Research Article
Volume 2 Issue 5 - 2018
Immunosuppression of Tumor Necrosis Factor α by Ethanol stem bark Extract of Boswellia dalzielii (h) Averts Macroscopic Gastric Mucosal Damage in Pylorus Ligated Gastric Ulcer Model in Albino Rats.
1Department of Human Physiology, College of Medical Sciences, University of Maiduguri, Nigeria
2Department of Veterinary Physiology and Biochemistry, Faculty of Veterinary Medicine, University of Maiduguri, Nigeria
3Department of Human Physiology, College of Medical Sciences, Gombe State University, Nigeria
4Department of Human Physiology College of Medical Sciences, Bingham University, Karu, Nigeria
2Department of Veterinary Physiology and Biochemistry, Faculty of Veterinary Medicine, University of Maiduguri, Nigeria
3Department of Human Physiology, College of Medical Sciences, Gombe State University, Nigeria
4Department of Human Physiology College of Medical Sciences, Bingham University, Karu, Nigeria
*Corresponding Author: Anas Husainy Yusuf, Department of Human Physiology, College of Medical Sciences, University of Maiduguri,
Nigeria.
Received: June 21, 2018; Published: July 04, 2018
Abstract
The immunomodulatory and cytoprotection potentials of the ethanol stem bark extract of Boswellia dalzielii was investigated in pylorus ligation gastric ulcer model in rats. Serum levels of tumor necrosis factor alpha (TNFα) and vascular endothelial growth factor (VEGF) after intragastric administration of the ethanol stem bark extract of Boswellia dalzielii were estimated using enzyme linked immunosorbent assay. Gastric acidity was determined by titration with 0.01 N sodium hydroxide. The results revealed that ethanol stem bark extract of Boswellia dalzielii plant and ranitidine respectively caused significant (p <. 0001) decrease in the level of tumor necrosis factor alpha and significantly up regulated the expression VEGF in serum. It also significantly (p <. 0001) decreased gastric free and total acidity and increased pH of gastric fluid. Hence, the ethanol stem bark extract of Boswellia dalzielii plant suppressed gastric acidity, down regulate the expression of TNFα, increased serum level of VEGF to avert gastric mucosal damage in rat.
Keywords: Cytokines; Gastric; Ulcer; Antioxidants; VEGF
Introduction
Functional and structural integrity of the gastric mucosal epithelium is maintained by mucus -bicarbonate-phospholipid layers. Hormonal, paracrine and autocrine mechanisms regulate secretion, lubrication, motility that avert damage of the gastric mucosa [1]. However, excess endogenous secretions of gastric hydrochloric acid and pepsin, release of gastric tissue endothelin, leukotrienes, [2,3] TNFα, interleukins, platelet activating factor (PAF) and neutrophils [4] along with exogenous agents like the Helicobacter pylori, non-steroidal anti-inflammatory drugs (NSAIDs), [5,6] refluxed bile and detergents [7] subvert gastric mucosal defence and entrench gastric ulcer [8]. The gastric mucosal barrier is disturbed through diverse complex physiological and biochemical processes that may involve decreasing gastric mucus and bicarbonate, reduce gastric microcirculation leading to oxygen and nutrient deficiency to the affected area, [9] accumulation of reactive oxygen species (ROS), oxidative stress, [8] release of TNFα a signaling agent responsible for the activation of inflammatory cascade, cellular damage and formation of gastric ulcer [10]. Gastric ulcer treatment with synthetic drugs such as proton pump inhibitors and histamine receptor blockers (H2), antacids etc. have failed to completely eradicate the disease but expose users to adverse effects like enteric infection, hypergastrinaemia, impotence, thrombocytopenia and arrhythmia [2]. It is imperative to search inward for medicinal plant that is effective, available, affordable and less toxic compared to present day synthetic agent used in the management of gastric ulcer.
The plant Boswellia dalzielii belongs to the family Burseracceae . It is called Frankincense in English and Ararraßi in Hausa. It is a tree that can grow up to 13-meter-high with papery stem bark that releases resin exudate upon incision. The plant is found in most villages and towns in Northern Nigeria. The leaves and stem bark extract of the tree are used for various traditional rituals and in the treatment of arthritis, diarrhea, snake bite and other inflammatory disorders. The Phytoconstituents of the aqueous and methanol stem bark extract of the plant include flavonoids, saponins, alkaloids [11,12].
In this study, attempt was made to evaluate the impact of oral administration of the ethanol stem bark extract of Boswellia dalzielii on the serum levels of TNFα, VEGF and gastric acid parameters in pylorus ligated rats.
Materials and Method
The chemicals used in this study were of ANALAR grade and obtained from BDH, London except otherwise indicated. Albino Wistar rats were obtained from the animal house, Department of Human Physiology, University of Maiduguri. The rats were fed with commercial diet (Growers mash, Vital feed, Ilorin) and allowed water ad libitum. They were allowed to acclimatized to the animal house condition in accordance with the rules and guidelines of the animal ethics committee of the Medical College (pre- clinical), University of Maiduguri.
Design of the study
Thirty Wistar albino rats of either sex (140-160g) were starved for 24 h but allowed drinking water to prevent dehydration. The rats were divided into six groups (I-VI) and kept in separate cages with wire mesh bottom to prevent coprophagy. Each group had five rats. One hour prior to treatments, drinking water was withdrawn from the rats. Group I received distilled water 5 ml/kg body weight of rat and served as control. Group II (negative control) received distilled water 5 ml/kg body weight of rat followed by pylorus ligation. Groups III-V were pretreated with ethanol stem bark extract of Boswellia dalzielii 100, 200 and 400 mg/kg respectively, followed by pylorus ligation. Rats in group VI were administered ranitidine 50 mg/kg body weight of rat followed by pylorus ligation. Treatments in all the groups were given into the stomach through gastric intubation using flexible plastic catheter gauge 8. The pylorus ligation was performed after one hour from treatment.
Thirty Wistar albino rats of either sex (140-160g) were starved for 24 h but allowed drinking water to prevent dehydration. The rats were divided into six groups (I-VI) and kept in separate cages with wire mesh bottom to prevent coprophagy. Each group had five rats. One hour prior to treatments, drinking water was withdrawn from the rats. Group I received distilled water 5 ml/kg body weight of rat and served as control. Group II (negative control) received distilled water 5 ml/kg body weight of rat followed by pylorus ligation. Groups III-V were pretreated with ethanol stem bark extract of Boswellia dalzielii 100, 200 and 400 mg/kg respectively, followed by pylorus ligation. Rats in group VI were administered ranitidine 50 mg/kg body weight of rat followed by pylorus ligation. Treatments in all the groups were given into the stomach through gastric intubation using flexible plastic catheter gauge 8. The pylorus ligation was performed after one hour from treatment.
Laparotomy through midline incision (3 cm) was performed under mild diethyl ether anesthesia. The pylorus was occluded with cotton thread ligature around the pyloric sphincter. The abdominal incision was closed in two layers with interrupted silk sutures. Rats were returned to their individual cages after the abdominal sutures. All the animals in the experiment were sacrificed after four hours by exsanguination via abdominal aorta. They were anesthetized with diethyl ether prior to euthenasia. The abdomen of each rat in the experiment was cut opened again, stomach removed, washed in warm normal saline and cut across the greater curvature and its content transferred to 10 ml measuring cylinder [13].
Evaluation of gastric ulcer in rat
Macroscopic ulcers seen on the gastric mucosa were evaluated and recorded accordingly using the following: grey colour of stomach was rated as zero (0) mark, red colour of stomach was scored 0.5, appearance of haemorrhagic streak was assigned a score of 1 while spot ulcer was rated 1.5, ulcer up to 2 mm was rated 2. Score of 3 was assigned to ulcer greater than 2 mm [2].
Macroscopic ulcers seen on the gastric mucosa were evaluated and recorded accordingly using the following: grey colour of stomach was rated as zero (0) mark, red colour of stomach was scored 0.5, appearance of haemorrhagic streak was assigned a score of 1 while spot ulcer was rated 1.5, ulcer up to 2 mm was rated 2. Score of 3 was assigned to ulcer greater than 2 mm [2].
Determination of gastric acidity
The content of each stomach subjected to pylorus ligation above was centrifuged at 2000 × g for five minutes. The supernatant (gastric juice) was measured and its pH was determined with digital pH meter (pHep, Hana). One milliliter of the gastric fluid was transferred into conical flask (25 ml). Sodium hydroxide (0.01N NaOH) was added to the gastric fluid from a burette. The mixture was thoroughly stirred with each addition of the base. The volume of base (0.01 N NaOH) used in the titration was recorded at pH 5 for free acidity and pH 7 for total acidity instead of using Tofers’ reagent and phenolphthalein indicator that changes colour at pH range of 4.5-5 and 8-9.
The content of each stomach subjected to pylorus ligation above was centrifuged at 2000 × g for five minutes. The supernatant (gastric juice) was measured and its pH was determined with digital pH meter (pHep, Hana). One milliliter of the gastric fluid was transferred into conical flask (25 ml). Sodium hydroxide (0.01N NaOH) was added to the gastric fluid from a burette. The mixture was thoroughly stirred with each addition of the base. The volume of base (0.01 N NaOH) used in the titration was recorded at pH 5 for free acidity and pH 7 for total acidity instead of using Tofers’ reagent and phenolphthalein indicator that changes colour at pH range of 4.5-5 and 8-9.
Calculations
Acidity= volume of NaOH ×Normality × 100 mEq/l/100g0.1
Note: Free and Titratable acidity were obtained when volume of base at pH 5 and 7 respectively were substituted in the equation for acidity.
Acidity= volume of NaOH ×Normality × 100 mEq/l/100g0.1
Note: Free and Titratable acidity were obtained when volume of base at pH 5 and 7 respectively were substituted in the equation for acidity.
Normality of NaOH = 36.5
Acid output (mEq) = Titratable acidity (ml) × gastric fluid volume (ml)
Acid output (mEq) = Titratable acidity (ml) × gastric fluid volume (ml)
Preparation of Serum for TNF-α and VEGF assays
The blood that has clot were spun for 20 minutes at 2000 x g to further retract the clots from the serum. The serum from each container was aspirated with Pasteur pipette, transferred to clean, sterile polypropylene tubes. The samples were kept in the refrigerator at 40c or -180c until required for electrolytes, creatinine, urea and liver enzyme tests [14]. All the experimental rats used were euthanized with overdose of anaesthetic ether at the end of the experiments [15].
The blood that has clot were spun for 20 minutes at 2000 x g to further retract the clots from the serum. The serum from each container was aspirated with Pasteur pipette, transferred to clean, sterile polypropylene tubes. The samples were kept in the refrigerator at 40c or -180c until required for electrolytes, creatinine, urea and liver enzyme tests [14]. All the experimental rats used were euthanized with overdose of anaesthetic ether at the end of the experiments [15].
Estimation of vascular endothelial growth factor (VEGF) in rat gastric mucosa
The level of VEGF in serum was measured using RayBio® Rat VEGF-A ELISA Kit according to the manufacturer’s protocol (Ray Biotech, 3607 Parkway Lane, Suite 100 Norcoss, GA 30082). VEGF level was expressed as pg/mg.
The level of VEGF in serum was measured using RayBio® Rat VEGF-A ELISA Kit according to the manufacturer’s protocol (Ray Biotech, 3607 Parkway Lane, Suite 100 Norcoss, GA 30082). VEGF level was expressed as pg/mg.
Estimation of pro-inflammatory cytokine TNF-α in rat gastric mucosa
The enzyme linked immunosorbent assay (ELISA) was used for the determination of TNF-α in serum. RayBio® Rat TNF-α ELISA kit was used according to manufacturer’s instruction (Ray Biotech, 3607 Parkway Lane, Suite 100 Norcoss, GA 30082). TNF-α present in a sample is bound to the wells by the immobilized antibody. The final product was incubated at room temperature. The absorbance was read at 450 nm. The TNF-α content was expressed as pg/mg of total protein.
The enzyme linked immunosorbent assay (ELISA) was used for the determination of TNF-α in serum. RayBio® Rat TNF-α ELISA kit was used according to manufacturer’s instruction (Ray Biotech, 3607 Parkway Lane, Suite 100 Norcoss, GA 30082). TNF-α present in a sample is bound to the wells by the immobilized antibody. The final product was incubated at room temperature. The absorbance was read at 450 nm. The TNF-α content was expressed as pg/mg of total protein.
Statistical analyses
Results were expressed as mean ± standard error of mean. One-way analysis of variance (ANOVA) followed by Dunnets’ post hoc test was used to evaluate differences among the groups. All analyses were performed using JMP Statistical Discovery (software) from SAS, version 11.0. A p-value of < 0.05 was considered statistically significant.
Results were expressed as mean ± standard error of mean. One-way analysis of variance (ANOVA) followed by Dunnets’ post hoc test was used to evaluate differences among the groups. All analyses were performed using JMP Statistical Discovery (software) from SAS, version 11.0. A p-value of < 0.05 was considered statistically significant.
Results
Effect of ethanol stem bark extract of Boswellia dalzielii on gastric ulcer induced by pylorus ligation in albino rats.
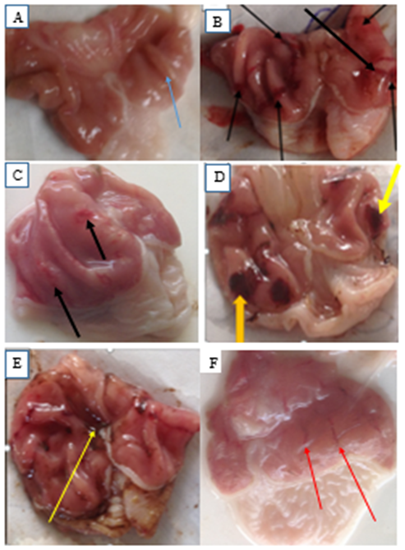
Four hours of pylorus ligation caused extensive and deep gastric ulcers in rats as shown in plate 1 (A-F). Pretreatment with graded doses of the ethanol stem bark extract of Boswellia dalzielii and ranitidine (50 mg/kg) respectively reduced gastric mucosal damage (figure 1C, D, E and F) caused by pylorus ligation when compared to pylorus control (plate 1B).
Four hours of pylorus ligation caused extensive and deep gastric ulcers in rats as shown in plate 1 (A-F). Pretreatment with graded doses of the ethanol stem bark extract of Boswellia dalzielii and ranitidine (50 mg/kg) respectively reduced gastric mucosal damage (figure 1C, D, E and F) caused by pylorus ligation when compared to pylorus control (plate 1B).
Effect of Ethanol Stem Bark Extract of Boswellia dalzielii plant on Gastric Mucosa after Pylorus Ligation in Rat.
Slide A is the groos presentation of the stomach in the sham control group with no pylorus ligation showing normal contour of the ruggae(blue arrows). Slide B depicts (blak arrows) deep gastric ulcers after pylorus ligation. Slides C, D, E and F shows the effect of pretreatment with 100, 200, 400 mg/kg of the Ethanol Stem Bark Extract of Boswellia dalzielii respctively and ranitidine 50 mg/kg followed by pylorus ligation after one hour. The yellow arrow shows blood clot and the red aaows shows haemmorragic streak.
Slide A is the groos presentation of the stomach in the sham control group with no pylorus ligation showing normal contour of the ruggae(blue arrows). Slide B depicts (blak arrows) deep gastric ulcers after pylorus ligation. Slides C, D, E and F shows the effect of pretreatment with 100, 200, 400 mg/kg of the Ethanol Stem Bark Extract of Boswellia dalzielii respctively and ranitidine 50 mg/kg followed by pylorus ligation after one hour. The yellow arrow shows blood clot and the red aaows shows haemmorragic streak.
Effect of ethanol stem bark extract of Boswellia dalzielii on gastric ulcer index induced by pylorus ligation in albino rats.
Pylorus ligation caused extensive gastric damage with large gastric ulcer in the pylorus control group. Pre-treatment with graded doses of the plant extract significantly (p < 0.05) decreased pylorus induced gastric ulcer score as shown in table 1. The plant extract at 400mg/kg protected the gastric mucosa against pylorus ligation induced damage more than ranitidine used in the study.
Pylorus ligation caused extensive gastric damage with large gastric ulcer in the pylorus control group. Pre-treatment with graded doses of the plant extract significantly (p < 0.05) decreased pylorus induced gastric ulcer score as shown in table 1. The plant extract at 400mg/kg protected the gastric mucosa against pylorus ligation induced damage more than ranitidine used in the study.
| Treatment (mg/kg) | Ulcer Index (mm) | % Protection |
| Control | Nil | Nil |
| Pylorus Ligation | 26.67 ± 1.07 | 33.75 |
| 100 | 17.66 ± 1.07a | 50 |
| 200 | 13.33 ± 1.07b | 53.70 |
| 400 | 7.33 ± 1.07b | 69.99 |
| Ranitidine 50 | 12.00 ± 1.07b | 62.96 |
Table 1: Effects of the ethanol stem bark extract of Boswellia dalzielii H. on pylorus ligation induced gastric ulcer index in albino rats.
Effects of the ethanol stem bark extract of Boswellia dalzielii H. on pylorus ligation induced gastric ulcer index in albino rats
Results are expressed as mean ± SEM (n=5). One-way ANOVA along with Dunnets’ post hoc tests were used for multiple statistical comparism.
Results are expressed as mean ± SEM (n=5). One-way ANOVA along with Dunnets’ post hoc tests were used for multiple statistical comparism.
A = p < .05 was considered statistically significant when compared to pylorus ligation control.
B = p < .0001was considered statistically significant when compared to pylorus ligation control
B = p < .0001was considered statistically significant when compared to pylorus ligation control
Effect of ethanol stem bark extract of Boswellia dalzielii on serum levels of VEGF and TNF α in pylorus ligation induced gastric ulcer in albino rats.
Pylorus ligation in rats up-regulated serum level of VEGF as shown in table 2. Pre-treatment with graded doses of the plant extract significantly (p < 0.0001) increased the serum level of VEGF in pylorus induced gastric ulcer when compared to sham control. The serum level of TNF α increased significantly (p < 0.0001) after pylorus ligation when compared to control. Oral administration of the graded doses of the plant extract prior to pylorus ligation has significantly (p) down regulated pylorus induced rise in serum TNF α as shown in table 2.
| Treatment | VEGF(pg/ml) | TNF α (pg/ml) |
| Sham Control | 9.2200 | 0.74 ± 0.50b |
| Pylorus control | 18.7567 | 9.08 ± 0.50a |
| 100 mg/kg | 21.2567a | 1.76 ± 0.50b |
| 200 mg/kg | 22.2900a | 1.80 ± 0.50b |
| Ranitidine (50 mg/kg) | 10.3750a | 2.59 ± 0.50b |
Table 2: Effect of ethanol stem bark extract of Boswellia dalzielii on serum level of VEGF and TNF α in pylorus ligation induced gastric ulcer in albino rats.
Effect of ethanol stem bark extract of Boswellia dalzielii on serum level of VEGF and TNF α in pylorus ligation induced gastric ulcer in albino rats
Results are expressed as mean ± SEM (n=5). One-way ANOVA along with Dunnets’ post hoc tests were used for multiple statistical comparism.
Results are expressed as mean ± SEM (n=5). One-way ANOVA along with Dunnets’ post hoc tests were used for multiple statistical comparism.
a = significant relative to sham control ( P < .0001), b = significant relative pylorus control (p < .0001)
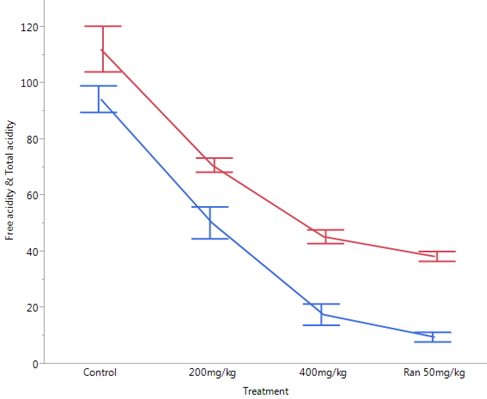
Figure 2: Effect of Ethanol Stem Bark Extract of Boswellia dalzielii
on Gastric Acid Parameters after Pylorus Ligation in Rats.
Effect of Ethanol Stem Bark Extract of Boswellia dalzielii on Gastric Acid Parameters after Pylorus Ligation in Rats.
A = total acidity, B = free acidity, a = statistically significant when compared to control
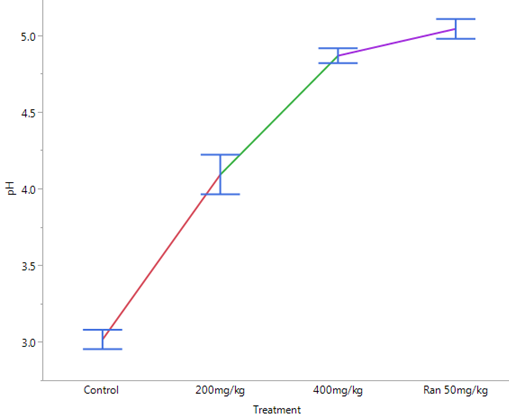
Figure 3: Effects of Ethanol Stem Bark Extract of Boswellia dalzielii
on Gastric pH after Pylorus Ligation in Albino Rats.
Results are expressed as mean ± SEM (n = 5). One-way ANOVA along with Dunnets’ post hoc tests were used for multiple statistical comparism.
A = statistically significant when compared to control (p < .0001)
A = statistically significant when compared to control (p < .0001)
Discussion
In the present study, pylorus ligation in rat has caused a perceptible gross gastric mucosal injury (Plate 1A-F) with increased gastric ulcer index in the pylorus control group when compared to the sham control (Table 1.0). Similar results were obtained in previous studies [16-18]. They reported the presence of severe disruption of the surface epithelium along with necrotic lesion that extends deeply in to the mucosa. The ethanol stem bark extract of Boswellia dalzielii significantly (p < 0.0001) reduced serum level of tumor necrosis factor alpha. The tumor necrosis factor (TNF)-α, TNFα, a potent immunomodulatory agent is involved in activation of transcription factor and oxidative stress mediated by neutrophil. It also promotes the synthesis and expression of adhesion molecules on gastric endothelium and neutrophil. Tumor necrosis factor (TNF)-α causes polymophornuuclear adhesion leading to gastric and intestinal mucosal epithelium damages [19]. The ethanol stem bark extract of Boswellia dalzielii probably act as an anti- TNFα by reducing its serum expression and averts damages to the gastric epithelia during pylorus ligation in rat.
The ethanol stem bark extract of Boswellia dalzielii significantly (p < 0.0001) increased the serum level of VEGF in this study. Vascular endothelial growth factor, an angiogenic agent, strengthen gastric mucosal defence and prevent damage to the epithelial mucosal gastric via establishment of perivascular edema around sub-epithelial capillaries in the gastric mucosa. This delays the absorption of toxic chemicals and minimize endothelial cells exposure [20,21]. Intragastric administration of graded doses of the ethanol stem bark extract of Boswellia dalzielii may have triggered the liberation of the VEGF to re-enforce the gastric mucosal barrier and minimize damage to gastric epithelia.
The plant extract significantly (p < 0.05) reduced free and total gastric acidity and gastric acid output and significantly (p < 0.0001) increased gastric fluid volume when compared to pylorus control. Previous studies [22] revealed similar result. They established that Boswellia extract significantly decreased gastric acidity and Malonaldehyde but elevates Superoxide dismutase, catalase and glutathione levels in pylorus ligated rats. Pylorus ligation ulcer is caused by accumulation of gastric acid and pepsin, mucosal digestion and disruption of gastric mucosal barrier. Other mechanisms of the mucosal damage by pyloric obstruction include starvation leading to decrease mucosal defence, increase vagal discharge leading to mast cell degranulation and depletion of histamine in gastric tissue and eventual gastric damage [23]. Gastric secretions in intact animal is regulated by neuronal and hormonal factors (Chantharangsikul., et al. 2009) [24].
The ethanol stem bark extract of Boswellia dalzielii may have acted on parietal cells, other cells and pathways to reduce gastric acidity, increase pH and avert mucosal damage as evidenced by reduced ulcer index (table 1). A ranitidine-like compound may be present in the plant extract and have probably acted on M1 and M3 muscuranic receptors and H2 receptors on the parietal cells to inhibit the synergic effect of endogenous acetylcholine and histamine. Thus, prevent the stimulation and release of gastric acid. Flavonoid in general attenuate histamine production and release by the mast cells through disruption of the histidine decarboxylase activity (Gao., et al. 2015) [24]. There is also the possibility of the bioactive component of the extract of Boswellia dalzielii to act on alpha adrenergic (α2) receptor to inhibit gastric acid secretion, gastric motility and increase blood flow or in concert with the muscarinic and histamine receptors or acted on entirely separate set of other mediators to reduce gastric acid secretion. Blockade of gastrin, pituituary adenylate cyclase acting peptide (PACAP), vasoactive intestinal peptide (VIP), γ -aminobutyric acid or cholecystokinin release will inhibit histamine, somatostatin, prostaglandin, calcitonin gene related peptide (CGRP) to reduce gastric acid secretion [24]. Hence, blockade of any of the above pathway by ethanol stem bark extract of Boswellia dalzielii may undermine gastric acid secretion. The role of ethanol stem bark extract of Boswellia dalzielii in the inhibition of gastric acid is indeed speculative. In vivo model like the pylorus ligation used in the study usually expose the plant material to intestinal mucosal absorption, biotransformation and distribution through the blood and to many other mediators of gastric acid secretion in the stomach and other parts of the body. Whereas; in vitro model is devoid of extraneous intervention and may be more precise in identifying pathways and mechanism of action.
Conclusion
The ethanol stem bark extract of Boswellia dalzielii averts pylorus ligation gastric mucosal damage in rat due to suppression of the release of tumor necrosis factor alpha, reduction in gastric acid production and increase in the level of vascular endothelial growth factor.
References
- Tarnawski A., et al. “Gastric cytoprotection beyond prostaglandins: cellular and molecular mechanisms of gastroprotective ulcer healing actions of antacids”.Current Pharmaceutical Design19(2013): 126–132.
- Gulia Y., et al. “Peptic ulcer disease review”. Pharmacologyonlie (2011): 48-70
- Maeng JH., et al. “Rabbit gastric ulcer models: comparison and evaluation of acetic acid-induced ulcer and mucosectomy-induced ulcer”. Laboratory Animal Research29.2 (2013): 96–102.
- Wantabe T., et al. “Acid Regulates Inflammatory Response in a Rat Model of Induction of Gastric Ulcer Recurrence by 1β.Gut”. 48 (2001): 774-781.
- Musumba C., et al. “Cellular and molecular mechanism of NSAIDs –induced peptic ulcers”.Alimentary Pharmacology and Therapeutics 30 (2009): 517-530.
- Keller R., et al. “Interrelation between ABH blood group O, Lewis (B) blood group antigen, Helicobacter pylori infection, and occurrence of peptic ulcer”. Zeitschrift für Gastroenterologie40.5 (2002): 273-6
- Davenport HW. “Physiological parameters of gastric mucosal barrier”. Digestive Diseases 21.2 (1976): 141-143
- Lain L., et al. “Gastric mucosal defense and cytoprotection: Bench to bedside”. Gastroenterology 135.1(2008): 41-60
- Srinath R., et al. “Pathophysiological mechanisms and preclinical models of Peptic ulcer”. International Journal of Pharmacy and Pharmaceutical Sciences 2.2 (2013): 771-779.
- Mahmoodi M., et al. “Impact of fumonisin B1 on the production of inflammatory cytokines by gastric and colon cell lines”. Iran J Allergy Asthma Immunology 1.2 (2012): 165-173.
- Nwinyi FC., et al. “Evaluation of the aqueous extract of Boswellia dalzielii stem bark for antimicrobial activities and gastrointestinal effects”. African Journal of Biotechnology 3.5 (2004): 284-288.
- Etuk EU, et al. “Anti-Diarrhoea effect of Boswellia dalzielli stem bark extract in albino rats”. Journal of Pharmacology and Toxicology 1.6 (2006): 591-596
- Ishii Y. “Critical studies of the pylorus ligated rat (Shay Rat)”. The Japanese Journal of Pharmacology 19 (1969):125-133.
- Decie JV., et al. “Practical Haematology(8th Ed). ELBS-Churchill Livingstone”. Edinburg (1994).
- Waynforth HB., et al. “Experimental and surgical techniques in rat”. 2nd edition London: Academic Press (1992).
- Matsui Y., et al. “Efficacy of vascular endothelial growth factor in the treatment of experimental gastric injury”. Digestion 66 (2002): 99-105
- Hussain R., et al. “Isolation, characterization, and in Silco, inivtro and antiulcer studies of isoimperatorin crystalized from Ostericum koreanum. Pharmaceutical Biology55.1 (2017): 218-225
- Mastwal A., et al. “Evaluation of antiulcer activity of the leaf extract of Osyris quadripartite decnne (santalacae) in rats”. Journal of Experimental Pharmacology 9 (2017)1-11.
- Wantabe T., et al. “Acid regulates”. inflammatory response in a rat model of induction of gastric ulcer recurrence by interleukin 1β. Gut 48(2001): 774-781.
- Suzuki N. “Relationship between VEGF and angiogenesisin spontaneous and indomethacin-delayed healing of acetic acid induced gastric ulcer in rats”. Journal of physiology and pharmacology 49 (1998): 515-527.
- Yusuf AH., et al. “Modulation of vascular endothelial growth factor and tumor necrosis factor alpha using ethanol stem bark extract of Boswellia dalzielii H attenuates ethanol-induced gastric ulcer in albino rats”. Eurasian Journal of Medicine and Oncology 2.2(2018): 97-104.
- Sharkawi SM., et al. “Prophylactic role of Echinacea, Green Tea and Boswellia Extracts in pyloric ligation-induced gastric ulcer in rats”.British Journal of Pharmacology and Toxicology 3 (2012): 197–204.
- Gao Y., et al. “Mechanism of anti-ulcerogenic effect of Ganoderma lucidum polysaccharides on indomethacin- induced lesions in the rat”. Life Sciences 72 (2002): 731-745.
- Chandrangsikul D., et al. “Gastric acid secretion by Ya-hom in isolated mouse whole stomach”. Asian Biomedicine 30.9 (2009): 663-673.
Citation:
Anas Husainy Yusuf., et al. “Immunosuppression of Tumor Necrosis Factor α by Ethanol stem bark Extract of Boswellia
dalzielii (h) Averts Macroscopic Gastric Mucosal Damage in Pylorus Ligated Gastric Ulcer Model in Albino Rats.”. Chronicles of Pharmaceutical
Science 2.5 (2018): 683-691.
Copyright: © 2018 Anas Husainy Yusuf., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.