Research Article
Volume 2 Issue 6 - 2018
Comparative Evaluation Quality of Different Brands of Ibuprofen 400 mg Tablets available in Yemeni’s Market
1Department of Chemistry, Sana’a University, Republic of Yemen
2Department of Pharmacy, Al-Razi University, Republic of Yemen
3Faculty of Pharmacy, Sana'a University, Republic of Yemen
2Department of Pharmacy, Al-Razi University, Republic of Yemen
3Faculty of Pharmacy, Sana'a University, Republic of Yemen
*Corresponding Author: Abdulmajed Alsaifi, Department of Chemistry, Sana’a University, Republic of Yemen.
Received: August 14, 2018; Published: August 28, 2018
Abstract
Background: Ibuprofen is an orally administered, non-steroidal anti-inflammatory agent used extensively in the treatment of arthritis. Many different brands and dosage forms of Ibuprofen are available in Yemeni’s Market that places health practitioners in a dilemma of drug substitution in case of non-availability of a particular brand. In this study we take samples of the Ibuprofen (400 mg) drug from five different pharmaceutical companies in the Sana'a market to evaluate the quality of different brands of Ibuprofen tablets with international standards.
Aim/Objective: The aim of the present study was to evaluate the quality control of five brands of Ibuprofen tablets formulation manufactured by different pharmaceutical companies and commonly prescribed in Sana'a city. The results and findings of the present study will be interpreted and discussed.
Methods: Five brands of Ibuprofen tablets (400 mg) were purchased from the retail pharmacy outlets and their pharmaceutical quality were assessed by using in-vitro tests as per the British Pharmacopoeia (BP) and unofficial standards as recommended by the manufacturers. The assessment of tablets included the evaluation of weight variation, friability, hardness, disintegration, dissolution test, water content and assay content by HPLC analysis.
Results: The results indicated that the only one brand from five brands of ibuprofen tablets was satisfactory as they met the requirements of the official and unofficial quality control tests; the four brands of ibuprofen tablets not meet the required standards of both BP and USP standards for hardness test.
Conclusion: It can be concluded that five brands of ibuprofen tablets that are available in the Yemeni’s Market were satisfactory as they met the requirements of the official and unofficial quality control tests.
Keywords: Quality control tests; Ibuprofen; Dissolution; Weight variation; Hardness; Friability and disintegration
Introduction
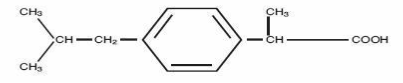
Ibuprofen (IBP) is one of the most potent orally active antipyretic, analgesic and non-steroidal anti-inflammatory drug (NSAID) used extensively in the treatment of acute and chronic pain, osteoarthritis, rheumatoid arthritis and related conditions. This compound is characterized by a better tolerability compared with other NSAIDs [1]. IBP is derived from propionic acid and has been approved as a nonprescription drug since 1983 [2]. It has a pKa value of 4.5 and is poorly soluble in water (0.078 μg/mL) [3-5]. Chemically Ibuprofen is 2-(4-Isobutylphenyl) propionic acid and its chemical structure [6] is given in the figure 1.
Indian Pharmacopoeia [7], British Pharmacopoeia [8] and European Pharmacopoeia [9] described titrimetric method for the estimation of Ibuprofen (IBU) in bulk form and titrimetric as well as liquid chromatographic method for the assay of tablet, cream, gel and oral suspension of Ibuprofen (IBU). United States Pharmacopoeia [10] described liquid chromatographic method for the estimation of Ibuprofen (IBU) in bulk form and in tablet and oral suspension formulation. Wane and Patel [11] pointed out that there are a number of methods have been described in the literature for the estimation of Ibuprofen (IBU) as single formulation which includes spectrophotometric, spectrofluorimetric, HPLC, HPTLC, and GLC. Also numerous methods have been reported for the estimation of IBU in multicomponent formulation which includes spectrophotometric, HPLC, UPLC, HPTLC and GC-MS.
The quality assessment of pharmaceutical products is a basic responsibility of Quality control (QC) unit. Quality control is concerned with the finished product and any signs of defects or deviations from set of standards in pharmacopoeias [12,13].
To confirm the safety, efficacy and effectiveness of any pharmaceutical dosage form it is required to perform in-vivo and In-vitro test. The in-vitro evaluations are performed as per the official pharmacopoeia of United States, India or other countries. The official in-vitro quality control tests those are required to confirm the quality of pharmaceutical products are friability, weight variation, disintegration, drug assay and In-vitro dissolution [14]. The aim of the present study is to evaluate the quality of different brands of Ibuprofen 400 mg Tablets available in Yemen’s Market.
Materials and Methods
Materials
Sampling
Comparative in-vitro quality control parameters between five commercially available tablet different brands of Ibuprofen were purchased from the retail pharmacies in Sana’a, Yemen. All brands were studied through the evaluation of average weight and weight variation, chemical content (assay), hardness, friability, and disintegration time and dissolution tests. All the tablet brands of Ibuprofen tablets (film coated) were labeled to contain 400 mg of Ibuprofen per tablet and coded as A, B, C, D, and E (table 1). The study was done by performing various test procedures associated to assess the quality of tablets. USP and British pharmacopoeia were used as standard for the evaluation study. All other reagents were of analytical grade and water was double distilled.
Sampling
Comparative in-vitro quality control parameters between five commercially available tablet different brands of Ibuprofen were purchased from the retail pharmacies in Sana’a, Yemen. All brands were studied through the evaluation of average weight and weight variation, chemical content (assay), hardness, friability, and disintegration time and dissolution tests. All the tablet brands of Ibuprofen tablets (film coated) were labeled to contain 400 mg of Ibuprofen per tablet and coded as A, B, C, D, and E (table 1). The study was done by performing various test procedures associated to assess the quality of tablets. USP and British pharmacopoeia were used as standard for the evaluation study. All other reagents were of analytical grade and water was double distilled.
| Code | Batch No. | Manufacture date | Expiry date | Labeled Strength (mg) |
| A | B651231 | 01/2015 | 02/2018 | 400 |
| B | B150307 | 03/2015 | 3/2018 | 400 |
| C | 2949 | 03/2015 | 03/2018 | 400 |
| D | XQ10660 | Apr 2018 | 400 | |
| E | 70 | 01/2015 | 01/2020 | 400 |
Table 1: Brands of Ibuprofen.
Methodology
Weight Variation
Twenty (20) tablets from each of the brands were weighed individually using an analytical weighing balance. The average weight for each brand as well as percentage deviations were calculated.
Twenty (20) tablets from each of the brands were weighed individually using an analytical weighing balance. The average weight for each brand as well as percentage deviations were calculated.
Chemical content (assay)
The estimation of drug content for Ibuprofen tablets was performed by HPLC.
The estimation of drug content for Ibuprofen tablets was performed by HPLC.
HPLC assay
The HPLC system (Agilent Infinity 1260, Agilent Technologies Inc., USA) has four gradient pumps incorporated with a solvent degasser, injector, column oven and a diode array detector. An Agilent ZORBAX Eclipse plus C18 100 mm × 4.6 mm, 3.5 μm column was used as the stationary phase.
The HPLC system (Agilent Infinity 1260, Agilent Technologies Inc., USA) has four gradient pumps incorporated with a solvent degasser, injector, column oven and a diode array detector. An Agilent ZORBAX Eclipse plus C18 100 mm × 4.6 mm, 3.5 μm column was used as the stationary phase.
Standard preparation
Pure ibuprofen powder (160 mg) was weighed into a 100 mL volumetric flask containing about 70 mL of HPLC grade methanol and sonicated for 20 min. The resultant solution was allowed to settle and made up to volume. A 5 mL aliquot of the solution was diluted to 100 mL to get a concentration of 80 μg/mL. The standard solution was run on the HPLC. Six injections were run for the standard to determine the system suitability and were calibrated to see the correlations and relative standard deviation [15].
Pure ibuprofen powder (160 mg) was weighed into a 100 mL volumetric flask containing about 70 mL of HPLC grade methanol and sonicated for 20 min. The resultant solution was allowed to settle and made up to volume. A 5 mL aliquot of the solution was diluted to 100 mL to get a concentration of 80 μg/mL. The standard solution was run on the HPLC. Six injections were run for the standard to determine the system suitability and were calibrated to see the correlations and relative standard deviation [15].
Sample preparation
Twenty tablets randomly selected from each brand were weighed and pulverized. The weight of powder equivalent to 400 mg ibuprofen was transferred into a 100 mL volumetric flask. About 70 mL of methanol was added and sonicated for 20 min. After dissolving, the solution was allowed to settle down and made up to volume. The solution was filtered through a 0.45 μm Sartorius nylon filter and 1 mL was taken and diluted to 50 mL with methanol in a volumetric flask to get a test solution of 80 μg/mL. The sample was analysed using HPLC. Two injections were run on each brand and the area of the ibuprofen peaks were quantified with the area of the peak of the ibuprofen standard to get the amount of the ibuprofen in percentage present in each brand. The mobile phase was also run with the test samples to serve as the blank [16]. Agilent Chem Station software was used to integrate and analyze HPLC peak responses for quantitation of the peaks by area percent.
Twenty tablets randomly selected from each brand were weighed and pulverized. The weight of powder equivalent to 400 mg ibuprofen was transferred into a 100 mL volumetric flask. About 70 mL of methanol was added and sonicated for 20 min. After dissolving, the solution was allowed to settle down and made up to volume. The solution was filtered through a 0.45 μm Sartorius nylon filter and 1 mL was taken and diluted to 50 mL with methanol in a volumetric flask to get a test solution of 80 μg/mL. The sample was analysed using HPLC. Two injections were run on each brand and the area of the ibuprofen peaks were quantified with the area of the peak of the ibuprofen standard to get the amount of the ibuprofen in percentage present in each brand. The mobile phase was also run with the test samples to serve as the blank [16]. Agilent Chem Station software was used to integrate and analyze HPLC peak responses for quantitation of the peaks by area percent.
Hardness Test
A tablet was placed vertically on the Monsanto Hardness tester. The load was then applied along the radial axis of the tablet. The weight or load required for breaking the tablet was noted down. Similarly it was done for 10 tablets.
A tablet was placed vertically on the Monsanto Hardness tester. The load was then applied along the radial axis of the tablet. The weight or load required for breaking the tablet was noted down. Similarly it was done for 10 tablets.
Friability
It was performed using Roche Friabilator, 10 tablets were weighed and placed in apparatus. The apparatus was rotated at a speed of 25 rpm. The apparatus was made to rotate for 4 min. The tablets were reweighed (W2) and compared with their initial weights and percentage friability was obtained. Percentage friability was calculated as: Percentage friability = [(W1–W2)/W1] x 100%.
It was performed using Roche Friabilator, 10 tablets were weighed and placed in apparatus. The apparatus was rotated at a speed of 25 rpm. The apparatus was made to rotate for 4 min. The tablets were reweighed (W2) and compared with their initial weights and percentage friability was obtained. Percentage friability was calculated as: Percentage friability = [(W1–W2)/W1] x 100%.
Disintegration Test
Six [6] tablets from each brand were employed for this test in a freshly prepared medium, 0.1N HCl at 37°C using the BP disintegration apparatus. The disintegration time was taken to be the time no particle remained on the basket of the system.
Six [6] tablets from each brand were employed for this test in a freshly prepared medium, 0.1N HCl at 37°C using the BP disintegration apparatus. The disintegration time was taken to be the time no particle remained on the basket of the system.
Dissolution Test
The dissolution test was carried out using USP apparatus II (paddle method) 5 in 6 replicates for each brand. The dissolution medium was 900 mL 0.1N HCL which was maintained at 37 ± 0.5°C. In all the experiments 5 mL of dissolution sample was withdrawn at 0, 10, 20, and 30 min and replaced with equal volume of dissolution medium to maintain sink condition. The sampling times were selected in due consideration for the short disintegration times. Samples were filtered and assayed by ultraviolet spectrophotometry at λmax of 221 nm. The concentration of each sample was determined from a predetermined calibration curve for Ibuprofen.
The dissolution test was carried out using USP apparatus II (paddle method) 5 in 6 replicates for each brand. The dissolution medium was 900 mL 0.1N HCL which was maintained at 37 ± 0.5°C. In all the experiments 5 mL of dissolution sample was withdrawn at 0, 10, 20, and 30 min and replaced with equal volume of dissolution medium to maintain sink condition. The sampling times were selected in due consideration for the short disintegration times. Samples were filtered and assayed by ultraviolet spectrophotometry at λmax of 221 nm. The concentration of each sample was determined from a predetermined calibration curve for Ibuprofen.
Data processing and analysis
After the completion of all test procedures data for all the individual tablets were recorded and separated on a different sheets according to the manufacturer. Finally data were analyzed by using the above mentioned mathematical formula and MS-Excel®, 2007.
After the completion of all test procedures data for all the individual tablets were recorded and separated on a different sheets according to the manufacturer. Finally data were analyzed by using the above mentioned mathematical formula and MS-Excel®, 2007.
Results
General aspect
The present study was conducted to assess the quality of Ibuprofen 400 mg tablets marketed in Yemen. To achieve this purpose, four different pharmaceutical companies (brands) A, B, C, D, and E were used (table 1). They were obtained from different retail pharmacies in Sana’a and then were subjected to a number of tests. A quality control study is very important to evaluate tablet properties. Different quality control parameters (e.g., weight variation, diameter, thickness, and content uniformity, friability, and dissolution tests) were performed to determine the differences among various conventional Ibuprofen tablets that are available in the Yemeni drug market.
The present study was conducted to assess the quality of Ibuprofen 400 mg tablets marketed in Yemen. To achieve this purpose, four different pharmaceutical companies (brands) A, B, C, D, and E were used (table 1). They were obtained from different retail pharmacies in Sana’a and then were subjected to a number of tests. A quality control study is very important to evaluate tablet properties. Different quality control parameters (e.g., weight variation, diameter, thickness, and content uniformity, friability, and dissolution tests) were performed to determine the differences among various conventional Ibuprofen tablets that are available in the Yemeni drug market.
Average Weight and Weight Variation
The weights of different brands of ibuprofen tablets were determined with the help of an electronic balance. Tablet weights should be controlled within a tight range. This will contribute to better tablet hardness and friability. The average weight and weight variation of the different brands of Ibuprofen tablets tested are shown in table 2 and Figure 2. It was found that the average weight of different five brands tablets of Ibuprofen tablets ranged from 557.7 mg ± 0.903 % (A brand) to 926 mg ± 4.44% (D brand) which indicates the use of different excipients with different weights. The weight uniformity test on the tablets indicated no significant differences in the weights of tablets from the different brands, hence conformed to the BP specification, i.e. that not more than two of the individual weights should deviate from the average weight by more than ± 5% [17] which proves that the five brands of Ibuprofen tablets those are available in the Trinidad pharmaceutical market passed the official weight variation test.
The weights of different brands of ibuprofen tablets were determined with the help of an electronic balance. Tablet weights should be controlled within a tight range. This will contribute to better tablet hardness and friability. The average weight and weight variation of the different brands of Ibuprofen tablets tested are shown in table 2 and Figure 2. It was found that the average weight of different five brands tablets of Ibuprofen tablets ranged from 557.7 mg ± 0.903 % (A brand) to 926 mg ± 4.44% (D brand) which indicates the use of different excipients with different weights. The weight uniformity test on the tablets indicated no significant differences in the weights of tablets from the different brands, hence conformed to the BP specification, i.e. that not more than two of the individual weights should deviate from the average weight by more than ± 5% [17] which proves that the five brands of Ibuprofen tablets those are available in the Trinidad pharmaceutical market passed the official weight variation test.
| Brands | Average weight (mg), % RSD |
Chemical content (%), % RSD |
| A | 557.7 ± 0.903 | 103.05 ± 1.86 |
| B | 635.9 ± 2.36 | 109 ± 4.64 |
| C | 683.55 ± 1.330 | 106.05 ±1.46 |
| D | 926 ± 4.44 | 99.95 ± 1.93 |
| E | 617.25 ± 0.6991 | 101.88 ± 0.926 |
Table 2: Average weight, % deviation from average weight, chemical content, and % deviation from chemical content of different brands of film coated Ibuprofen tablets.
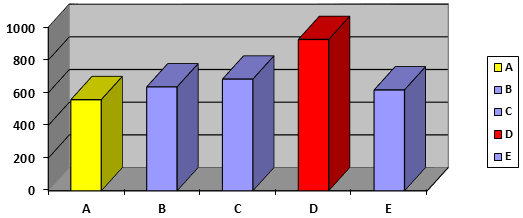
Figure 2: Comparison of different brands weight variation of different brands of film coated Ibuprofen tablets.
Chemical Content (Assay)
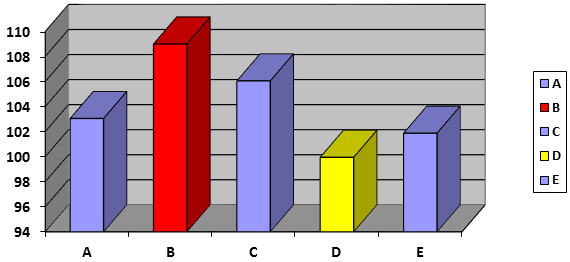
The results of the assay of chemical content using HPLC analysis to determine the amount of Ibuprofen. The average percent chemical content (assay) values of the different brands of Ibuprofen tablets tested shown in table 2 and figure 3. The results of the assay of chemical content of Ibuprofen tablets showed that the active content of all the brands were between 99.95 ± 1.93 % (D brand) and 109 ± 1.46 % (B brand) of the labeled amount specified for Ibuprofen tablets. According to USP – 30, content of ibuprofen tablets must be not lower than 90.0 % and not more than 110.0 % of the labeled amount of active drug [USP] [18]. The BP stipulates a 95%–105 % of active drug content [17]. The brands of A, D, and E ibuprofen tablets are within the limits recommended by the BP, and USP. While, the five Ibuprofen tablet brands (A, B, C, D, and E) are within the limits recommended by the USP. The assay results reveal the active drug content of five Ibuprofen tablet brands met the required standard.
The results of the assay of chemical content using HPLC analysis to determine the amount of Ibuprofen. The average percent chemical content (assay) values of the different brands of Ibuprofen tablets tested shown in table 2 and figure 3. The results of the assay of chemical content of Ibuprofen tablets showed that the active content of all the brands were between 99.95 ± 1.93 % (D brand) and 109 ± 1.46 % (B brand) of the labeled amount specified for Ibuprofen tablets. According to USP – 30, content of ibuprofen tablets must be not lower than 90.0 % and not more than 110.0 % of the labeled amount of active drug [USP] [18]. The BP stipulates a 95%–105 % of active drug content [17]. The brands of A, D, and E ibuprofen tablets are within the limits recommended by the BP, and USP. While, the five Ibuprofen tablet brands (A, B, C, D, and E) are within the limits recommended by the USP. The assay results reveal the active drug content of five Ibuprofen tablet brands met the required standard.
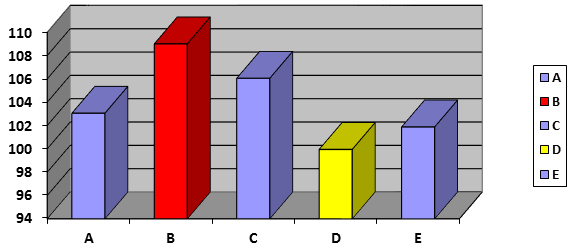
Figure 3: Comparison of assay content (Percent %) of different brands of film coated Ibuprofen tablets.
Hardness Test
Hardness of a tablet refers to the amount of pressure applied by a tablet press during tablet compression. Normally, the greater the pressure applied the harder the tablets, also the characteristics of granulation also have a role in tablet hardness. In general, tablets should be sufficiently hard to resist breaking during normal handling and yet soft enough to disintegrate properly after swallowing [19]. Tablet hardness of 4 kg F is considered to be the minimum for a satisfactory tablet [20]. Sufficient tablet hardness is essential to ensure resistance to damage during handling, packaging and transportation. The average values of hardness of the different brands of Ibuprofen tablets tested shown in table 3 and figure 4. Average hardness was found in the range of 6.73 ± 0.87 % kg/cm2 (D brand) to 17.52 ± 0.40 % kg/cm2 E (E brands). Although BP recommends a crushing strength of 5–8 kg, an overly hard tablet would lower disintegration time significantly and in turn dissolution [17]. The hardness of the tablets showed that all brands gave the highest crushing strength except the brand of D ibuprofen tablets is within the limits recommended by the BP. Also, hardness is referred to as non-compendia test.
Hardness of a tablet refers to the amount of pressure applied by a tablet press during tablet compression. Normally, the greater the pressure applied the harder the tablets, also the characteristics of granulation also have a role in tablet hardness. In general, tablets should be sufficiently hard to resist breaking during normal handling and yet soft enough to disintegrate properly after swallowing [19]. Tablet hardness of 4 kg F is considered to be the minimum for a satisfactory tablet [20]. Sufficient tablet hardness is essential to ensure resistance to damage during handling, packaging and transportation. The average values of hardness of the different brands of Ibuprofen tablets tested shown in table 3 and figure 4. Average hardness was found in the range of 6.73 ± 0.87 % kg/cm2 (D brand) to 17.52 ± 0.40 % kg/cm2 E (E brands). Although BP recommends a crushing strength of 5–8 kg, an overly hard tablet would lower disintegration time significantly and in turn dissolution [17]. The hardness of the tablets showed that all brands gave the highest crushing strength except the brand of D ibuprofen tablets is within the limits recommended by the BP. Also, hardness is referred to as non-compendia test.
| Brands | Hardness (kg/cm2) % RSD | Friability (%) | Disintegration time (min) | Dissolution (30 min), % RSD |
| A | 8.61 (± 1.265) | 0.1 | 2.00 | 119.82 ± 6.65 |
| B | 11.1 (± 0.74) | 0.03 | 4.05 | 89.5 ± 8.56 |
| C | 14.67 (± 0.45) | 0.1 | 3.20 | 90.72 ± 13.35 |
| D | 6.73 (± 0.87) | 0.02 | 16.15 | 89 ± 11.75 |
| E | 17.52 (± 0.40) | 0.06 | 2.40 | 99.3 ± 6.65 |
Table 3: Hardness (kg/cm2), % deviation from hardness, friability percent (%), disintegration time (min), dissolution (30 min), and % deviation from dissolution of different brands of film coated Ibuprofen tablets.
Friability Test
It is a measure of the resistance of the tablets to shipping and abrasion by tumbling them in a rotating drum. After tumbling, the integrity of the tablets and the weight loss are evaluated. According to BP specifications, the total weight loss should not exceed one percent and no tablet shows any type of breakage or crack [17]. The average values of friability of the different brands of Ibuprofen tablets tested shown in table 3 and figure 5. The average values of friability ranging from 0.02% (D brand) to 0.1% (A and C brands). The results of friability test are less than 1% which and as per the pharmacopoeia the limit is 1% of the initial weight of the tablets so all the brands of Ibuprofen passed to the friability test that means all these brands of ibuprofen tablets those are available in Trinidad pharmaceutical market are having good strength and can tolerate the shocks during transportation handling of these tablets.
It is a measure of the resistance of the tablets to shipping and abrasion by tumbling them in a rotating drum. After tumbling, the integrity of the tablets and the weight loss are evaluated. According to BP specifications, the total weight loss should not exceed one percent and no tablet shows any type of breakage or crack [17]. The average values of friability of the different brands of Ibuprofen tablets tested shown in table 3 and figure 5. The average values of friability ranging from 0.02% (D brand) to 0.1% (A and C brands). The results of friability test are less than 1% which and as per the pharmacopoeia the limit is 1% of the initial weight of the tablets so all the brands of Ibuprofen passed to the friability test that means all these brands of ibuprofen tablets those are available in Trinidad pharmaceutical market are having good strength and can tolerate the shocks during transportation handling of these tablets.
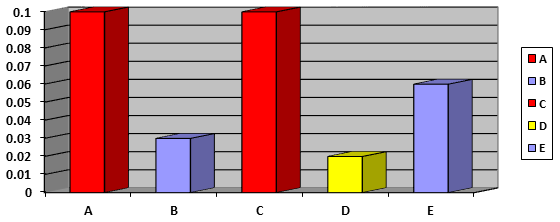
Figure 5: Comparison of friability percent (%) of different brands of film coated Ibuprofen tablets.
Disintegration Time
Tablet disintegration time is one of the very important physicochemical properties in solid dosage forms. The disintegration test measures the time required for tablets to disintegrate into particles. This is a necessary condition for dissolution and could be the rate-determining step in the process of drug absorption. The average values of disintegration of the different brands of film coated Ibuprofen tablets tested shown in table 3 and in figure 6. The results of disintegration time (min) of Ibuprofen tablets were ranging from 2.00 min (A brand) to 16.15 min (D brand).
Tablet disintegration time is one of the very important physicochemical properties in solid dosage forms. The disintegration test measures the time required for tablets to disintegrate into particles. This is a necessary condition for dissolution and could be the rate-determining step in the process of drug absorption. The average values of disintegration of the different brands of film coated Ibuprofen tablets tested shown in table 3 and in figure 6. The results of disintegration time (min) of Ibuprofen tablets were ranging from 2.00 min (A brand) to 16.15 min (D brand).
Disintegration is the break down process of tablet into smaller particles and is the first step towards dissolution, used to determine the disintegration time of the medication in the human body [21]. British Pharmacopeia specifies that uncoated tablets should disintegrate within 15 min and film coated tablet disintegrate within in 30 min while USP specification for disintegration is 30 min both for uncoated and film coated tablets. The results of disintegration test shows that the disintegration time of four brands (A, B, C, and E) of film coated Ibuprofen tablet is less than 10 minutes which is less than 30 minutes (standard disintegration time for coated tablet), means are disintegrate time very fast so the onset action will be fast. While, the disintegration time of D brand is about 16 min 15 sec which is less than 30 minutes. It proves that all these brands of film coated Ibuprofen tablet passes the pharmaceutical quality control limits as per the US pharmacopoeia [22].
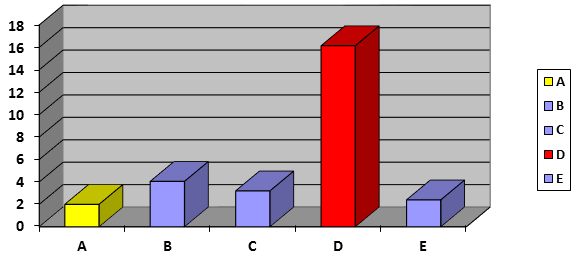
Figure 6: Comparison of disintegration time (min) of different brands of film coated Ibuprofen tablets.
Dissolution Test
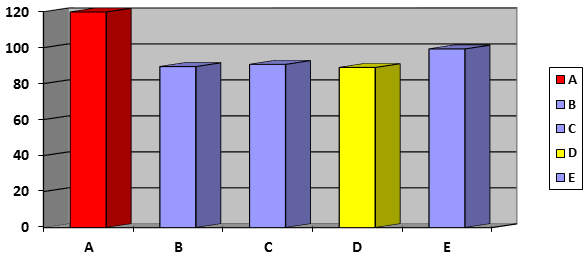
Dissolution is considered one of the most important quality control tests performed on pharmaceutical dosage forms and is now developing into a tool for predicting bioavailability, and in some cases, replacing clinical studies to determine bioequivalence. Dissolution behavior of drugs has a significant effect on their pharmacological activity. A dissolution test was intended to determine the percent release of the samples in 30 minutes time and the results show that all are in accordance to the pharmacopeias standards. The average values of dissolution of the different brands of Ibuprofen tablets tested shown in table 3 and graphically in figure 7. The obtained dissolution content at 30 minutes was found to be ranged from 89% ± 11.75% (D brand) to 119.82% ± 6.65% (A brand). The USP stated that not less than 80% of the labeled amount of ibuprofen should be released within 1h [22]. All brands of Ibuprofen tablets showed more than 80% of the labeled amount of ibuprofen was released after 1h. Thus, all brands of Ibuprofen tablets which are uncoated meet the USP specifications.
Dissolution is considered one of the most important quality control tests performed on pharmaceutical dosage forms and is now developing into a tool for predicting bioavailability, and in some cases, replacing clinical studies to determine bioequivalence. Dissolution behavior of drugs has a significant effect on their pharmacological activity. A dissolution test was intended to determine the percent release of the samples in 30 minutes time and the results show that all are in accordance to the pharmacopeias standards. The average values of dissolution of the different brands of Ibuprofen tablets tested shown in table 3 and graphically in figure 7. The obtained dissolution content at 30 minutes was found to be ranged from 89% ± 11.75% (D brand) to 119.82% ± 6.65% (A brand). The USP stated that not less than 80% of the labeled amount of ibuprofen should be released within 1h [22]. All brands of Ibuprofen tablets showed more than 80% of the labeled amount of ibuprofen was released after 1h. Thus, all brands of Ibuprofen tablets which are uncoated meet the USP specifications.
Discussion
In this study, five different brands of ibuprofen tablets (film coated) (400 mg) were obtained from different retail pharmacies in Sana’a and then were subjected to a number of tests. A quality control study is very important to evaluate tablet properties. Different quality control parameters (e.g., weight uniformity weight, thickness, and assay content, friability, and disintegration time and dissolution tests) were performed to determine the differences among various conventional ibuprofen tablets that are available in the Yemeni drug market.
The pharmacopeia compliance with regard to uniformity of weight of each brand studied is important since the uniformity of dosage unit can be demonstrated by either weight variation or content uniformity study [22]. These either reflect indirectly or measure directly the amount of drug substance in the tablet [23]. The uniformity of weight determination for five brands of ibuprofen tablets gave values that are within limits (table 2). There was different mean weight of all brands because of different excipient used in the different brands. The weight uniformity test for all the brands revealed values that complied with official specifications for weight uniformity as none of the brands deviated by up to ± 5% from the mean value (table 2 and 4) [24]. For assurance of uniform potency of tablets, weight variation is not sufficient. The potency of tablets is expressed in terms of gram, milligrams, or micrograms of drug per tablet and is given as the label strength of the product [25]. Although there was compliance of the weight uniformity test with each brand, the differences in tablet weight (a reflection of their sizes), among the brands may have some negative psychological effects on clinicians and their patients since they could raise some doubts on the general equivalence of the different brands of ibuprofen tablets (400 mg) available. The World Health Organization model formulary advises that a patient should be placed on a particular brand, probably due to pharmacokinetic and psychological reasons [26].
Every unit of tablet should contain the amount of drug substance equivalent to its label amount. For the evaluation of content, assay should be performed. The weight variation test is simplified and alternative to content uniformity test to assure therapeutic utility [27] and is an indicator of variations in the drug content [28]. The results of the assay of chemical content using HPLC analysis to determine the amount of ibuprofen present in each brand were presented in table 2 and 4. The content of Ibuprofen active ingredient in the samples ranged from 99.95% to 109%. The USP specifications for assay are that the drug content should be less than 90% and not more than 110 % [24]. Assay values of A, B, C, D, and E brands of ibuprofen tablets within the limits recommended by the USP. The assay results reveal the active drug content of five Ibuprofen tablet brands brand met the required standard. Therefore, the assay results ascertain the presence and compendia quality of the drug in A, B, C, D, and E products. The differences of the values may be due to different additives and manufacturing mechanism used in different factories [25].
The hardness of the tablets is an essential criterion in the determination of the ability of the tablets to resist chipping, abrasion, or breakage under conditions of storage, transportation, and handling [25]. Average hardness was found in the range of 6.73 ± 0.87% kg/cm2 (D brand) to 17.52 ± 0.40% kg/cm2 E (E brands).
Tablet hardness of 4 kgF is considered to be the minimum for a satisfactory tablet [29]. Sufficient tablet hardness is essential to ensure resistance to damage during handling, packaging and transportation. Although BP recommends a crushing strength of 5–8 kg, an overly hard tablet would lower disintegration time significantly and in turn dissolution [17]. The hardness of the tablets (table 3 and 4) showed that all brands gave the highest crushing strength except the brand of D ibuprofen tablets is within the limits recommended by the BP. However, a minimum hardness of 4 kg is essential and 6.0 kg or more will produce tablets of high compact nature [30]. This related to one or combined factors effect on hardness. All ibuprofen brands have a hardness within the acceptable range and, therefore, comply with the specification of USP.
A difference in tablet hardness reflects difference in tablet density and porosity. In which turn are supposed to result in different release pattern of the drug by affecting the rate of penetration of dissolution fluid at the surface of the tablet and formation of gel barrier [31].
The tablet hardness is usually assessed as an in-process-control parameter during tablet manufacture, because it is one of the most relevant tablet properties, characterizing the compatibility of tableting materials and the mechanical tablet strength to withstand potential stresses during tableting, packaging, shipping and dispensing [32].
Another measure of tablet strength, its friability is often measured because tablet hardness is not an absolute parameter of strength since some tablets tend to cap on attrition, losing their crown portions when compressed into very hard tablets [25]. The Pharmacopoeia (USP 30, NF 25) states that the friability value of tablets should be less than 1% and as such all the brands of ibuprofen had passed this friability specification 0.02–0.1% (table 3 and 4). All uncoated brands had mean of the percentage loss in weight of less than 1.0% and no tablet cracked, split or broken in the course of the test. So all brands showed compliance with BP 2005 specification [33]. From the Friability and Hardness tests and analysis of quality, it can be opined that there are considerable variations in the product formulations of both the companies because of the combination of other drugs and excipients [34].
Disintegration time is the rate determining step in drug absorption. The type and amount of excipients used by different manufacturers may influence disintegration and consequently the bioavailability of the drug. The disintegration times of the different brands could not be predicted from their tablet crushing strength values. The results of disintegration time (min) of Ibuprofen tablets were ranging from 2.00 min (A brand) to 16.15 min (D brand) (table 3 and 4). All the brands were complied with the USP specifications.
The satisfactory disintegration time exhibited by all the brands maybe indicative of good bioavailability as the tablets will be broken down immediately in the gastrointestinal tract presenting increase surface area for dissolution and absorption of the drug. The disintegration time could not be predicted from the tablet hardness values as different manufacturers adopt different formulation techniques and excipients to manipulate the disintegration and release properties of their tablets [35].
Tablets with increased hardness values tend to have increasing disintegration time.From this study, that there is no relationship between disintegration time and hardness. In fact, the relationship between tablet hardness and disintegration is a complex one where drug particle size, difference in excipients used and the formulation process followed by different manufacturers could impart different characteristics to the tablet in its solid or hydrated solution form [36].
Dissolution of drug from oral solid dosage form is a necessary criterion for drug bioavailability (i.e., the drug must be solubilized in the aqueous environment of gastrointestinal tract to be absorbed). For this reason, dissolution testing of solid oral drug products has emerged assuring product uniformity. The results of dissolution tests in terms of dissolution efficiency and time to dissolve 50% [25].
Dissolution behavior of drugs has a significant effect on their pharmacological activity. In fact, a direct relationship between in vitro dissolution rate of many drugs and their bioavailability has been demonstrated and is generally referred to as in vitro-in vivo correlation. Solid dosage forms may or may not disintegrate when they interact with gastrointestinal fluid following oral administration depending on their design [19].
From results of dissolution test was observed that all brands meet pharmacopoeia specification of dissolution test. The results revealed that all brands exhibit good release of the drug to the site of absorption and may have good bioavailability.
As there are many factors affecting on the dissolution taken as a whole, this gives each product certain dissolution characteristics which varies from brand to another. So it is not surprising to observe variation in vitro dissolution among three brands of ibuprofen tablets (B, C, and D brands). While, the value of dissolution of a brand revealed a high variation in vitro dissolution.
The formulation of the product can have a significant effect on the rate of disintegration and dissolution and may be influenced by the physicochemical properties of the active ingredients and excipients as well as the manufacturing process [37].
The type and amount of excipients used in tablet formulation as well as the manufacturing process are all known to affect both the disintegration and dissolution parameters [38].
The formulation of the drug product can have a significant effect on the quality parameters such as weight variation, hardness, friability, disintegration time, dissolution profile etc. This also includes the physiochemical properties of the active ingredients and excipients as well as the procedures used in the manufacturing process [39,40].
From the results of disintegration and dissolution tests (table 4) show that increase in disintegration significantly increased drug release rate. Fu., et al. reported that, Orally Disintegrating Tablets (ODT) was designed to have a rapid disintegration and dissolution, so that tablets generally have high porosity to ensure rapid absorption of water into the tablet [41].
Although all uncoated brands of Ibuprofen tablet have very high hardness, they still exhibited very good quality control parameters such as dissolution profile, disintegration time and chemical content determination. This indicates that hardness test is not a critical quality control parameter [42].
Therefore, quality of all the brands of ibuprofen tablets was confirmed regarding their weight uniformity, content of active ingredient, hardness, friability, disintegration time, dissolution rate, and were complied with the USP specifications,
| Brands | A | B | C | D | E |
| Average weight (mg) | 557.7 | 635.9 | 683.55 | 926 | 617.25 |
| Assay | 103.05 | 109 | 106.05 | 99.95 | 101.9 |
| Friability (%) | 0.1 | 0.03 | 0.1 | 0.02 | 0.06 |
| Hardens | 8.61 | 11.11 | 14.67 | 6.73 | 17.52 |
| Disintegration(sec) | 2.00 | 4.05 | 3.20 | 16.15 | 2.40 |
| Dissolution | 119.82 | 89.5 | 90.72 | 89 | 99.3 |
Table 4: Compression of quality control test between different brands of film coated Ibuprofen tablets.
Conclusion
According to the present study, it was clearly demonstrated that all five brands of the ibuprofen tablets (film coated) comply with USP specifications for in vitro quality control tests of uniformity of weight, uniformity of content, friability, disintegration time, and dissolution except hardens test for four brands (A, B, C, and E) is highest. The USP and BP specification of maximum hardens value of 10 kg/cm2, where the lower value of hardens is 6.73 kg/cm2 and the higher value is 17.52 kg/cm2. But Hardness is referred to as non-compendia test.
Bioequivalence studies are recommended for more investigation. Also such studies may be more important in developing countries where counterfeit and substandard drugs have become a major challenge to health care services.
Conflict of interest
The authors declare that they have no competing interests
The authors declare that they have no competing interests
References
- Manjusha. et al. “Comparative in Vitro Evaluation of Conventional Ibuprofen Marketed Formulation”. Journal of PharmaSciTech 2.2(2013): 75-80.
- Rainsford K. “Ibuprofen: from invention to an OTC therapeutic mainstay”. International Journal of Clinical Practice 67.178(2013): 9-20.
- Rivera-Leyva J., et al. “Comparative studies on the dissolution profiles of oral ibuprofen suspension and commercial tablets using biopharmaceutical classification system criteria”. Indian Journal of Pharmaceutical Sciences 74.19 (2012): 312.
- Avdeef A., et al. “pH-Metric log P 11. pKa determination of water-insoluble drugs in organic solvent-water mixtures”. Journal of Pharmaceutical and Biomedical Analysis 20.1(1999): 631-641.
- Herzfeldt C., et al. “Dissociation constants, solubility’s and dissolution rates of some selected nonsteroidal anti-inflammatories”. Drug Development and Industrial Pharmacy 9.1(1983): 767-93.
- Benjamin JO., et al. “The strucyclooxynenase-2”. Journal of Structural Biology 189.1 (2015): 62-66.
- “Indian Pharmacopoeia, I.P. Commission, Ghaziabad”. (2010): 1331-1479.
- “British Pharmacopoeia, Her Majesty's Stationary Office, London”. (2009): 767-791.
- “European Pharmacopoeia, Council of Europe (EDQM), France” 2119.
- “United States Pharmacopoeia, U.S.P.Convention, and XXIV Rockville” (2009): 1865.
- YOGITA B., et al. “Development and Validation of Spectrophotometric Methods for The Estimation of Ibuprofen and Famotidine”. International Journal of Pharmacy and Pharmaceutical Sciences 5(201 3): 358-363.
- Stuart AV., et al. “Comparing the dissolution profiles of seven metformin formulations in simulated intestinal fluid”. Dissolution Technologies 22.1 (2015): 17-22.
- “Pharmacopeia U. United States Pharmacopeia and National Formulary (USP 37–NF 32)”. Chapter (2014).
- Bhosale AV., et al. “Formulation and in-vitro evaluation of microbially triggered ibuprofen delivery for colon targeting”. International Journal of PharmTech Research 1(2009): 328-333.
- Allison Crouter., et al. “The Effect of Moisture on the Flow ability of Pharmaceutical Excipients”. Journal of the American Association of Pharmaceutical Scientists 15.1 (2011): 65-74.
- Umapathi P., et al. “Quantitative determination of metformin hydrochloride in tablet formulation containing croscarmellose sodium as disintegrant by HPLC and UV spectrophotometry”. Tropical Journal of Pharmaceutical Research 11.1 (2012): 107-16.
- “British Pharmacopoeia Commission. British pharmacopoeia Vol. III. London: The Stationery Office Limited”. (2009): 6578-85.
- “The United States Pharmacopeia and National Formulary U.S. Pharmacopeia National Formulary USP 30 NF 25, the United States Pharmacopeia, Inc”. (2007): 276, 2327.
- Neha Mathur., et al. “Evaluation of Quality Control Parameters on Various Brands of Paracetamol Tablet Formulation”. World Journal of Pharmacy and Pharmaceutical Sciences 4.7 (2015): 976-984.
- Rudnic ED., et al. “Oral solid dosage forms. In: Alfonso RG, ed. Remington: The Science and Practice of Pharmacy, 20th edn”. Lippincot Williams and Wilkins Inc., Philadelphia (2000): 858-893.
- Cardot JM., et al. “In Vitro-In Vivo Correlation: Importance of Dissolution in IVIVC”. Dissolution Technologies, February 14.2 (2007): 15-19.
- “U. S. Pharmacopeial Convention. U.S. Pharmacopeia National Formulary: USP 34 NF 29 (United States Pharmacopeia/National Formulary)”. Rockville: United States Pharmacopeia. (2011).
- Alderborn G. “Tablets and compaction. In: Aulton ME, ed. Pharmaceutics: The Science of Dosage Form Design”. Longman Group UK” (2002) 397-448.
- Samar A., et al. “Comparative Evaluation of the Pharmaceutical and Chemical Equivalence of Some Commercial Brands of Acetaminophen Tablets”. Life Science Journal 10.3 (2013): 2385-2391.
- Tapan Kumar Giri., et al. “Comparative in Vitro Evaluation of Conventional Ibuprofen Marketed Formulation”. Journal of PharmaScienceTechnology 2.2 (2013):75-80
- Couper MR., et al. “WHO model formulary. Geneva: World Health Organization”. (2002): 236.
- Lachman L., et al. “The Theory and Practice of Industrial Pharmacy; Third Edition”. (1986): 293-345.
- Aulton M. “Pharmaceutics: The Science of Dosage Form Design”. International Student Edition. (2002): 347-668.
- Rudnic ED., et al. “Oral solid dosage forms. In: Alfonso RG, ed. Remington: The Science and Practice of Pharmacy, 20th edition. Lippincott Williams and Wilkins Inc., Philadelphia”. (2000): 858-893.
- Gupta AK. “Introduction to pharmaceutics-I, 2nd ed.; India: CBS Publishers & Distributors”. (1999): 126-130.
- Shanmugam, S., et al. “Formulation and Invitro Evaluation of Sustained Release Matrix Tablets of Ibuprofen”. Research Journal of Pharmaceutical, Biological and Chemical Sciences 4.2 (2013): 1656-1664.
- Wu, C.-Y., et al. “A simple predictive model for the tensile strength of binary tablets”. European Journal of Pharmaceutical Sciences 25 (2005): 331-336.
- “British Pharmacopoeia” The Stationery Office, London (2005).
- Anand Kishore K. “A Comparative Study on Quality Evaluation and Analysis of Tablets”. Asian-American Journal of Chemistry 1.1(2013): 22-24.
- Sylvester Okhuelegbe Eraga., et al. “A comparative UV−HPLC analysis of ten brands of ibuprofen tablets”. Asian Pacific Journal of Tropical Biomedicine 5.10 (2015): 880-884.
- Liya Teklu., et al. “QUALITY EVALUATION OF PARACETAMOL TABLETS OBTAINED FROM THE COMMON SHOPS (KIOSKS) IN ADDIS ABABA, ETHIOPIA”. International Journal of Pharmaceutical Sciences and Research 5.8 (2014): 3502-3510.
- Race I. “Drug Formulation”, John Wiley, New York (1988):337.
- Mu’az, J., et al. “Comparative in vitro evaluation of the pharmaceutical and chemical equivalence of multi-source generic ciprofloxacin hydrochloride tablets around maiduguri metropolitan area”. Nigerian Journal of Pharmaceutical Sciences 8.2 (2009): 102-106.
- Ofori-Kwakye, K., et al. “Formulation and quality evaluation of two conventional release tablet formulations”. International Journal of Pharmaceutical Sciences Review and Research 4.1 (2010): 94-99.
- Kalakuntla, R., et al. “Effect of various super disintegrants on hardness, disintegration and dissolution of drug from dosage form”. Journal of Advanced Scientific Research 1.1 (2010): 15-19.
- Fu Y., et al. “Orally Fast Disintegrating Tablets: Developments, Technologies, Taste-Making and Clinical Studies, Critical Reviews™ in Therapeutic Drug Carrier Systems”. 21.6(2004): 433-475.
- Adegbolagun OA., et al. “Comparative evaluation of the biopharmaceutical and chemical equivalence of some commercially available brands of ciprofloxacin hydrochloride tablets”. Tropical Journal of Pharmaceutical Research 6.3 (2007): 737-745.
Citation:
Abdulmajed Alsaifi., et al. “Comparative Evaluation Quality of Different Brands of Ibuprofen 400 mg Tablets available in Yemeni’s
Market”. Chronicles of Pharmaceutical Science 2.6 (2018): 724-736.
Copyright: © 2018 Abdulmajed Alsaifi., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.