Research Article
Volume 3 Issue 2 - 2019
The Inhibitory Effect of Euphorbia Hirta Extracts against Some Wound Bacteria Isolated From Yemeni Patients
1Biology Department, Faculty of Science, Sana'a University, Yemen
2Pharmacology Department, Faculty of Pharmacy, Aden University, Yemen
2Pharmacology Department, Faculty of Pharmacy, Aden University, Yemen
*Corresponding Author: Wadhah Hassan Ali Edrees, Biology Department, Faculty of Science, Sana'a University, Yemen.
Received: December 09, 2018; Published: January 23, 2019
Abstract
The search for novel natural products from medicinal plants against bacterial strains resistant to multidrug is promising and urgent need to overcome the resistance complication. This study was carried out to investigate the antibacterial activities of Euphorbia hirta extracts against some bacteria isolated from surgical wounds of the hospitals located at Aden City, Yemen. The methanolic and aqueous extracts of the leaves and stems of Euphorbia hirta were determined for their antimicrobial activity by agar well diffusion method against Escherichia coli, Pseudomonas aeruginosa, Proteus mirabilis, and Staphylococcus aureus. The phytochemical screening was conducted to determine the plant constituents. The results showed that the extracts have a significant inhibitory effect on most bacterial species, especially P. mirabilis bacteria. Also, the E. hirta methanolic extracts were more efficient than aqueous extracts in the inhibition of tested bacteria at a concentration of 150 μL. However, the results revealed that the ciprofloxacin antibiotic had a higher inhibitory effect than other antibiotics used. Also, both of methanolic and aqueous extracts of E. hirta were showed more effect than antibiotics on most bacterial species, especially when concentration at 150 μL. The phytochemical screening revealed the presence of reducing sugars, terpenoids, alkaloids, steroids, tannins, oils, saponin, cardiac glycosides, anthroquinones, and flavanoids. The present findings showed that E. hirta possesses interesting inhibitory properties against bacteria associated with surgical wounds infection.
Keywords: Euphorbia hirta; Phytochemical; Aqueous and Methanolic Extracts; Antimicrobial Activity
Introduction
Wounds, resulting from microbial infection, are the most common public health problems that are responsible between 70 to 80% of mortality in the world [1,2]. The common wound pathogens includes bacteria, fungi, protozoa and viruses among which the most common are Streptococcus pyogens, Staphylococcus aureus, Pseudomonas aeuruginosa, Proteus, Escherichia coli and Enterococcus [3,4]. These microorganisms are present in hospital and community acquired infections, decreasing antibiotic therapy options especially Staphylococcus aureus and Pseudomonas aeruginosa. Due to increasing of resistance against several antimicrobial drugs, searching for new therapeutic alternatives using medicinal plants play a significant role for obtaining new drugs [5,6]. Medicinal plants are part and parcel of human society to combat diseases, from the dawn of civilization. They usually contain many biological active ingredients and are used primarily for treating mild or chronic ailments. According to world health organization (WHO), about 80% of the world population relies chiefly on the plant based traditional medicine especially for their primary healthcare needs [7]. Therapeutical properties of medical plants are very useful in healing various diseases owing to their attributes having wide biological and medicinal activities [8]. Herbal molecules are safe and would overcome the resistance produced by the pathogens as they exist in a combined form or in a pooled form of more than one molecule in the protoplasm of the plant cell [9,10]. The antimicrobial compounds found in plants may prevent bacterial infections by different mechanisms than the commercial antibiotics and therefore may have clinical value in treating resistant microorganism strains [11]. In this study, it was investigated the antibacterial activities of the E. hirta aqueous and methanolic extracts against four bacteria isolated from surgical wounds of Yemeni patients.
Materials and Methods
Collection of plant material
The fresh plant Euphorbia hirta that used in this study was collected from a different area in Dhi Al-Sufal located in Ibb City, Yemen. The taxonomical identification of the plant was confirmed by Dr. AbdulNaser Al-Gifri of the Biology Department, Faculty of Science, and Aden University, Yemen.
The fresh plant Euphorbia hirta that used in this study was collected from a different area in Dhi Al-Sufal located in Ibb City, Yemen. The taxonomical identification of the plant was confirmed by Dr. AbdulNaser Al-Gifri of the Biology Department, Faculty of Science, and Aden University, Yemen.
Preparation of plant extracts
Aqueous extract
A weighed quantity of 40 g of plant powder (in each of leaves and steams) was extracted in 400 mL of sterile water and soaked for two days (48h). After that, the mixture was filtered through double layered of clean muslin cloth and then Whatman No. 1 filter paper. The filtrate was then evaporated to dryness using an oven at 45ºC to remove the excess solvent. Subsequently, the solution was filter sterilized with 0.45 µm of mixed cellulose ester membranes (Millipore USA). The extracts were kept in a sterile bottle under refrigerated condition (4ºC) until needed for use [12]. 1g of extract was dissolved in 10 mL of sterile water and this gave 100 mg/mL. Thereafter three serial dilutions were made from the original stock of 10 mL (containing 100 mg/mL), according to the method described by Akujobi., et al. [13], using the solvents to obtain the following concentrations: 5 mg/mL (50 µL), 10 mg/mL (100 µL), and 15 mg/mL (150 µL) which were used for the antimicrobial sensitivity test.
Aqueous extract
A weighed quantity of 40 g of plant powder (in each of leaves and steams) was extracted in 400 mL of sterile water and soaked for two days (48h). After that, the mixture was filtered through double layered of clean muslin cloth and then Whatman No. 1 filter paper. The filtrate was then evaporated to dryness using an oven at 45ºC to remove the excess solvent. Subsequently, the solution was filter sterilized with 0.45 µm of mixed cellulose ester membranes (Millipore USA). The extracts were kept in a sterile bottle under refrigerated condition (4ºC) until needed for use [12]. 1g of extract was dissolved in 10 mL of sterile water and this gave 100 mg/mL. Thereafter three serial dilutions were made from the original stock of 10 mL (containing 100 mg/mL), according to the method described by Akujobi., et al. [13], using the solvents to obtain the following concentrations: 5 mg/mL (50 µL), 10 mg/mL (100 µL), and 15 mg/mL (150 µL) which were used for the antimicrobial sensitivity test.
Methanolic extract
The preparation of methanolic extraction followed the Naga., et al. [14], method. 40g of dried powder (in each of leaves and steams) plant was soaked into a flask containing 400 mL of methanol 96% and shaken for 6 hours. The mixture was filtered by Whatman No.1 filter paper and evaporated to dryness using a rotary evaporator at 45ºC and pressure 25 mm Hg [15]. 1g of extract was dissolved in 10 mL of Daimethylsulfoxide to gives 100 mg/mL and the solution was filter sterilized with 0.45 µm of mixed cellulose ester membranes (Millipore USA). After that, the prepared different concentrations (50, 100, 150 mg/mL) were prepared of the standard solution with a concentration of 100 mg/Ml. Then, the extracts were stored in the refrigerator in airtight containers until use [12].
The preparation of methanolic extraction followed the Naga., et al. [14], method. 40g of dried powder (in each of leaves and steams) plant was soaked into a flask containing 400 mL of methanol 96% and shaken for 6 hours. The mixture was filtered by Whatman No.1 filter paper and evaporated to dryness using a rotary evaporator at 45ºC and pressure 25 mm Hg [15]. 1g of extract was dissolved in 10 mL of Daimethylsulfoxide to gives 100 mg/mL and the solution was filter sterilized with 0.45 µm of mixed cellulose ester membranes (Millipore USA). After that, the prepared different concentrations (50, 100, 150 mg/mL) were prepared of the standard solution with a concentration of 100 mg/Ml. Then, the extracts were stored in the refrigerator in airtight containers until use [12].
Source of bacteria species
Four types of bacteria species used in this study are E. coli, P. aeruginosa, P. mirabilis, and S. aureus which have been isolated from the wounds of surgeries for patients from different hospitals located in Aden City, Yemen. The Isolated bacteria were subjected to standard microbiological identification tests based on morphological characteristics for the colony, and biochemical tests to confirm their identity/purity according to the criteria of Bergey’s Manual of Systematic Bacteriology, 2nd edition [16].
Four types of bacteria species used in this study are E. coli, P. aeruginosa, P. mirabilis, and S. aureus which have been isolated from the wounds of surgeries for patients from different hospitals located in Aden City, Yemen. The Isolated bacteria were subjected to standard microbiological identification tests based on morphological characteristics for the colony, and biochemical tests to confirm their identity/purity according to the criteria of Bergey’s Manual of Systematic Bacteriology, 2nd edition [16].
Antibiotics susceptibility testing of extracts
The method described by Emeruwa [17] was used. In briefly, 100 µL of a 20-hour culture of bacteria adjusted to 1.0×108 CFU/mL was spread using a sterile glass spreader onto a Mueller Hinton agar plate. The wells approximately 6 mm in diameter and 2.5 mm deep were bored on the surfaces of the agar medium using a sterile cork borer. Each concentration of extract was pipetted individually into one of the holes. The plates were allowed to stand for one hour for pre-diffusion of the extracts to occur before incubating overnight at 37ºC for 24h. The zones of inhibition that developed were measured in mm (millimeter) and the results were recorded [17].
The method described by Emeruwa [17] was used. In briefly, 100 µL of a 20-hour culture of bacteria adjusted to 1.0×108 CFU/mL was spread using a sterile glass spreader onto a Mueller Hinton agar plate. The wells approximately 6 mm in diameter and 2.5 mm deep were bored on the surfaces of the agar medium using a sterile cork borer. Each concentration of extract was pipetted individually into one of the holes. The plates were allowed to stand for one hour for pre-diffusion of the extracts to occur before incubating overnight at 37ºC for 24h. The zones of inhibition that developed were measured in mm (millimeter) and the results were recorded [17].
Phytochemical screening of the plant material
Phytochemical screening was carried out on the powdered plant material for the presence of bioactive components such as terpenoids, tannins, alkaloids, cardiac glycosides, carbohydrates, anthroquinones, steroids, oils, flavonoids, and saponins [17-19].
Phytochemical screening was carried out on the powdered plant material for the presence of bioactive components such as terpenoids, tannins, alkaloids, cardiac glycosides, carbohydrates, anthroquinones, steroids, oils, flavonoids, and saponins [17-19].
Results
Antibacterial activity of plants extract
The present study showed that the extract of leaves and stems of E. hirta, both aqueous and methanol, observed the inhibitory effects on all bacterial species isolated. The results of the antibacterial screening of the different concentrations of the aqueous extract of leaves on the test isolates are shown in Table (1) and Figure (1).
The present study showed that the extract of leaves and stems of E. hirta, both aqueous and methanol, observed the inhibitory effects on all bacterial species isolated. The results of the antibacterial screening of the different concentrations of the aqueous extract of leaves on the test isolates are shown in Table (1) and Figure (1).
The aqueous extract of plant leaves was showed the minimum effect on growth of S. aureus and E. coli bacteria at 50 μL and 100 μL in two diameters inhibitory of 6.1 mm, while it was showed the maximum effect on the S. aureus and P. mirabilis at 150 μL in diameters inhibitory of 12 mm and 12.8 mm, respectively, (Table 1).
However, the aqueous extracts of the stems were showed the minimum effect on the S. aureus bacteria at 50 μL in inhibitory diameter of 7 mm and a maximum effect of P. mirabilis when at 150 μL in diameter of 20.3 mm (Table 1). The results showed that the increase in the concentration of aqueous extract increased the zone of growth inhibition of tested bacteria.
| Pathogen organisms | Aqueous extract | |||||
| Leaves | Stems | |||||
| 50µL (5mg) | 100µL (10mg) | 150µL (15mg) | 50µL (5mg) | 100µL (10mg) | 150µL (15mg) | |
| E. coli | 6.1 | 6.1 | 9.6 | 15,1 | 16.3 | 17.7 |
| P. mirabilis | 6 | 9.7 | 12.8 | 13.9 | 16.3 | 20.3 |
| P. aeruginosa | 6 | 6.3 | 9.9 | 7.3 | 9.7 | 13.1 |
| S. aureus | 6.1 | 9.1 | 12 | 7 | 9.4 | 13.3 |
Table 1: effect of aqueous extract of E. hirta on the isolated bacteria.
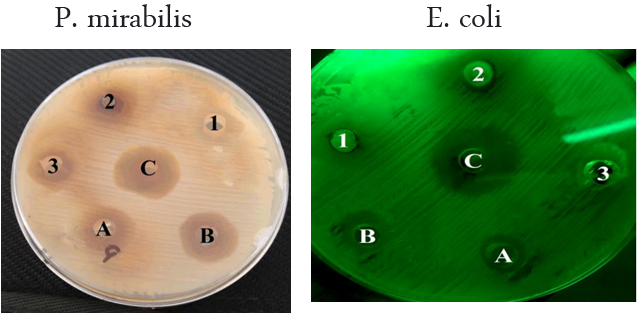
Concentration of extracts
Stems: (A)= 50 µL, (B)=100 µL, (C)=150 µL Leaves: (1)=50 µL, (2)=100 µL, (3)=150 µL
Figure 1: the effect of E. hirta methanolic aqueous on isolated bacteria.
Stems: (A)= 50 µL, (B)=100 µL, (C)=150 µL Leaves: (1)=50 µL, (2)=100 µL, (3)=150 µL
Figure 1: the effect of E. hirta methanolic aqueous on isolated bacteria.
In the methanolic extract of leaves, it was showed the minimum effect on the S. aureus bacteria when at 50 μL with an inhibitory diameter of 7.1 mm and the maximum effect of P. mirabilis bacteria at 150 μL was recorded with an inhibitory diameter of 13.9 mm as shown in Table (2)and Figure. (2).
While, in the methanolic extract of the stems was showed the minimum inhibitory effect on growth of P. aeruginos and S. aureus bacteria at 50 μL in inhibitory diameters of 7.3 mm and 7.9 mm, respectively, and the maximum effect of the P. mirabilis at 150 μL in inhibitory diameter of 21.1 mm (Table 2).
| Pathogen organisms | Methanolic extract | |||||
| Leaves | Stems | |||||
| 50µL (5mg) | 100µL (10mg) | 150µL (15mg) | 50µL (5mg) | 100µL (10mg) | 150µL (15mg) | |
| E. coli | 6 | 7.3 | 9.7 | 14.6 | 16.1 | 19.7 |
| P. mirabilis | 6.3 | 9.3 | 13.9 | 10.4 | 16.7 | 21.1 |
| P. aeruginosa | 6.2 | 7.1 | 10.3 | 7.3 | 9.6 | 10.9 |
| S. aureus | 7.1 | 13 | 13 | 7.9 | 10.6 | 13.7 |
Table 2: Effect methanolic extract of E. hirta on the isolated bacteria.
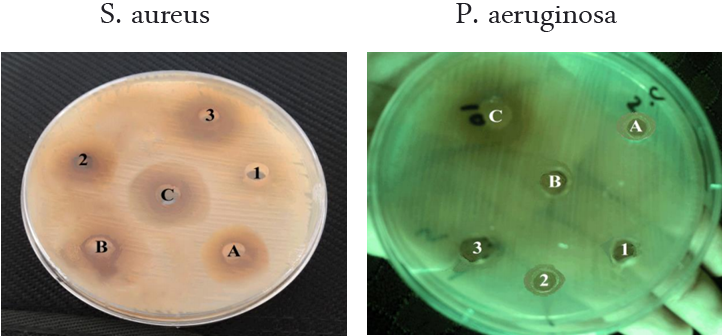
Concentration of extracts
Stems: (A)= 50 µL, (B)=100 µL, (C)=150 µL Leaves: (1)=50 µL, (2)=100 µL, (3)=150 µL
Figure 2: The effect of E. hirta methanolic extract on isolated bacteria.
Stems: (A)= 50 µL, (B)=100 µL, (C)=150 µL Leaves: (1)=50 µL, (2)=100 µL, (3)=150 µL
Figure 2: The effect of E. hirta methanolic extract on isolated bacteria.
Phytochemical constituents
It was observed that the methanol extract contained all the active compounds excepting the anthraqunones and saponins, while the anthroquinones, oil, and saponins were not detected in the aqueous extract as showed in the table (3).
It was observed that the methanol extract contained all the active compounds excepting the anthraqunones and saponins, while the anthroquinones, oil, and saponins were not detected in the aqueous extract as showed in the table (3).
| Chemical compounds | Name of the test | Aqueous extract | Methanolic extract | ||
| Leaves | Stems | Leaves | Stems | ||
| ALKaloids | Hager's | + | + | + | + |
| Anthroquinones | Chloroform layer | - | - | - | - |
| Cardiac glycoside | Killer-Killani's | + | + | + | + |
| Flavonoids | Ammonia (modified) | + | + | + | + |
| Oils | Solubility | - | - | + | + |
| Reducing sugar | Fehling's | + | + | + | + |
| Saponins | Frothing | - | - | - | - |
| Steroids | Salkowski | + | + | + | + |
| Tanins | FeCl3 | + | + | + | + |
| Terpenoids | Salkowski (modified) | + | + | + | + |
- = Compound not detected; + = compound detected
Table 3: Phytochemical screening of E. hirta extract.
Table 3: Phytochemical screening of E. hirta extract.
Discussion
The results obtained showed that all the bacteria tested were susceptible to both methanolic and aqueous extracts at different concentrations of E. hirta. It was observed that the methanolic extracts are more effective than aqueous extracts. Also, the steam extracts more effective than the leaf extracts. These findings are consistent with results of Ogueke., et al. [20] who indicated that the E. hirta extract inhibited the growth of S. aureus, E. coli and P. aeruginosa in varying degrees. Also, the study by Nazeer, [21] showed that the fresh latex extract of E. hirta was inhibited the growth of E. coli and P. aeriginosa, while the S. aureus is resistant. S. aureus produces the enzyme penicillinase which converts the antibiotic penicillin to penicillinic acid which is no longer inhibitory to its growth [22]. Other workers have also shown that extracts of E. hirta inhibit the growth of various microorganisms at different concentrations [13, 23-25]. The current study raveled that the phytochemical screening of extract was found to contain the tannins, flavonoids, alkaloids and cardiac glycosides and free of saponins. These results are in accordance obtained from the extraction of E. hirta with different solvents by many workers [20,24]. Some workers have also identified tannins, flavonoids, and alkaloids in the extracts of the plant [23, 26-27].
Plants are rich in a wide variety of secondary metabolites such as alkaloids, tannins, terpenoids, and flavonoids having been found in vitro since they have antimicrobial properties and may serve as an alternative, effective, cheep, and safe antimicrobial for the treatment of microbial infections [28]. The observed antibacterial properties corroborate its use in traditional medicine. Traditionally extracts of the plant are used in sore and wound healing, as ear drop for boils in the ear and treatment of boils. They are also used in the control of diarrhea and dysentery [29,30].
Conclusions
The aqueous and ethanol extracts of E. hirta show promise and can form a primary platform as an alternative therapy in the control of wounds pathogens. Further phytochemical and Pharmacological studies will be required to isolate the active constituents and estimate the antimicrobial activities against a wide range of bacterial pathogens.
References
- Gottrup, F., et al. ''An overview of surgical site infections: An etiology, incidence and risk factors”. European Wound Management Association Journal 5.2 (2005):11-15.
- Odimegwu DC., et al. “Wound healing and antibacterial activities of the extract of Dissotis theifoila (Melastomataceae) stem formulated in a simple ointment base”. Journal of Medicinal Plants Research 2.1(2008): 11-16.
- Khan SS and Chaghtai SA. “Ethnobotanical study of some plants used for curing skin afflictions”. Ancient Science of Life 1.4 (1982): 236-238.
- Mulu A., et al. “Pattern and multiple drug resistance of bacterial pathogens isolated from wound infection at University of Gondar teaching hospital, Northwest Ethiopia”. The Ethiopian Medical Journal 44.2 (2006): 125-131.
- Reynolds R. “Antimicrobial resistance in the UK and Ireland”. Journal of Antimicrobial Chemotherapy 64.1 (2009): 19-23.
- Anuradha SD., et al. “Prevalence of metallo-β-Lactamase Producing Pseudomonas aeruginosa and Acinetobacter species in a tertiary care hospital”. Indian Journal of Critical Care Medicine 14.4 (2010): 217-219.
- Divya N., et al. “Antibacterial activity of medicinal plant against wound infected pathogens”. International Journal of Pharmaceutical Sciences and Research 5. 11 (2014): 4942-4947.
- Kalemba D and Kunicka A. “Antibacterial and antifungal properties of essential oils”. Current Medicinal Chemistry, Current Medicinal Chemistry 10.10 (2003): 813-829.
- Lai PK and Roy J. “Antimicrobial and chemopreventive properties of herbs and spices”. Current Medicinal Chemistry 11.11 (2004): 1451-1460.
- Tapsell LC., et al. “Health benefits of herbs and spices; the past, the present, the future”. The Medical journal of Australia 185.4 (2006): 4-24.
- Eloff JN. “Antibacterial assays of plant extracts by agar diffusion assay may give misleading results”. International Society of Ethnopharmacology, Pretoria. South African Journal of Botany 20. 69 (2003): 229-235.
- Abu-Shanab BA., et al. “In vitro activity of certain drugs in Combination with plant extracts against Staphylococcus aureus infections”. Pakistan Journal of Medical Sciences 24. 4 (2008): 541-544.
- Akujobi C., et al. “Antibacterial activities and preliminary phytochemical screening of four medicinal plants”. Journal of Applied Sciences 7.3 (2004): 4328 – 4338.
- Naga et al. “Preliminary phytochemical screening and antibacterial studies of the flowers of Antigonon leptopus”. Annals of Biological Research 1.4 (2010): 229-233.
- Parekh J and Chanda S. “In vitro antimicrobial activity of Trapa natans L. fruit rind extracted in different solvents”.The African Journal of Biotechnology 6.6 (2007):1905-1909.
- Don J., et al. Bergey’s manual of systematic bacteriology. 2nd edition. Michigan State University, East Lansing, USA. 2(B,C). (2004).
- Emeruwa KC. “Antimicrobial substances from Cacaya papaya fruit extracts”. Journal of Natural Products 45.2 (1982): 123–127.
- Trease GE and Evans WC. Pharmacognosy. 11th edition. Braillar Tiriden Company, Macmillan publishers. (1996): 56-109.
- Edeoga HO., et al. “Phytochemical constituents of some Nigerian medicinal plants”. The African Journal of Biotechnology 4.7 (2005): 685-688.
- Ogueke C., et al. “Antibacterial activities and toxicological potentials of crude ethanolic extracts of Euphorbia hirta”. Journal of American Science 3.3 (2007): 11-16.
- Nazeer BS. “Antimicrobial activity of E. hirta L”. Indian Journal of Research 6.1 (2017): 1-2.
- Singleton P. Bacteria in Biology, Biotechnology and Medicine. 4th edition. John Wiley and Sons Ltd, New York. (1999).
- Abubakar EM. “Antibacterial activity of crude extracts of Euphorbia hirta against some bacteria associated with enteric infections”. Journal of Medicinal Plants Research 3.7 (2009): 498-505.
- Singh G and Kumar P. “Phytochemical study and screening for antimicrobial activity of flavonoids of Euphorbia hirta”. International Journal of Applied Basic Medical Research 3.2 (2013):111–116.
- Nikunj BP and Kaushik CP. “Antibacterial activity of Euphorbia hirta L. ethanomedicinal plant against gram negative UTI pathogens”. International Journal of Pharmaceutical Research and Allied Sciences 3.2 (2014): 24-29.
- Abo KA. “Isolation of ingenol from the lattices of Euphorbia and Elaeophorbia species”. Fitoterapia 61.5 (1990): 462–463.
- Yoshida T., et al. “Euphorbin E, a hydrolysable tannin dimmer of highly oxidized structure from Euphorbia hirta”. Chemical and Pharmaceutical Bulletin 38.4 (1990): 1113–1115.
- Cowan MM. “Plant products as antimicrobial agents”. Clinical Microbiology Reviews 12.4 (1999): 564–582.
- Kokwaro JO. “Current status of utilization and conservation of medicinal plants in Africa south of the Sahara”. Acta Horticulture 332 (1993):121-129.
- Igoli JO., et al. “Traditional medicine practice amongst the Igede people of Nigeria”. Part II.African Journal of Traditional, Complementary and Alternative Medicines 2. 2 (2005): 134–152.
Citation:
Wadhah Hassan Ali Edrees., et al. “The Inhibitory Effect of Euphorbia Hirta Extracts against Some Wound Bacteria Isolated From Yemeni Patients”.
Chronicles of Pharmaceutical Science 3.1 (2018): 780-786.
Copyright: © 2018 Meda Nagesh., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.