Research Article
Volume 2 Issue 6 - 2018
Polymerization in Static Hetrogeneous Monomer - Water System in the Absence of Emulsifier
Scientific and Technological Center for Organic and Pharmaceutical Chemistry of the National Academy of Sciences of Armenia, 0014, Yerevan, Azatutyan Ave 26
*Corresponding Author: AA Hovhannisyan, Scientific and Technological Center for Organic and Pharmaceutical Chemistry of the National Academy of Sciences of Armenia, 0014, Yerevan, Azatutyan Ave 26.
Received: August 04, 2018; Published: August 13, 2018
Abstract
The topology of formation of polymer-monomer particles, polymerization of vinyl acetate and styrene in a heterogeneous static monomer-water system, in the presence of hydroquinone and asobisisibutironitrile (AIBN) has been studied by photo and spectrophotometric observation methods. It was established that, in a static heterogeneous monomer-water system, regardless of the nature of the monomer and initiator, the dispersion of the monomer and the generation of latex particles in the form of micro droplets of the monomer occur at the interface. It is suggested that the stability of the resulting colloidal system is due to the terminal hydrophilic groups of polymer and oligomer molecules formed as a result of radical reactions taking place in the aqueous phase. The concluded assumption is that the stability of the obtained colloidal system is due to the polymer and oligomer end hydrophilic groups of in the aqueous phase.
Keywords: Heterogeneous styrene; Latex particles; Oligomer; Vinyl acetate
Introduction
Polymerization In a static heterogeneous styrene-water system leads to the formation of latex particles [1-3]. A hypothesis was set forth that the work of dispersing a monomer, and thus the generation of latex particles, is due to the heat of the polymerization reactions proceeding at the interface [2,3]. It remains unclear how realistic this mechanism is, since in the aqueous phase it is possible to accumulate polymer molecules and their homogeneous nucleation [4]. This issue is especially relevant for the polymerization of monomers, solubility of which in water may lead to the formation of large polymer molecules. The reason of stability of latex particles to coagulation is still unclear.
To clarify these circumstances the topology of dispersed particle formation in polymerization processes of vinyl acetate (VA) in emulsifier free statistic monomer-potassium per sulfate (PP) aqueous solution was investigated. VA –water and styrene-water systems were also investigated, in the presence of an inhibitor and an oil-soluble initiator. Under these conditions the polymerization processes are localized in separate zones of the heterogeneous system and such an approach helps in obtaining more advanced topological picture of the latex particles formation.
Experimental Part and Discussion of The Results
Polymerization in a static monomer-water system was carried out in thermo stated U-shaped tubes, which allowed sampling for analyses from the aqueous phase without perturbing the phase boundary. The temperature of the experiments is 60°C. The processes of polymerization and the formation of dispersed particles in the static monomer-water system are accompanied by turbidity of the aqueous phase, which makes it possible to follow the dynamics of this process by photographing the tubes and measuring the optical density of the aqueous phase.
Polymerization in a static monomer-water system was carried out in thermo stated U-shaped tubes, which allowed sampling for analyses from the aqueous phase without perturbing the phase boundary. The temperature of the experiments is 60°C. The processes of polymerization and the formation of dispersed particles in the static monomer-water system are accompanied by turbidity of the aqueous phase, which makes it possible to follow the dynamics of this process by photographing the tubes and measuring the optical density of the aqueous phase.
The topological picture of the dynamics of turbidity of the aqueous phase in the vinyl acetate system is an aqueous solution of potassium per sulfate is shown in Figure 1. The dynamics of the development of the process is clearly visible on these pictures: the dispersed particles that originate in the polymerization process are first localized in a narrow zone of the water phase near the interface, then gradually spread into the interior of the water phase. Earlier, an analogous picture was obtained in the polymerization of styrene [2,3] and the results of Figure 1 suggest that the process of nucleation of dispersed particles at the phase interface does not depend on the nature of the monomer but as the result of polymerization reactions in the boundary layer monomer.

Figure 1: Topological picture of the turbidity of the aqueous phase in the
solution of vinyl acetate in methanol - 0.4% aqueous solution of potassium per
sulfate at different observation times: a - 30 min., b - 50, c - 70, d - 100, e - 130.
To locate possible polymerization reactions in the aqueous phase, an inhibitor (hydroquinone) was introduced into the system. Hydroquinone was initially dissolved in the monomeric phase (0.4% by weight of the monomer). Systems such as styrene-water and VA-water were studied. In the styrene-water system, the change in the optical density of the aqueous phase and the formation of the emulsion began after approximately 60 minutes of observation, and in the VA-water system, the turbidity of the aqueous phase was not observed at all. In Figure 2 photo of test tubes of the two systems after 10 days of temperature control is illustrated.

Figure 2: VA - water and styrene - water systems containing hydroquinone
in the monomeric phase and 0.4% potassium per sulfate K2S2O8 in the
aqueous phase, after 10 days of temperature control at T 60°C.
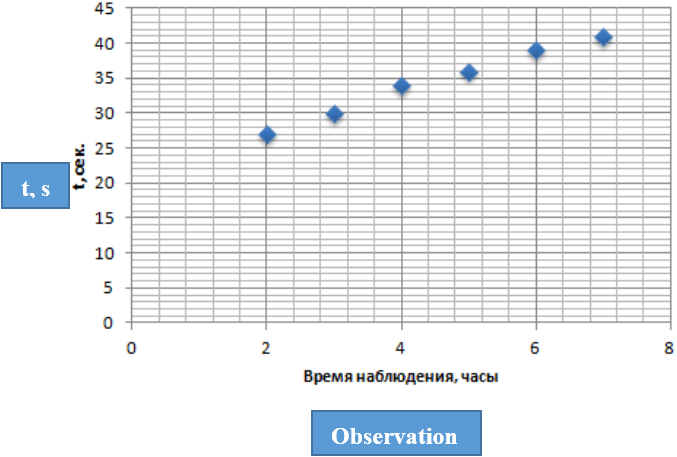
Figure 3: Dependence of the viscosity of the aqueous phase on the time of
thermo stating (observation) of the system. t is the time of the outflow of
aqueous phase samples in a viscometer.
The absence of the process of formation of dispersed particles in the VA-water system is possible due to the formation of water-soluble oligomers with sulfate end groups. To clarify this issue, PP was replaced by asobisisibutironitrile (AIBN). AIBN was introduced into the aqueous phase as a methanol solution. The studies were carried out in U-shaped test tubes. On the one side, VA was layered on the water, and on the other - a saturated solution of AIBN in methanol. Thus, polymerization was initiated by the diffusion of AIBN into the aqueous phase.
Phase compositions: VA-5 ml, water phase: water-20 ml, saturated solution of AIBN in methanol – 5 ml in these series of experiments, samples were taken from the test tubes, and the viscosity of the upper zone of the aqueous phase from the VA-water side was measured. The results of the measurement are shown in Figure 3. These results show that the viscosity of the upper zone of the water phase increases with time. The increase in viscosity was not accompanied by turbidity of the medium, which indicates the accumulation of water-soluble oligomers in the water phase. A change in the viscosity of the monomeric phase, during the time of the experiments, was not observed.
The inhibition of the formation of polyvinyl acetate latex particles with hydroquinone indicates that the length of polymer molecules produced in the aqueous phase and the nature of their terminal groups play a decisive role in the process of formation and stabilization of latex particles. From this result it follows that the chemistry of stability of emulsifier-free latex should be directly dependent on both the nature of the monomer and the initiator. According to [5], the thermal decomposition of AIBN into dimethylcyanomethyl radicals proceeds according to the monomolecular mechanism and does not depend on the nature of the solvent:
(CN)(CH3)2CN=NC (CH3)2(CN) ð 2(CN) (CH3)2C* + N2 (1)
In the static system, with the diffusion of AIBN and vinyl acetate molecules into the aqueous phase, the dimethylcyanomethyl radicals interact with both the monomer molecules and the water molecules:
(CN) (CH3)2C* + M ð CN) (CH3)2CM* (2)
(CN)(CH3)2C* + HOH ð (CN) (CH3)2CH + HO* (3)
(CN)(CH3)2CN=NC (CH3)2(CN) ð 2(CN) (CH3)2C* + N2 (1)
In the static system, with the diffusion of AIBN and vinyl acetate molecules into the aqueous phase, the dimethylcyanomethyl radicals interact with both the monomer molecules and the water molecules:
(CN) (CH3)2C* + M ð CN) (CH3)2CM* (2)
(CN)(CH3)2C* + HOH ð (CN) (CH3)2CH + HO* (3)
Where, M is the monomer molecule, *is the sign of the radical. As a result of reaction 3, polymerization of BA in the aqueous phase can be initiated by hydroxyl radicals, as a result of which surface-active oligomers with OH end groups can be formed:
HO* + M ð HOM* (4)
The flow of reaction 3 was confirmed by the presence of an absorption band characteristic of hydroxyl groups in the obtained IR spectra of the polymers.
The flow of reaction 3 was confirmed by the presence of an absorption band characteristic of hydroxyl groups in the obtained IR spectra of the polymers.
The possibility of obtaining stable polymer dispersions in the polymerization of VA will largely depend on the hydrophilic-lipophilic balance (HLB) of the oligomers formed in the aqueous phase.
Usually, the calculation of HLB of diphasic molecules is carried out according to Davis formula [6]:
HLB = 7 + aA – bB
Where A and B are numbers characterizing the hydrophobicity of the polar and the hydrophobicity of alkyl groups in the molecule. a and b are equal to the numbers of the polar and nonpolar groups of the molecule.
The values of A and B for different groups of organic diphasic molecules are given in [6]:
A for (– O–), (– OH) and (– C = O) groups are 1.3, 1.9 and 0.3, respectively, and B for
Usually, the calculation of HLB of diphasic molecules is carried out according to Davis formula [6]:
HLB = 7 + aA – bB
Where A and B are numbers characterizing the hydrophobicity of the polar and the hydrophobicity of alkyl groups in the molecule. a and b are equal to the numbers of the polar and nonpolar groups of the molecule.
The values of A and B for different groups of organic diphasic molecules are given in [6]:
A for (– O–), (– OH) and (– C = O) groups are 1.3, 1.9 and 0.3, respectively, and B for
CH3–, – CH2 – and – C= groups is equal to – 0.475.
Substituting these values of A and B in the Davis formula for the HLB of VA oligomers with one and two hydroxyl end groups, we obtain:
HLB = 7 + 1.9 + n (1.6) + n (– 1.9) = 8.9 + n (– 0.3)
HLB = 7 + 3.8 + n (1.6) + n (– 1.9) = 10.8 + n (– 0.3)
where n number of monomer units in oligomers
Substituting these values of A and B in the Davis formula for the HLB of VA oligomers with one and two hydroxyl end groups, we obtain:
HLB = 7 + 1.9 + n (1.6) + n (– 1.9) = 8.9 + n (– 0.3)
HLB = 7 + 3.8 + n (1.6) + n (– 1.9) = 10.8 + n (– 0.3)
where n number of monomer units in oligomers
The calculated values of the HLB are given in Table 1.
| N | HLB oligomers of VA with one terminal OH group | of HLB oligomers of VA with two OH groups |
| 1 | 8.6 | 10.5 |
| 4 | 7.7 | 9.6 |
| 10 | 5.9 | 7.8 |
| 15 | 4.4 | 6.3 |
| 20 | 2.9 | 4.8 |
Table 1
Dependence of HLB oligomers of VA with hydroxyl end groups on the number of monomer units (n) of oligomers according to literature data, substances whose HLB values are in the range of 5 to 20 can be used as stabilizers. The calculated HLB values of the oligomers shown in Table 1 show that in the static VA-water system, when polymerization is initiated by an oil-soluble initiator, stable latexes can be obtained under the condition of initiation of polymerization by hydroxyl radicals. To create such conditions, systems containing the minimum and maximum amount of VA in the aqueous phase were investigated. Polymerization was carried out in a two-phase system of VA - (water + a saturated solution of AIBN in methanol).
Two series of experiments were carried out. In the first series, the monomer concentration in the aqueous phase, at the beginning of the polymerization, was zero. In the second series, the water was initially saturated with a monomer. Measurements of induction periods of turbidity of aqueous phases for both systems gave the same result, which indicated the limiting role of diffusion of oligomer radicals during the formation of the dispersed phase. Figure 4 shows photographs of the resulting dispersions, after 10 days storage, at room temperature. As can be seen from the figure, the dispersion obtained without preliminary dissolution of the VA in the aqueous phase (tube 2) was fairly stable, whereas in the second series (tube 1) in 10 days, the polymer completely separated from the aqueous phase. In both systems, the dry residue of the aqueous phase was 2%.
The obtained experimental results allow us to conclude that when using oil-soluble initiators (as well as inhibitors and other chain transmitters) stable polyvinyl acetate latexes can be synthesized. The optical density of the solutions was measured on a SF-26 spectrophotometer, the dry residue was determined by the gravimetric method, the IR spectra were examined with a SPEKORD UR 75 instrument, the viscosity of the solutions was measured using Ubbelohde viscometer, and electron micrographs were obtained with the Tescan Vega electron microscope.

Figure 4: Latexes obtained by the polymerization of VA in the monomer-AIBN
solution in alcohol- water, after 10daysofstorage.
- The aqueous phase at the beginning of the polymerization was saturated with monomer,
- No monomer was in the aqueous phase at the beginning of the polymerization.
Conclusion
The polymerization of vinyl acetate and styrene in a heterogeneous static monomer-water system, shows that, regardless of the nature of the monomer and initiator, polymerization processes proceeding at the interface disperse the system and produce a latex whose stability to coagulation is due to the hydrophilic balance of the oligomer molecules synthesized in the aqueous phase.
References
- Oganesyan AA., et al. “Theoretical Foundations of Chemical Engineering”. 47.5 (2013): 600–603.
- Hovhannisyan AA., et al. “Journal of Chemistry and Chemical Engineering USA 9.5(2015): 363-368.
- Hovhannisyan AA., et al. “On the Theory of Emulsion Polymerization Lambert, Academic Publishing, Germ. (2016): 68.
- Fitch R.M., et al. Polymer Chemistry Prepare II 2 (1970) 807-810.
- Lewis FM., et al. Journal of Chemistry and Chemical Engineering 71 (1949): 747.
- Adamson AA. Physical Chemistry of Surfaces, Welly (1990): 777.
Citation:
AA Hovhannisyan., et al. “Polymerization in Static Hetrogeneous Monomer - Water System in the Absence of Emulsifier”.
Chronicles of Pharmaceutical Science 2.6 (2018): 718-723.
Copyright: © 2018 AA Hovhannisyan., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.