Research Article
Volume 4 Issue 1 - 2019
Constitution and Assessment of Nabumetone Loaded Buccal Films
1Adarsh Vijendra Institute of Pharmaceutical Sciences, Shobhit University, Gangoh, Saharanpur-247341, Uttar Pradesh, India
2Division of Pharmacy, SGRRITS, Dehradun-248001 Uttarakhand, India
3Baddi University, Baddi, Solan (Hp), India
2Division of Pharmacy, SGRRITS, Dehradun-248001 Uttarakhand, India
3Baddi University, Baddi, Solan (Hp), India
*Corresponding Author: Pranshu Tangri, Division of pharmaceutical sciences, Shri Guru Ram Rai University, Dehradun, Uttarakhand, India.
Received: April 19, 2019; Published: July 10, 2019
Abstract
The current research work aims at the formulation and evaluation of nabumetone loaded buccal films followed by its quality control. Nabumetone is a nonsteroidal anti-inflammatory drug (NSAID) of the arylalkanoic acid family. Ten bathces of buccal atrips were prepared using polymers like HPMC, Eudragit, sodium alginate, and sodium CMC in varying proportions. A total amount of upto 4% of the polymers was used. All the formulations were subjected for quality control parameters like folding endurance, content uniformity, swelling index, surface pH, mucoadhesive strength studies, in-vitro permeation, ex-vivo permeation and stability studies. The prepared formulations showed zero order controlled release following super case II transport mechanism suggesting controlled release by swelling and erosion mechanism. The formulations showed optimum results and showed good control over dug release along with correlation between in-vitro and ex-vivo studies.
Keywords: Nabumetone; Buccal films; Bioadhesionve
Introduction
The buccal region of the oral cavity is an attractive target for administration of the drug of choice, particularly in overcoming deficiencies associated with the latter mode of administration. Problems such as high first-pass metabolism and drug degradation in the gastrointestinal environment can be circumvented by administering the drug via the buccal route. Moreover, rapid onset of action can be achieved relative to the oral route and the formulation can be removed if therapy is required to be discontinued. It is also possible to administer drugs to patients who unconscious and less co-operative. To prevent accidental swallowing of drugs adhesive mucosal dosage forms were suggested for oral delivery, which included adhesive tablets, adhesive gels, adhesive patches and many other dosage forms with various combinations of polymers, absorption enhancers (Ahuja., et al. 2000). Natural polymers have recently gained importance in pharmaceutical field.
Advantages (De Vries., et al. 1992)
- It is richly vascularised and additional reachable for administration and removal of formulations.
- Patient accessibility is high.
- Retentive dosage forms are suitable for administration.
- Improves bioavailability by eliminating first pass metabolism.
- Surface of buccal mucosa achieves a fast cellular recovery.
- Low enzyme activity.
The objective for selecting nabumetone and formulating its buccal films is that it belongs to BCS class II and has low solubility which causes low absorption and low oral bioavailability of 35%, hence to increase the oral bioavailability and absorption characeteristics buccal films of nabumetone have been formulated. (Abdul., et al. 2004) (Ahmed., et al. 2002)
Materials and Methods
Materials
Nabumetone was obtained as a gift sample from APS Biotech, Roorkee, Uttarakhand. All other chemicals and reagents used were of analytical grade and quality.
Nabumetone was obtained as a gift sample from APS Biotech, Roorkee, Uttarakhand. All other chemicals and reagents used were of analytical grade and quality.
Methods
a) Development of buccal strips
Mucoadhesive buccal patches of nabumetone were prepared by solvent casting method. Various polymers were used based on their mucoadhesion strength and residence time. Trial batches were prepared using these polymers alone and in combination in concentration ranging from 0.4% to 6%. Based on the results of trail batches a high concentration of 3.5% and a low concentration of 1.5% was optimized for buccal patches. Each formulation contains four % polymer concentration which was optimized based on the results of tensile strength, mucoadhesion residence time of trial batches. (Disabato., et al. 1996) Nabumetone buccal patches contain a mixture of HPMC-K4M/Eudragit RS 100 and Sodium Alginate/Sodium CMC
a) Development of buccal strips
Mucoadhesive buccal patches of nabumetone were prepared by solvent casting method. Various polymers were used based on their mucoadhesion strength and residence time. Trial batches were prepared using these polymers alone and in combination in concentration ranging from 0.4% to 6%. Based on the results of trail batches a high concentration of 3.5% and a low concentration of 1.5% was optimized for buccal patches. Each formulation contains four % polymer concentration which was optimized based on the results of tensile strength, mucoadhesion residence time of trial batches. (Disabato., et al. 1996) Nabumetone buccal patches contain a mixture of HPMC-K4M/Eudragit RS 100 and Sodium Alginate/Sodium CMC
Procedure
The nabumetone loaded strips for buccal drug delivery were prepared by solvent casting method using polymers different ratios. The polymeric solutions were prepared in double distilled water with constant stirring. The polymeric solutions were filtered through a nylon gauze to remove debris and suspended particles. The resultant solution was left overnight at room temperature to ensure a clear, bubble-free solution. The solution was poured into a glass petri dish having 8 cm diameter. 50mg/sqcm equivalent of drug was added to each film. The backing membrane of ethyl cellulose was prepared for each of the films. Dried films were carefully removed, checked for any imperfections or air bubbles and cut into patches of 1sqcm in diameter by using fabricated punch. (Dhaneshwar., et al. 2010) (Lavelle., et al. 2001)
The nabumetone loaded strips for buccal drug delivery were prepared by solvent casting method using polymers different ratios. The polymeric solutions were prepared in double distilled water with constant stirring. The polymeric solutions were filtered through a nylon gauze to remove debris and suspended particles. The resultant solution was left overnight at room temperature to ensure a clear, bubble-free solution. The solution was poured into a glass petri dish having 8 cm diameter. 50mg/sqcm equivalent of drug was added to each film. The backing membrane of ethyl cellulose was prepared for each of the films. Dried films were carefully removed, checked for any imperfections or air bubbles and cut into patches of 1sqcm in diameter by using fabricated punch. (Dhaneshwar., et al. 2010) (Lavelle., et al. 2001)
| Ingredients | NP1 | NP2 | NP3 | NP4 | NP5 | NP6 | NP7 | NP8 | NP9 | NP10 |
| Diacerein (mg) | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 | 50 |
| HPMC | 3 % | 2.5% | 2% | 1.5% | 1% | - | - | - | - | - |
| Sodium CMC | 1% | 1.5% | 2% | 2.5% | 3% | - | - | - | - | - |
| Eudragit RS 100 | - | - | - | - | - | 3 % | 2.5% | 2% | 1.5% | 1% |
| Sodium Alginate | - | - | - | - | - | 1% | 1.5% | 2% | 2.5% | 3% |
| Glycerine | 2% | 2% | 2% | 2% | 2% | - | - | - | - | - |
| Propylene Glycol | - | - | - | - | - | 2% | 2% | 2% | 2% | 2% |
| PVP K 100 (mg) | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 | 10 |
| Solvent | q.s. | q.s | q.s | q.s | q.s | q.s | q.s | q.s | q.s | q.s |
Table 1: Formulations Chart.
b) Evaluation of buccal patches
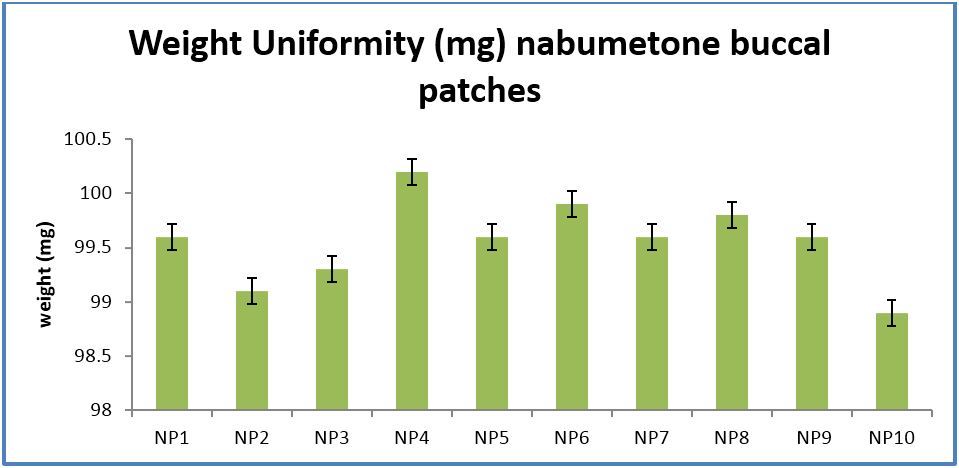
The prepared buccal strips were evaluated for parameters like Physical appearance, Thickness, Weight variation, Flatness, Folding endurance, Moisture uptake, Moisture content, Swelling study, Drug content determination. In-vitro permeation and Ex-vivo permeation using franz diffusion cell and stability study of optimized formulations. (Roldo., et al. 2004) (Rowe., et al. 2004) (Remunan., et al. 1988)
The prepared buccal strips were evaluated for parameters like Physical appearance, Thickness, Weight variation, Flatness, Folding endurance, Moisture uptake, Moisture content, Swelling study, Drug content determination. In-vitro permeation and Ex-vivo permeation using franz diffusion cell and stability study of optimized formulations. (Roldo., et al. 2004) (Rowe., et al. 2004) (Remunan., et al. 1988)
Results and Discussion
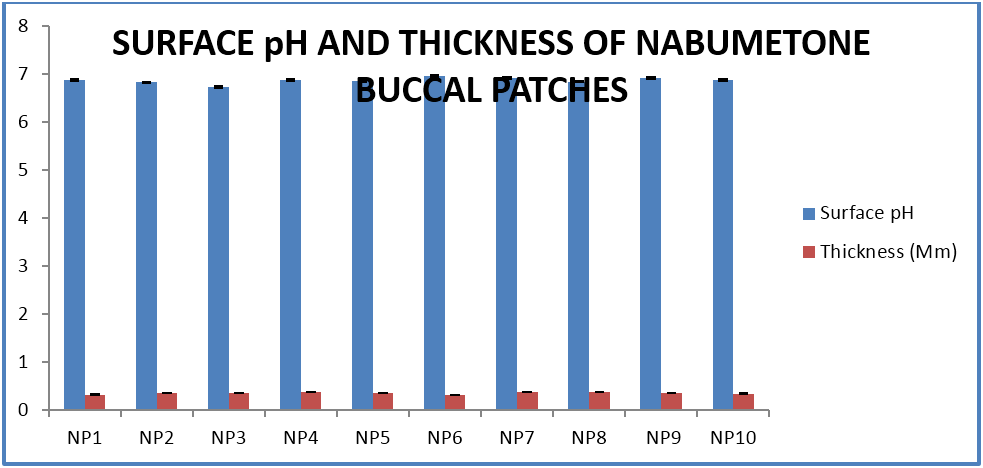
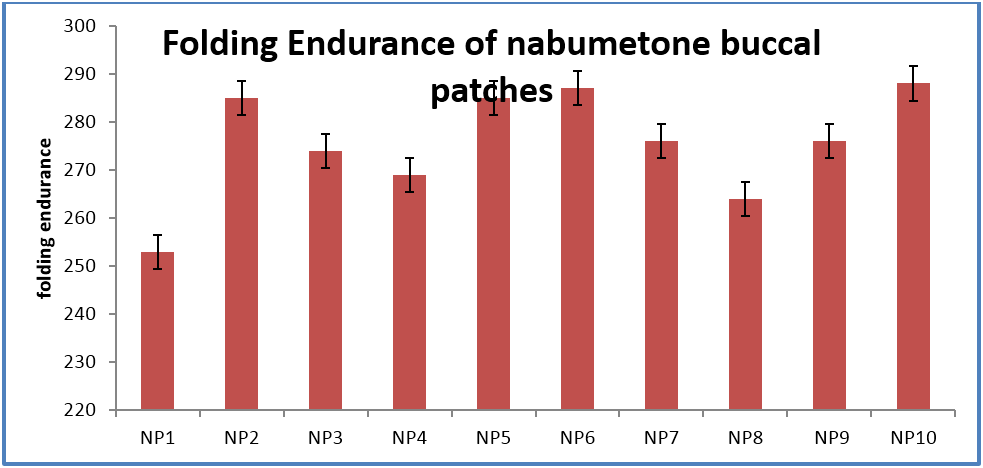
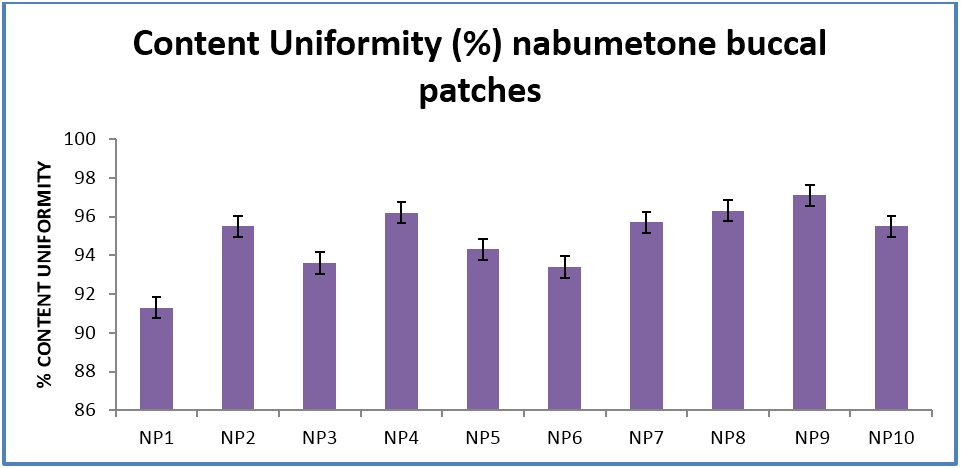
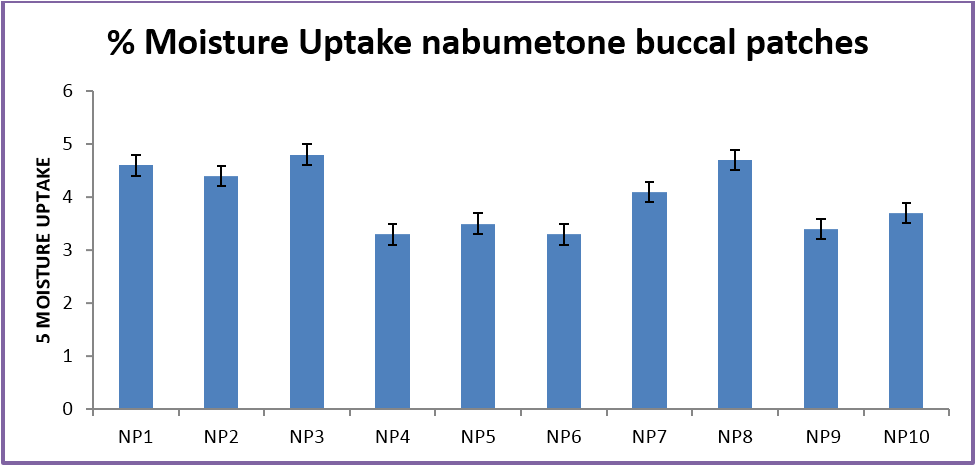
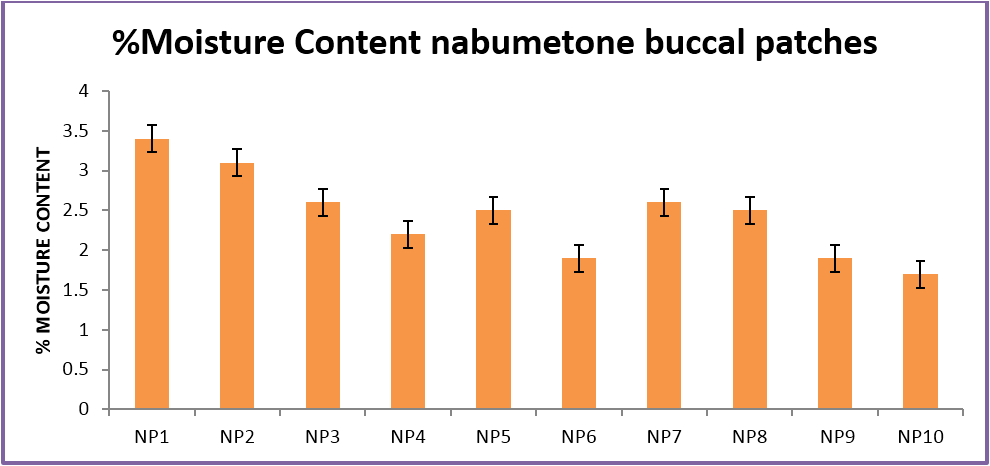
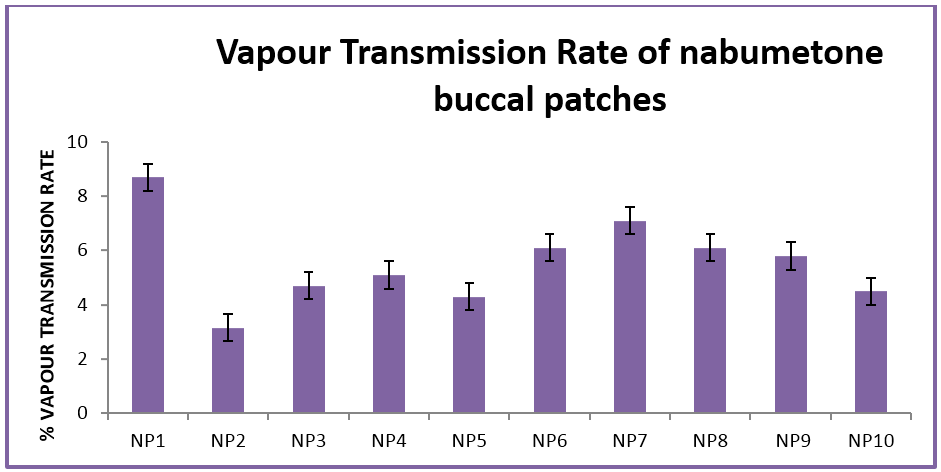
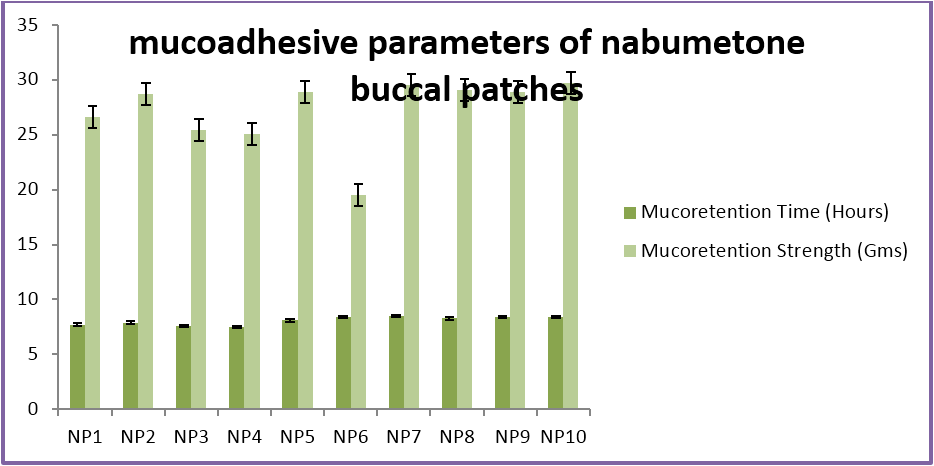
The buccal patches were evaluated for parameters like pH, thickness, folding endurance, content uniformity, % moisture content, % moisture uptake, Vapour transmission rate, % swelling , bioadhesive strentgth and bioadhesive retention time. The formulations showed good and satisfactory results of all parameters which were within the ranges of the accepatance criteria.
Nabumetone buccal patches showed thickness of 0.33-0.38 mm, pH 6.73-6.91, folding endurance 253-288, content uniformity of 91.3-97.1%, moisture content of 1.9%-3.4 %, moisture uptake of 3.3-4.8%, vapour transmission of 3.1-8.7%, retention time of 7.5 - 8.5 hrs and bioadhesive strength of 25.1-29.5 gm.
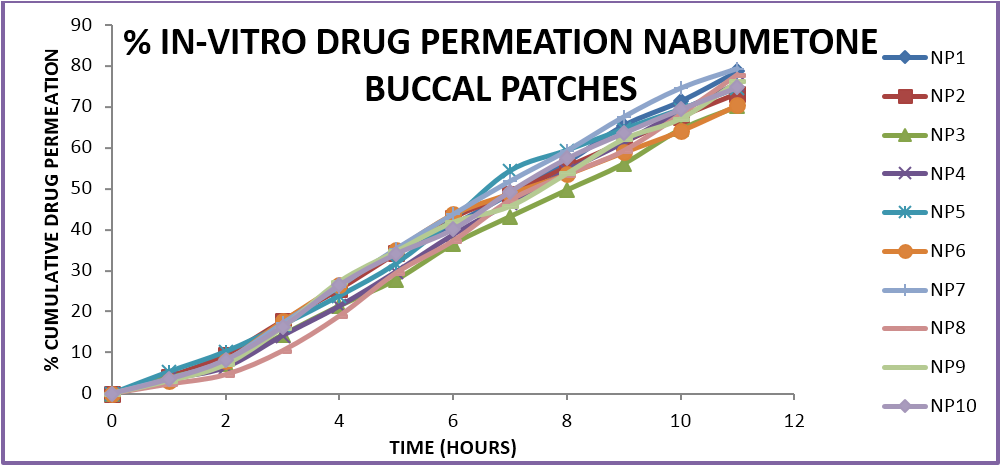
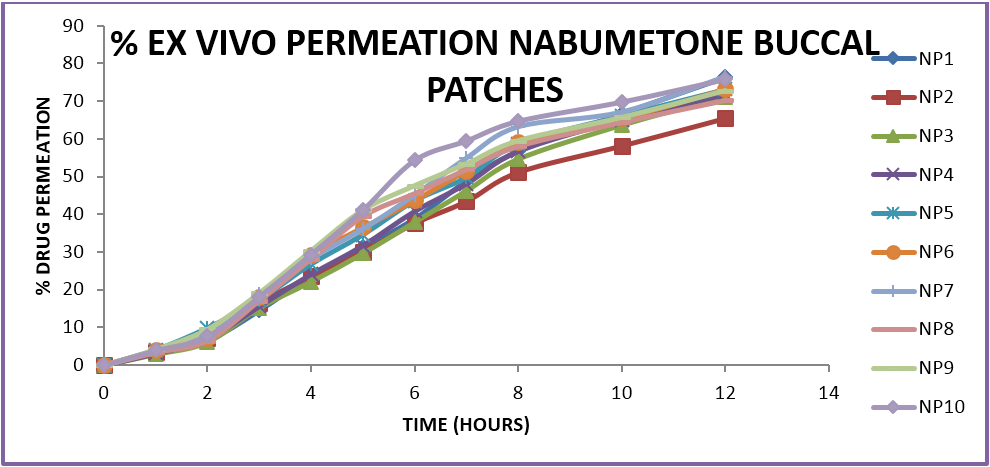
The prepared formulations were evaluated for in-vitro drug release and ex-vivo permeation studies using goat/sheep mucosa in a Franz diffusion cell. The results were analysed and the percent drug released, percent drug permeation was determined. The mechanism of drug release and the best fit models of drug release. The formulations show variation in drug release profiles from 60%-80% within a time period of 7-11 hours. The variation is due to the difference in the amounts of polymers and the various combinations used. The optimum formulations showed a controlled zero order release profile in which the mechanism of release is dependant on the erosion and dissolution of the polymeric matrix. The formulations containing HPMC in combination with carbopol and sodium alginate show a better controlled release as compared to other formulations. In case of buccal patches the formulations with a ratio of 1:3 of two polymers showed optimum release profiles. The formulations do not show more than 80% release in case of ex-vivo permeation which may be due to the variation in permeation across a biological membrane as compared to the in-vitro release characteristics and further in case of sustained or controlled release formulations release of less than 80% in 12 hrs is acceptable unlike in conventional dosage forms where a minimum 80% release is required witjin 12 hrs.
| Parameter | NP1 | NP2 | NP3 | NP4 | NP5 | NP6 | NP7 | NP8 | NP9 | NP10 |
| Surface pH | 6.87 ± .2 | 6.82 ± 0.1 | 6.73 ± 0.3 | 6.87 ± 0.4 | 6.85 ± 0.2 | 6.95 ± 0.2 | 6.91 ± 0.3 | 6.84 ± 0.1 | 6.91 ± 0.2 | 6.87 ± 0.2 |
| Thickness (Mm) | 0.33 ± .003 | 0.36 ± .002 | 0.36 ± .003 | 0.38 ± .001 | 0.36 ± 0.01 | 0.32 ± 0.004 | 0.38 ± 0.004 | 0.38 ± 0.003 | 0.36 ± 0.004 | 0.35 ± 0.005 |
| Folding Endurance | 253 ± 3 | 285 ± 4 | 274 ± 5 | 269 ± 4 | 285 ± 2 | 287 ± 2 | 276 ± 3 | 264 ± 5 | 276 ± 6 | 288 ± 3 |
| Content Uniformity (%) | 91.3 ± 0.14 | 95.5 ± 0.33 | 93.6 ± 0.53 | 96.2 ± 0.07 | 94.3 ± 0.02 | 93.4 ± 0.1 | 95.7 ± 0.44 | 96.3 ± 0.22 | 97.1 ± 0.32 | 95.5 ± 0.31 |
| % Moisture Uptake | 4.6 ± .2 | 4.4 ± .2 | 4.8 ± .4 | 3.3 ± .1 | 3.5 ± .4 | 3.3 ± 0.1 | 4.1 ± .1 | 4.7 ± .1 | 3.4 ± .3 | 3.7 ± .2 |
| %Moisture Content | 3.4 ± .05 | 3.1 ± .03 | 2.6 ± .01 | 2.2 ± 0.15 | 2.5 ± .03 | 1.9 ± .05 | 2.6 ± .04 | 2.5 ± .06 | 1.9 ± .04 | 1.7 ± .02 |
| Mucoretention Time (Hours) | 7.7 ± .2 | 7.9 ± .1 | 7.6 ± .2 | 7.5 ± .1 | 8.1 ± .2 | 8.4 ± .2 | 8.5 ± .3 | 8.3 ± .2 | 8.4 ± .2 | 8.4 ± .2 |
| Mucoretention Strength (Gms) | 26.6 ± .12 | 28.72 ± .2 | 25.4 ± .22 | 25.1 ± .24 | 28.9 ± .25 | 29.5 ± .16 | 29.5 ± .17 | 29.1 ± .13 | 28.9 ± .15 | 29.7 ± .16 |
Table 2: Evaluation Parameters.
Conclusion
The shortlisted formulations were further analyzed for stability studies according to ICH guidelines under two set of conditions viz. (65% RH and 25˚C) and (75% RH with 40˚C). The studies were conducted in a humidity chamber. The results of the stability study indicated no significant changes in the physical appearance and also in the drug content at 5% level. The formulations were also analysed for in-vitro drug release where no significant change was seen after 90 days in the t50% and t80% values.
All the formulations were statistically analysed and suitable limits of standard deviation along with error bars have been applied. The research work can be very useful in the management of osteoarthritis and arthritis. These formulations can provide better patient compliance, higher bioavailability, lower frequency of dosage administration, decreased systemic adverse effects.
The research work was completed with satisfactory results in all the aspects and parameters of the dosage forms. The buccal mucosa offers several advantages for controlled drug delivery for extended periods of time. The mucosa is well supplied with both vascular and lymphatic drainage and first-pass metabolism in the liver and pre-systemic elimination in the gastrointestinal tract are avoided. The area is well suited for a retentive device and appears to be acceptable to the patient. With the right dosage form design and formulation, the permeability and the local environment of the mucosa can be controlled and manipulated in order to accommodate drug permeation.
References
- Ahuja A., et al. “Mucoadhesive drug delivery systems”. Drug Dev Ind Pharm 19 (2000): 143–194.
- Abdul S and Poddar SS. “A flexible technology for modified release of drugs: multi layered tablets”. J Control Release 97 (2004): 393–405.
- Ahmed A., et al. “Bioadhesive microdevices for oral drug delivery: a feasibility study”. Biomed Microdevices 3.2 (2002): 89–96.
- De Vries ME., et al. “Developments in buccal drug delivery”. Crit Rev Ther Drug Carrier Syst 8 (1992): 271–303.
- Desmukh GD., et al. “Critical review on buccal mucoadhesive drug delivery system”. Am J Pharm Tech Res 1 (2011): 66-80.
- Dhaneshwar K., et al. “A review on oral mucosal drug delivery system”. Int J Pharm Sci Res (2010): 50–56.
- Disabato-mordarski T and Kleinberg I. “Measurement and comparison of the residual saliva on various oral mucosal and dentition surfaces in humans”. Arch Oral Biol 41 (1996): 655–665.
- Lavelle EC. “Targeted delivery of drugs to the gastrointestinal tract”. Crit Rev Ther Drug Carrier Syst 18.4 (2001): 341–386.
- Remunan-Lopez C., et al. “Design and evaluation of chitosan/ethylcellulose mucoadhesive bilayered devices for buccal drug delivery”. J Control Release 55 (1988): 143–152.
- Roldo M., et al. “Mucoadhesive thiolated chitosans as platforms for oral controlled drug delivery: synthesis and in vitro evaluation”. Eur J Pharm Biopharm 57 (2004): 115–121.
- Rowe CW., et al. “Multimechanism oral dosage forms fabricated by three dimensional printing”. J Control Release 66.1 (2004): 11–17.
Citation:
Pranshu Tangri., et al. “Constitution and Assessment of Nabumetone Loaded Buccal Films”. Chronicles of Pharmaceutical Science 4.1 (2019): 22-29.
Copyright: © 2019 Pranshu Tangri., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.