by a validated LC-MS/MS method, discussed below. Dolutegravir standard and Dolutegravir-d3 standard were received from Clearsynth Labs Limited, India. HPLC-grade solvents such as methanol, acetonitrile were purchased from Labs can and all other chemicals and reagents such as Ammonium acetate and Ammonia were purchased from Merck Millipore.Deionised water was prepared using a Millipore water system. All these reagents and chemicals were used without further purification.
Solid-phase extraction was adopted for the sample preparation. At the time of analysis, the samples were thawed at room temperature and 50µl of Dolutegravir-d3 solution (4035 ng/mL) as the internal standard was vertex-mixed with 200 µL plasma sample for 30 seconds. Then, these samples were added with 200 uL of 2% Ammonia solution and mixed. Prelabelled Strata-X SPE cartridges were activated with 1 mL of methanol, equilibrated with 1 mL of deionized water and loaded the plasma samples onto respective prelabelled SPE cartridges and applied positive pressure to pass the plasma samples through SPE cartridges. Then, the sample SPE cartridges were washed with 1 mL of deionized water. The sample SPE cartridges were left drying for 2 minutes and samples are eluted with 1 mL of methanol into prelabelled collection tubes. The eluted samples were evaporated for 20 minutes at 50°C and 20 psi nitrogen gas. The residues were dissolved in 400µL mobile phase and 10 µL samples were injected to the chromatographic system.
The chromatographic separations were achieved using a Peerless basic C18 analytical column (5µm, 4.6 x 100 mm). The mobile phase consisted of Acetonitrile-Methanol-5 mM Ammonium acetate buffer pH 3.0 (45%:25%:30%). The flow rate of the mobile phase was set at 1.0 ml/ min. The column oven temperature was 35˚C. The autosampler temperature was 10°C.
The Shimadzu HPLC system was coupled to an API 3000 MS/ MS triple-quadrupole system detector equipped with a turbo ion spray ionization (ESI) source (Applied Biosystems MDS, SCIEX, Canada). The turbo ion spray ionization source was operated in a positive mode. The ion spray voltage was adjusted to 5000 V. The mass spectrometer was operated at a unit resolution for both Q1 and Q3 in multiple reaction monitoring (MRM) mode. The transition of precursor to product ion was monitored at 420.00→ 277.00 for Dolutegravir and 423.10→ 277.00 for internal standard (Dolutegravir-d3). The method had a total run time of 4.5 minutes. Chromatograms processing, data generation and concentrations back calculations were all performed by the Analyst software (Applied Biosystems, MDS, SCIEX, Canada).
Analysis of Samples for Emtricitabine and Tenofovir Alafenamide
Samples were analyzed by a validated LC-MS/MS method, discussed below.
Emtricitabine and Tenofovir Alafenamide standards were received from Simson Pharma Pvt. Limited, India and Tenofovir Alafenamide-D5 and Emtricitabine-15ND2 standards were received from Vivan Life Sciences Pvt. Limited, India.
Samples were analyzed by a validated LC-MS/MS method, discussed below.
Emtricitabine and Tenofovir Alafenamide standards were received from Simson Pharma Pvt. Limited, India and Tenofovir Alafenamide-D5 and Emtricitabine-15ND2 standards were received from Vivan Life Sciences Pvt. Limited, India.
HPLC-grade solvents such as Methanol was purchased from Labs can and all other chemicals and reagents such as Orthophosphoric acid and Formic acid were purchased from Merck Millipore. Deionized water was prepared using a Millipore water system. All these reagents and chemicals were used without further purification.
Solid-phase extraction was adopted for the sample preparation. At the time of analysis, the samples were thawed at room temperature and 50µl of Internal standard solution (containing 500 ng/mL Tenofovir Alafenamide-D5 and 10000 ng/ml Emtricitabine-15ND2) was vertex-mixed with 200 µL plasma sample for 30 seconds. Then, these samples were added with 200 uL of 2%v/v Orthophosphoric acid in water solution and mixed. Prelabelled Strata-X SPE cartridges were activated with 1 mL of methanol, equilibrated with 1 mL of deionized water and loaded the plasma samples onto respective prelabelled SPE cartridges and applied positive pressure to pass the plasma samples through SPE cartridges. Then, the sample SPE cartridges were washed with 1 mL of deionized wáter and 1ml of 10%v/v methanol in wáter solution. The sample SPE cartridges were left drying for 2 minutes and samples are eluted with 1 mL of methanol into prelabelled collection tubes. The eluted samples were evaporated for 20 minutes at 50°C and 15 psi nitrogen gas. The residues were dissolved in 2.0mL mobile phase and 10 µL samples were injected to the chromatographic system.
The chromatographic separations were achieved using a Waters symmetry C18 analytical column (5µm, 4.6x100 mm). The mobile phase consisted of Methanol-0.05% Formic acid (60%:40%). The flow rate of the mobile phase was set at 1.0 ml/ min. The column oven temperature was 35˚C. The autosampler temperature was 10°C.
The Shimadzu HPLC system was coupled to an API 4000 MS/ MS triple-quadrupole system detector equipped with a turbo ion spray ionization (ESI) source (Applied Biosystems MDS, SCIEX, Canada). The turbo ion spray ionization source was operated in a positive mode. The ion spray voltage was adjusted to 5500 V. The mass spectrometer was operated at a unit resolution for both Q1 and Q3 in multiple reaction monitoring (MRM) mode. The transition of precursor to product ion was monitored at 248.20→ 130.00 for Emtricitabine, 251.20→ 131.00 for Emtricitabine-15ND2, 477.20→ 346.30 for Tenofovir Alafenamide and 482.40→ 351.10 for Tenofovir Alafenamide-d5. The method had a total run time 3.0 minutes. Chromatograms processing, data generation and concentrations back calculations were all performed by the Analyst software (Applied Biosystems, MDS, SCIEX, Canada).
The chromatographic method was validated according to international guidelines to establish selectivity, accuracy, precision, recovery, calibration curve, and stability.
Sample concentrations, C, were calculated using the following formula x = y-c/m
Where,x is analyte Concentration in sample; y is ratio of analyte peak area / Internal standard peak area; m is slope of the calibration curve; and c is y-axis intercept value
Where,x is analyte Concentration in sample; y is ratio of analyte peak area / Internal standard peak area; m is slope of the calibration curve; and c is y-axis intercept value
Pharmacokinetics and Statistical Analyses
Plasma concentrations of Dolutegravir, Emtricitabine and Tenofovir Alafenamide at various time intervals following oral administration of the 2 products were determined for each volunteer and mean values were calculated. A non-compartment model was used to determine the following pharmacokinetic parameters: Cmax, Tmax, AUC0–t, AUC0-inf, kel and t1/2. Cmax and Tmax were obtained directly from the concentration–time curve; AUC was calculated using the linear trapezoidal method. Elimination rate constant (kel) was calculated by applying a log-linear regression analysis to at least the last 3 quantifiable Darunavir concentrations, and then t1/2 was calculated as 0.693/ke. All of these pharmacokinetic parameters were determined using the software Phoenix® WinNonlin® Version 6.4.
Plasma concentrations of Dolutegravir, Emtricitabine and Tenofovir Alafenamide at various time intervals following oral administration of the 2 products were determined for each volunteer and mean values were calculated. A non-compartment model was used to determine the following pharmacokinetic parameters: Cmax, Tmax, AUC0–t, AUC0-inf, kel and t1/2. Cmax and Tmax were obtained directly from the concentration–time curve; AUC was calculated using the linear trapezoidal method. Elimination rate constant (kel) was calculated by applying a log-linear regression analysis to at least the last 3 quantifiable Darunavir concentrations, and then t1/2 was calculated as 0.693/ke. All of these pharmacokinetic parameters were determined using the software Phoenix® WinNonlin® Version 6.4.
Pharmacokinetic data for the 2 formulations were log-transformed before statistical analysis, which was based on the 90% CIs for the ratio of the geometric means for these log-transformed pharmacokinetic parameters of the 2 formulations (test/reference).
The lower boundary (LB) and higher boundary (HB) of 90% CIs were calculated using the following equations [9]:
LB = e(MD-[t-value X SD/√n])
HB = e(MD+ [t-value X SD/√n])
Where MD is the mean difference and SD refers to the standard deviation of the transformed metric; n is the number of patients in the study;
LB = e(MD-[t-value X SD/√n])
HB = e(MD+ [t-value X SD/√n])
Where MD is the mean difference and SD refers to the standard deviation of the transformed metric; n is the number of patients in the study;
If the 90% CIs for the ratios of Cmax, AUC0–t and AUC0-inf values of the test and reference formulations fell within the range of 80.00 to 125.00, then these formulations were considered bioequivalent, as recommended by the US FDA [10].
Results
Assay and In vitro Dissolution Studies: The mean percentage of the active ingredient in the test and reference formulations for Dolutegravir was found to be 100.9% and 101.7% (1%), respectively. Dolutegravir mean in vitro drug release (dissolution) profiles of the test and reference tablet formulations were determined (Figure 1) and the data were subjected to a simple model independent approach of dissolution profile comparison. The f1 and the f2 were found to be 11.40 and 53.52, respectively (Table 3).
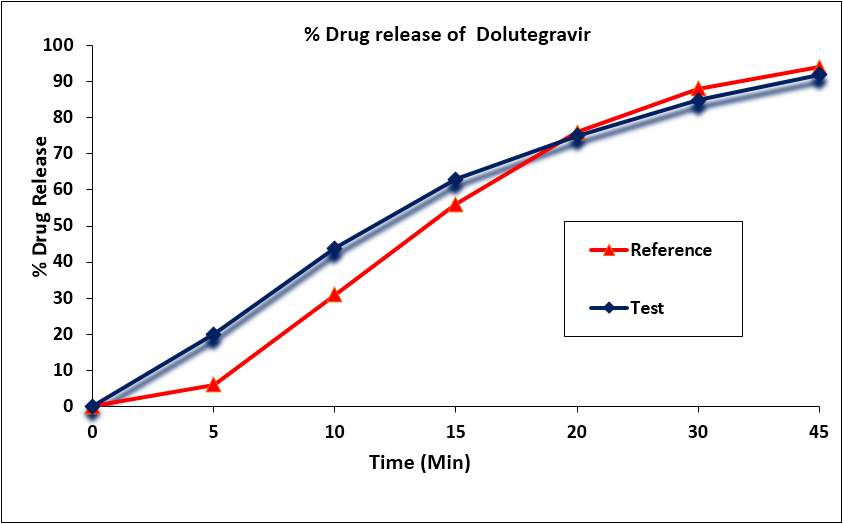
Figure 1: Dolutegravir in vitro drug release profile of test and reference
formulations. Each point represents mean (SD) of 12 Tablets.
| Time (min) | Mean % Release | Difference Factor (f1) | Similarity Factor (f2) | |
| Test Formulation | Reference Formulation | |||
| 0 | 0 | 0 | 11.40 | 53.52 |
| 5 | 20 | 6 | ||
| 10 | 44 | 31 | ||
| 15 | 63 | 56 | ||
| 20 | 75 | 76 | ||
| 30 | 85 | 88 | ||
| 45 | 92 | 94 | ||
Table 3: Comparison of the dissolution profile of Dolutegravir from 2 Dolutegravir, Emtricitabine and Tenofovir Alafenamide Tablet formulations (n = 12, where n is the number of tablets tested of each formulation).
The mean percentage of the active ingredient in the test and reference formulations for Emtricitabine was found to be 100.3% and 99.0% respectively. Emtricitabine mean in vitro drug release (dissolution) profiles of the test and reference tablet formulations were determined (Figure 2) and the data were subjected to a simple model independent approach of dissolution profile comparison. The f1 and the f2 were found to be 5.81 and 56.10, respectively (Table 4).
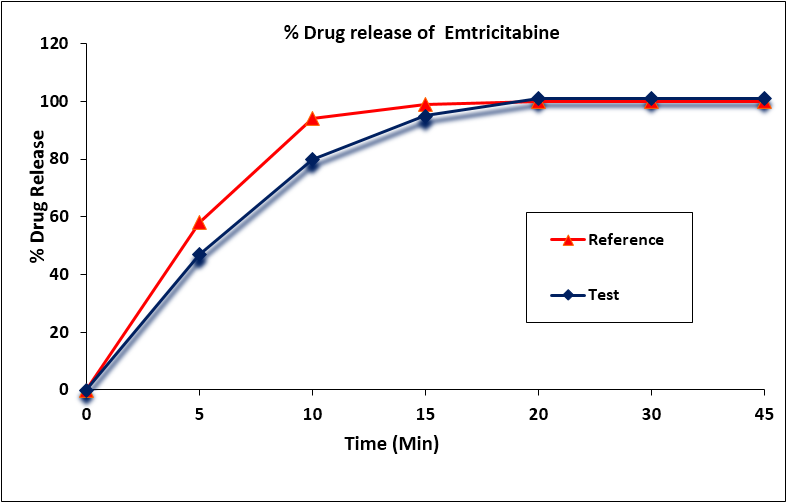
Figure 2: Emtricitabine in vitro drug release profile of test and reference
formulations. Each point represents mean (SD) of 12 Tablets.
| Time (min) | Mean % Release | Difference Factor (f1) | Similarity Factor (f2) | |
| Test Formulation | Reference Formulation | |||
| 0 | 0 | 0 | 5.81 | 56.10 |
| 5 | 47 | 58 | ||
| 10 | 80 | 94 | ||
| 15 | 95 | 99 | ||
| 20 | 101 | 100 | ||
| 30 | 101 | 100 | ||
| 45 | 101 | 100 | ||
Table 4: Comparison of the dissolution profile of Emtricitabine from 2 Dolutegravir, Emtricitabine and Tenofovir Alafenamide Tablet formulations (n = 12, where n is the number of tablets tested of each formulation).
The mean percentage of the active ingredient in the test and reference formulations for Tenofovir Alafenamide was found to be 100.1% and 99.8% (1%), respectively. Tenofovir Alafenamide mean in vitro drug release (dissolution) profiles of the test and reference tablet formulations were determined (Figure 3) and the data were subjected to a simple model independent approach of dissolution profile comparison. The f1 and the f2 were found to be 5.11 and 58.51, respectively (Table 5).
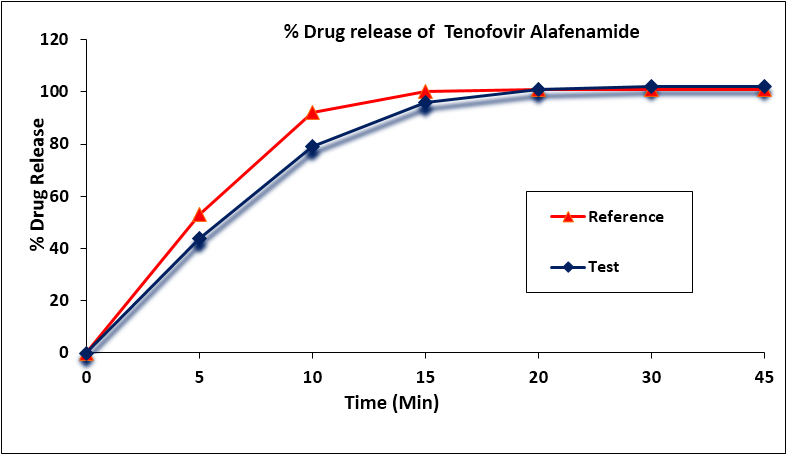
Figure 3: Tenofovir Alafenamide in vitro drug release profile of test and reference formulations. Each point represents mean (SD) of 12 Tablets.
| Time (min) | Mean % Release | Difference Factor (f1) | Similarity Factor (f2) | |
| Test Formulation | Reference Formulation | |||
| 0 | 0 | 0 | 5.11 | 58.51 |
| 5 | 44 | 53 | ||
| 10 | 79 | 92 | ||
| 15 | 96 | 100 | ||
| 20 | 101 | 101 | ||
| 30 | 102 | 101 | ||
| 45 | 102 | 101 | ||
Table 5: Comparison of the dissolution profile of Tenofovir Alafenamide from 2 Dolutegravir, Emtricitabine and Tenofovir Alafenamide Tablet formulations (n = 12, where n is the number of tablets tested of each formulation).
Tolerability: Both test (fixed dose combination of Dolutegravir, Emtricitabine and Tenofovir Alafenamide Tablets 50mg/200mg/25mg) and reference (Tivicay (Dolutegravir) Tablets 50mg and Descovy (Emtricitabine and Tenofovir Alafenamide Tablets) 200mg/25mg)) formulations were safe and well tolerated by all the healthy volunteers in this study. No unexpected incidents occurred that influenced study outcomes, and all 12 volunteers continued to study end and were discharged in good health.
Validation of the Analytical Method
Dolutegravir: The LC-MS/MS method developed for the quantification of Dolutegravir in plasma was linear in the range of 20.0 to 8996.4 ng/mL , where at the LLOQ accuracy obtained was 101.5% (less than 20% deviation) and 97.3%-101.5% for standard points higher than LLOQ (less than 15% deviation), and 75% ratio of total standard points were accepted. The correlation coefficients (r) of all standard curves were more than 0.9996 for plasma samples. The lower limit of quantification was 20.0 ng/mL for Dolutegravir in plasma. LLOQ response is identifiable, discrete and reproducible with precision and ac-curacy of ± 20%. Specificity of the method was verified by the absence of any co-eluted peaks of endogenous plasma component at the retention times of the drug or the internal standard.
Dolutegravir: The LC-MS/MS method developed for the quantification of Dolutegravir in plasma was linear in the range of 20.0 to 8996.4 ng/mL , where at the LLOQ accuracy obtained was 101.5% (less than 20% deviation) and 97.3%-101.5% for standard points higher than LLOQ (less than 15% deviation), and 75% ratio of total standard points were accepted. The correlation coefficients (r) of all standard curves were more than 0.9996 for plasma samples. The lower limit of quantification was 20.0 ng/mL for Dolutegravir in plasma. LLOQ response is identifiable, discrete and reproducible with precision and ac-curacy of ± 20%. Specificity of the method was verified by the absence of any co-eluted peaks of endogenous plasma component at the retention times of the drug or the internal standard.
All samples passed the acceptance criteria. The intra-day accuracies were 93.0% to 103.7% for LLOQ and 94.8%-106.6% for QC's above LLOQ and the inter-day accuracies were 97.9% for LLOQ and 99.1%-102.9% for QC's above LLOQ. The intra-day precision was 5.5% to 9.7% for LLOQ and 2.0%–5.8% for QC's above LLOQ. The inter-day precision was 8.7% for LLOQ and 3.7%-6.1% for QC's above LLOQ. This results support the fact that the method is accurate and precise, where deviations obtained were less than 20% for LLOQ and less than 15% for the QC's above the LLOQ. The average extraction recoveries of Dolutegravir determined at 59.7, 2899.5 and 6903.5 ng/mL were 97.6%, 95.1% and 94.6% respectively, while that of internal standard (Dolutegravir-D3) was 96.4% with high degree of precision, accuracy and reproducibility. Dolutegravir was stable with absolute percentages of deviation of calculated vs theoretical concentration being less than 15% for auto sampler, freeze-thaw, short-term and long-term stabilities determined at two concentrations of 59.7 and 6903.5 ng/mL. The stability was within the limit of 85.00% - 115.00% & the CV% less than 15.00%
Emtricitabine: The LC-MS/MS method developed for the quantification of Emtricitabine in plasma was linear in the range of 8.00 to 4008.41 ng/mL , where at the LLOQ accuracy obtained was 98.5% (less than 20% deviation) and 94.9%-103.7% for standard points higher than LLOQ (less than 15% deviation), and 75% ratio of total standard points were accepted. The correlation coefficients (r) of all standard curves were more than 0.9988 for plasma samples. The lower limit of quantification was 8.00 ng/mL for Emtricitabine in plasma. LLOQ response is identifiable, discrete and reproducible with precision and ac-curacy of ±20%. Specificity of the method was verified by the absence of any co-eluted peaks of endogenous plasma component at the retention times of the drug or the internal standard. All samples passed the acceptance criteria. The intra-day accuracies were 98.2% to 104.9% for LLOQ and 92.0%-99.7% for QC's above LLOQ and the inter-day accuracies were 102.5% for LLOQ and 92.6%-99.0% for QC's above LLOQ. The intra-day precision was 4.2% to 5.2% for LLOQ and 0.8%–3.7% for QC's above LLOQ.
The inter-day precision was 5.4% for LLOQ and 2.0%-2.7% for QC's above LLOQ. This results support the fact that the method is accurate and precise, where deviations obtained were less than 20% for LLOQ and less than 15% for the QC's above the LLOQ. The average extraction recoveries of Emtricitabine determined at 23.98, 1255.38 and 3061.90 ng/mL were 70.4%, 73.6% and 75.3% respectively, while that of internal standard (Emtricitabine-15ND2) was 76.1% with high degree of precision, accuracy and reproducibility. Emtricitabine was stable with absolute percentages of deviation of calculated vs theoretical concentration being less than 15% for auto sampler, freeze-thaw, short-term and long-term stabilities determined at two concentrations of 23.98 and 3061.90 ng/mL. The stability was within the limit of 85.00%-115.00% & the CV% less than 15.00%
Tenofovir Alafenamide
The LC-MS/MS method developed for the quantification of Tenofovir Alafenamide in plasma was linear in the range of 2.00 to 500.48 ng/mL , where at the LLOQ accuracy obtained was 97.8% (less than 20% deviation) and 96.7%-103.8% for standard points higher than LLOQ (less than 15% deviation), and 75% ratio of total standard points were accepted. The correlation coefficients (r) of all standard curves were more than 0.9990 for plasma samples. The lower limit of quantification was 2.00 ng/mL for Tenofovir Alafenamide in plasma. LLOQ response is identifiable, discrete and reproducible with precision and ac-curacy of ± 20%. Specificity of the method was verified by the absence of any co-eluted peaks of endogenous plasma component at the retention times of the drug or the internal standard.
The LC-MS/MS method developed for the quantification of Tenofovir Alafenamide in plasma was linear in the range of 2.00 to 500.48 ng/mL , where at the LLOQ accuracy obtained was 97.8% (less than 20% deviation) and 96.7%-103.8% for standard points higher than LLOQ (less than 15% deviation), and 75% ratio of total standard points were accepted. The correlation coefficients (r) of all standard curves were more than 0.9990 for plasma samples. The lower limit of quantification was 2.00 ng/mL for Tenofovir Alafenamide in plasma. LLOQ response is identifiable, discrete and reproducible with precision and ac-curacy of ± 20%. Specificity of the method was verified by the absence of any co-eluted peaks of endogenous plasma component at the retention times of the drug or the internal standard.
All samples passed the acceptance criteria. The intra-day accuracies were 94.3% to 103.2% for LLOQ and 92.3%-97.9% for QC's above LLOQ and the inter-day accuracies were 99.6% for LLOQ and 94.6%-96.1% for QC's above LLOQ. The intra-day precision was 1.6% to 3.6% for LLOQ and 1.5%–3.0% for QC's above LLOQ. The inter-day precision was 4.9% for LLOQ and 2.6%-2.7% for QC's above LLOQ. This results support the fact that the method is accurate and precise, where deviations obtained were less than 20% for LLOQ and less than 15% for the QC's above the LLOQ. The average extraction recoveries of Tenofovir Alafenamide determined at 5.98, 157.11 and 381.80 ng/mL were 77.3%, 78.2% and 74.0% respectively, while that of internal standard (Tenofovir Alafenamide-D5) was 83.6% with high degree of precision, accuracy and reproducibility. Tenofovir Alafenamide was stable with absolute percentages of deviation of calculated vs theoretical concentration being less than 15% for auto sampler, freeze-thaw, short-term and long-term stabilities determined at two concentrations of 5.98 and 381.80 ng/mL. The stability was within the limit of 85.00% - 115.00% & the CV% less than 15.00%
Pharmacokinetics and Statistical Analyses
Dolutegravir, Emtricitabine and Tenofovir Alafenamide mean plasma concentration–time profiles after administration of the test and reference formulations under fasting conditions in Mexican healthy volunteers are shown in Figure III, Figure IV & Figure V respectively. Mean (SD) values of various pharmacokinetic parameters of Dolutegravir for the test and reference formulations respectively, for Cmax were 3281.4 (967.30) and 2610.3 (831.73) ng/mL; AUC0-t were 52878.5 (15006.77) and 44650.6 (15370.93) ng·h/mL; AUC0-inf were 54701.0 (15231.16) and 46612.5 (15771.97) ng·h/mL; t1/2 were 14.42 (2.810) and 15.02 (2.958) h; Tmax were 2.85 (1.245) and 2.92 (1.737) h; Kel were 0.0498 (0.01021) and 0.0478 (0.00938) h-1. Similarly, the 90% CIs for the ratios of Cmax, AUC0–t and AUC0-inf for the 2 formulations respectively were 105.85-151.20, 103.17-143.11 and 102.16-142.02 which along with summary statistics such as median, range, and %CV, are given in Table 6.
Dolutegravir, Emtricitabine and Tenofovir Alafenamide mean plasma concentration–time profiles after administration of the test and reference formulations under fasting conditions in Mexican healthy volunteers are shown in Figure III, Figure IV & Figure V respectively. Mean (SD) values of various pharmacokinetic parameters of Dolutegravir for the test and reference formulations respectively, for Cmax were 3281.4 (967.30) and 2610.3 (831.73) ng/mL; AUC0-t were 52878.5 (15006.77) and 44650.6 (15370.93) ng·h/mL; AUC0-inf were 54701.0 (15231.16) and 46612.5 (15771.97) ng·h/mL; t1/2 were 14.42 (2.810) and 15.02 (2.958) h; Tmax were 2.85 (1.245) and 2.92 (1.737) h; Kel were 0.0498 (0.01021) and 0.0478 (0.00938) h-1. Similarly, the 90% CIs for the ratios of Cmax, AUC0–t and AUC0-inf for the 2 formulations respectively were 105.85-151.20, 103.17-143.11 and 102.16-142.02 which along with summary statistics such as median, range, and %CV, are given in Table 6.
| Pharmacokinetic Parameters | Test Formulation | Reference Formulation | P value | T/R Point Estimate (90% CI) |
| Cmax (ng/mL) | ||||
| Mean (SD) | 3281.4 (967.30) | 2610.3 (831.73) | 0.0379 | 126.51 (105.85-151.20) |
| Range | 1757.7-4202.0 | 1056.6-4299.0 | ||
| Median | 3523.8 | 2516.9 | ||
| %CV | 29.48 | 31.86 | ||
| AUC0-t (hr.ng/mL) | ||||
| Mean (SD) | 52878.5 (15006.77) | 44650.6 (15370.93) | 0.0563 | 121.51 (103.17-143.11) |
| Range | 31849.2-77143.4 | 16289.8-72204.0 | ||
| Median | 52542.0 | 44730.8 | ||
| %CV | 28.38 | 34.42 | ||
| AUC0-inf(hr.ng/mL) | ||||
| Mean (SD) | 54701.0 (15231.16) | 46612.5 (15771.97) | 0.0677 | 120.45 (102.16-142.02) |
| Range | 33272.9-80511.8 | 16980.2-73751.9 | ||
| Median | 54427.6 | 47518.7 | ||
| %CV | 27.84 | 33.84 | ||
| Tmax (h) | ||||
| Mean (SD) | 2.85 (1.245) | 2.92 (1.737) | -- | -- |
| Range | 1.33-5.00 | 0.67-6.00 | ||
| Median | 3.00 | 3.00 | ||
| %CV | 43.73 | 59.55 | ||
| t1/2 (hr) | ||||
| Mean (SD) | 14.42 (2.810) | 15.02 (2.958) | -- | -- |
| Range | 9.53-20.04 | 10.88-20.04 | ||
| Median | 13.96 | 14.89 | ||
| %CV | 19.48 | 19.69 | ||
| Kel (hr-1) | ||||
| Mean (SD) | 0.0498 (0.01021) | 0.0478 (0.00938) | ||
| Range | 0.0346-0.0727 | 0.0346-0.0637 | ||
| Median | 0.0497 | 0.0465 | ||
| %CV | 20.49 | 19.63 |
Table 6: Pharmacokinetic parameters and 90% CIs for the ratios of the geometric means of their log-transformed values of Dolutegravir for the 2 Dolutegravir, Emtricitabine and Tenofovir Alafenamide tablet formulations under fasting conditions (n = 12, where n is the number of volunteers).
Mean (SD) values of various pharmacokinetic parameters of Emtricitabine for the test and reference formulations respectively, for Cmax were 3105.84 (798.152) and 3022.30 (1009.968) ng/mL; AUC0-t were 12470.63 (1363.406) and 11857.29 (1268.583) ng·h/mL; AUC0-inf were 12741.18 (1413.171) and 12433.11 (1549.186) ng·h/mL; t1/2 were 13.85 (4.330) and 16.85 (11.105) h; Tmax were 1.55 (1.246) and 1.63 (0.977) h; Kel were 0.0541 (0.01464) and 0.0510 (0.01821) h-1. Similarly, the 90% CIs for the ratios of Cmax, AUC0–t and AUC0-inf for the 2 formulations respectively were 89.84 - 120.82, 99.95 - 110.68 and 98.48 - 107.01 which along with summary statistics such as median, range, and %CV, are given in Table 7.
| Pharmacokinetic Parameters | Test Formulation | Reference Formulation | P value | T/R Point Estimate (90% CI) |
| Cmax (ng/mL) | ||||
| Mean (SD) | 3105.84 (798.152) | 3022.30 (1009.968) | 0.6265 | 104.19 (89.84-120.82) |
| Range | 1566.26-4240.92 | 1424.58-5580.80 | ||
| Median | 2919.37 | 2976.37 | ||
| %CV | 25.70 | 33.42 | ||
| AUC0-t(hr.ng/mL) | ||||
| Mean (SD) | 12470.63 (1363.406) | 11857.29 (1268.583) | 0.1030 | 105.18 (99.95-110.68) |
| Range | 10541.04-15041.55 | 9800.10-13600.84 | ||
| Median | 11975.83 | 11685.26 | ||
| %CV | 10.93 | 10.70 | ||
| AUC0-inf(hr.ng/mL) | ||||
| Mean (SD) | 12741.18 (1413.171) | 12433.11 (1549.186) | 0.2797 | 102.65 |
| Range | 10669.41-15379.40 | 9921.47-15302.18 | (98.48-107.01) | |
| Median | 12147.15 | 12473.81 | ||
| %CV | 11.09 | 12.46 | ||
| Tmax (h) | ||||
| Mean (SD) | 1.55 (1.246) | 1.63 (0.977) | -- | -- |
| Range | 0.67-5.00 | 0.67-4.00 | ||
| Median | 1.17 | 1.42 | ||
| %CV | 80.19 | 60.12 | ||
| t1/2 (hr) | ||||
| Mean (SD) | 13.85 (4.330) | 16.85 (11.105) | -- | -- |
| Range | 9.29-24.40 | 8.48-47.04 | ||
| Median | 13.64 | 12.55 | ||
| %CV | 31.27 | 65.89 | ||
| Kel (hr-1) | ||||
| Mean (SD) | 0.0541 (0.01464) | 0.0510 (0.01821) | ||
| Range | 0.0284-0.0746 | 0.0147-0.0817 | ||
| Median | 0.0509 | 0.0553 | ||
| %CV | 27.08 | 35.68 |
Table 7: Pharmacokinetic parameters and 90% CIs for the ratios of the geometric means of their log-transformed values of Emtricitabine for the 2 Dolutegravir, Emtricitabine and Tenofovir Alafenamide tablet formulations under fasting conditions (n = 12, where n is the number of volunteers).
Mean (SD) values of various pharmacokinetic parameters of Tenofovir Alafenamide for the test and reference formulations respectively, for Cmax were 205.13 (120.804) and 203.08 (125.170) ng/mL; AUC0-t were 149.11 (61.520) and 158.27 (68.473) ng·h/mL; AUC0-inf were 150.81 (61.914) and 160.08 (68.546) ng·h/mL; t1/2 were 0.32 (0.097) and 0.37 (0.093) h; Tmax were 0.78 (0.474) and 1.02 (0.649) h; Kel were 2.3342 (0.73062) and 2.0069 (0.52527) h-1. Similarly, the 90% CIs for the ratios of Cmax, AUC0–t and AUC0-inf for the 2 formulations respectively were 79.86 - 129.70, 83.66 - 107.42 and 83.58 - 107.34 which along with summary statistics such as median, range, and %CV, are given in Table 8.
| Pharmacokinetic Parameters | Test Formulation | Reference Formulation | P value | T/R Point Estimate (90% CI) |
| Cmax (ng/mL) | ||||
| Mean (SD) | 205.13 (120.804) | 203.08 (125.170) | 0.8983 | 101.77 (79.86-129.70) |
| Range | 65.70-458.03 | 73.67-452.50 | ||
| Median | 181.19 | 169.35 | ||
| %CV | 58.89 | 61.64 | ||
| AUC0-t(hr.ng/mL) | ||||
| Mean (SD) | 149.11 (61.520) | 158.27 (68.473) | 0.4566 | 94.80 (83.66-107.42) |
| Range | 44.84-254.60 | 40.02-301.02 | ||
| Median | 155.66 | 155.19 | ||
| %CV | 41.26 | 43.26 | ||
| AUC0-inf(hr.ng/mL) | ||||
| Mean (SD) | 150.81 (61.914) | 160.08 (68.546) | 0.4497 | 94.72 (83.58-107.34) |
| Range | 45.61-255.82 | 40.83-302.49 | ||
| Median | 157.26 | 157.25 | ||
| %CV | 41.05 | 42.82 | ||
| Tmax (h) | ||||
| Mean (SD) | 0.78 (0.474) | 1.02 (0.649) | -- | -- |
| Range | 0.33-2.00 | 0.17-2.50 | ||
| Median %CV |
0.75 61.05 |
0.84 63.91 |
||
| t1/2 (hr) | ||||
| Mean (SD) | 0.32 (0.097) | 0.37 (0.093) | -- | -- |
| Range | 0.17 - 0.54 | 0.21 - 0.53 | ||
| Median | 0.32 | 0.37 | ||
| %CV | 29.91 | 25.50 | ||
| Kel (hr-1) | ||||
| Mean (SD) | 2.3342 (0.73062) | 2.0069 (0.52527) | ||
| Range | 1.2859-4.0156 | 1.3152-3.2418 | ||
| Median | 2.2111 | 1.9030 | ||
| %CV | 31.30 | 26.17 |
Table 8: Pharmacokinetic parameters and 90% CIs for the ratios of the geometric means of their log-transformed values of Tenofovir Alafenamide for the 2 Dolutegravir, Emtricitabine and Tenofovir Alafenamide tablet formulations under fasting conditions (n = 12, where n is the number of volunteers).
Discussion
Aspects of this study design, such as use of a single dose; recruitment of healthy volunteers; fasting, standardized diet, fluid intake etc. and the study conditions were consistent with the regulatory guidelines [10, 11] to bring uniformity in the testing conditions for the 2 formulations.
The mean values for Cmax, Tmax, AUC0-t, AUC0-inf, Kel and t1/2 of the 2 formulations did not differ significantly (P ≥ 0.05), suggesting that the plasma profiles generated by the test formulation were not significantly different from those produced by the reference formulation.
Based on the observed T/R ratios and 90% CI’s of Cmax, AUC0-t and AUC0-inf, it was evident that the test formulation showed similar in-vivo profile to that of reference formulation under fasting conditions w.r.t Emtricitabine and Tenofovir Alafenamide. However, the in vitro dissolution studies found that the formulations were pharmaceutically equivalent with respect to dosage form.
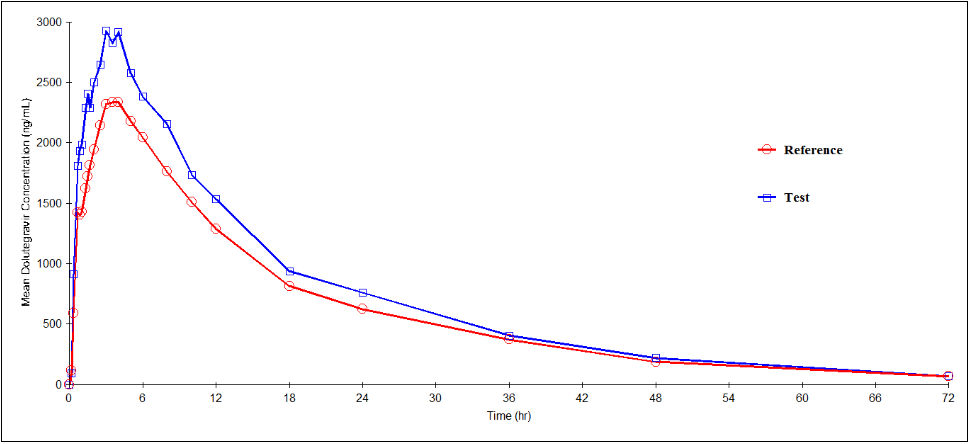
Figure 4: Plasma concentration versus time profile of Dolutegravir after 50mg/200mg/25mg oral dose of test and reference formulations under fasting conditions. Each point represents mean (ng/mL).
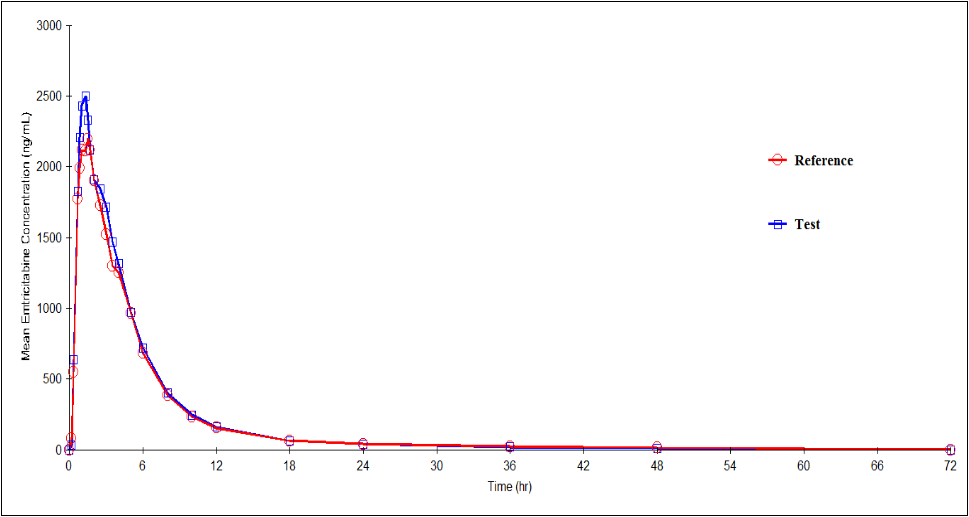
Figure 5: Plasma concentration versus time profile of Emtricitabine after 50mg/200mg/25mg oral dose of test and reference formulations under fasting conditions. Each point represents mean (ng/mL).
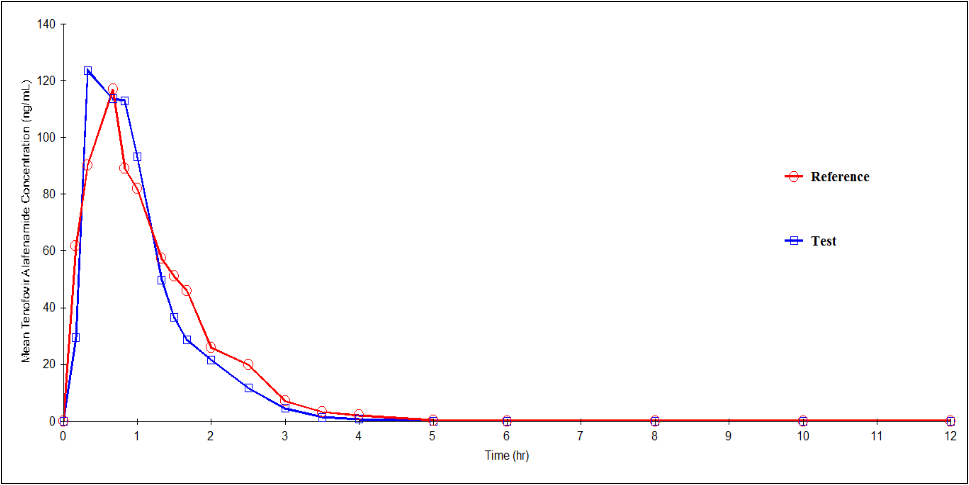
Figure 6: Plasma concentration versus time profile of Tenofovir Alafenamide after 50mg/200mg/25mg oral dose of test and reference formulations under fasting conditions. Each point represents mean (ng/mL).
Conclusions
The results of this single-dose fasting study showed that test formulation developed as fixed dose combination of Dolutegravir, Emtricitabine and Tenofovir Alafenamide Tablets 50 mg/200 mg/25 mg was found to be not bioequivalent to reference formulation w.r.t Dolutegravir and bioequivalent to reference formulation w.r.t Emtricitabine and Tenofovir Alafenamide in healthy male and female volunteers. No statistically significant differences were found among in vitro dissolution profiles, relative bioavailability and pharmacokinetic parameters. The study also concluded that test formulation developed as fixed dose combination of Dolutegravir, Emtricitabine and Tenofovir Alafenamide has similar in-vitro behavior to that of reference formulation. The in-vivo behavior of test formulation was found to be similar for Emtricitabine and Tenofovir Alafenamide except for Dolutegravir. Both test and reference formulations were safe and well tolerated.
Acknowledgments
All authors contributed equally to this study. Axis Clinicals Ltd, Mexico was selected by Aurobindo Pharma Ltd, India to design and perform this bioequivalence study and to determine whether the test formulation developed by Aurobindo is bioequivalent to reference formulation. The authors have indicated that they have no other conflicts of interest regarding the content of this article.
All authors contributed equally to this study. Axis Clinicals Ltd, Mexico was selected by Aurobindo Pharma Ltd, India to design and perform this bioequivalence study and to determine whether the test formulation developed by Aurobindo is bioequivalent to reference formulation. The authors have indicated that they have no other conflicts of interest regarding the content of this article.
References
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/204790lbl.pdf.
- Ivy Song., et al. Antimicrobial Agents and Chemotherapy 56 (2012): 1627-1629.
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021500s010,021896s004lbl.pdf
- Molina JM and Cox SL. Drugs Today 41 (2005): 241-252.
- https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/208464s001lbl.pdf
- http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_- _Public_assessment_report/human/004169/WC500223216.pdf
- Chow SC and Liu JP. “Design and Analysis of Bioavailability and Bioequivalence Studies”. 3rd edition. Boca Raton, FL: Chapman & Hall/CRC (2008).
- Shargel L and Yu ABC. Applied Bio pharmaceutics and Pharmacokinetics. Stamford, Conn: Appleton& Lange Norwalk (1999).
- Balthasar JP. “Bioequivalence and bioequivalence testing”. American Journal of Pharmaceutical Education 63(1999): 194-198.
- Food and Drug Administration (FDA). Guidance for industry: bioavailability and bioequivalence studies for orally administered drug products — general considerations. Rockville, Md: United States Department of Health and Human Services, FDA, Center for Drug Evaluation and Research (CDER), Revision 1; (2003).
- European Medicines Agency (EMEA). “Guideline on the investigation of bioequivalence”. [CPMP/EWP/QWP/1401/98], Rev.1/Corr**.London, UK; 20 January2010.
Citation:
Akula Thukaram Bapuji., et al. “Bioequivalence Study of Dolutegravir, Emtricitabine and Tenofovir Alafenamide Tablets
50mg/200mg/25mg in Healthy Volunteers under Fasting Conditions”. Chronicles of Pharmaceutical Science 2.3 (2018): 554-571.
Copyright: © 2018 Akula Thukaram Bapuji., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.