Research Article
Volume 1 Issue 2 - 2018
Osteoporosis among Patients with Type 1 and 2 Diabetes Mellitus
1Department of Endocrinology, King Fahad Armed Forces Hospital, Jeddah, Kingdom of Saudi Arabia
2College of medicine, Um Al Qura University
2College of medicine, Um Al Qura University
*Corresponding Author: Khalid S Aljabri, Department of Endocrinology, King Fahad Armed Forces Hospital, Jeddah, Kingdom of Saudi Arabia, PO Box 9862. Jeddah 21159. Kingdom of Saudi Arabia.
Received: March 30, 2018; Published: May 10, 2018
Abstract
Background and Objective: The incidence of diabetes mellitus is increasing. Osteoporosis has become an alarming health problem throughout the entire world. This study was undertaken to estimate the frequency of osteoporosis among Saudi patients with type 1 and 2 diabetes mellitus.
Methods: Eligible patients were patients with type 1 diabetes mellitus (T1DM) and type 2 diabetes mellitus (T2DM). All patients were recruited from the population of the Diabetic Centre at King Fahad Armed Forces Hospital. Eligible patients met with investigators for a complete history and physical examination, to have baseline laboratory assessments including glycosylated hemglobin (HbA1c). Bone mineral density (BMD) in the current study was measured using the lumber spine, neck of femur and hip.
Main Results: Among 53 cases of individuals with diabetes mellitus who participated in this study, 27 cases (50.9%) were T1DM and 26 (49.1%) were T2DM. BMD showed that Osteoporosis was found in 26 cases (49.1%). 17 cases (65.4%) of T1DM has osteoporosis compared to 9 cases (34.6%) of T2DM, p = 0.0. Male were more frequent with osteoporosis as compared to more frequent osteopenia in female, (figure 2). The mean age difference between T1DM and T2DM was -8.2 (95% confidence interval (CI); -13.4, -3.1), p = 0.002. Mean HbA1c and diabetes duration differences between T1DM and T2DM were -1.1 (95% CI; -2.3, 0.008), p = 0.05 and 6.7 (95% CI; 1.6, 11.8), p = 0.01 subsequently. The mean lumbar spine BMD in the T1DM group was -1.9 ± 0. 9 g/cm2 and in T2DM group was -1. 5 ± 1.0 g/cm2 (P = 0.2). The mean femur neck BMD in T1DM group was -1.5 ± 1.0 g/cm2 and in T2DM group was -1.3 ± 0.7 g/cm2, the difference was not statistically significant (P = 0.4). The mean hip BMD in the T1DM group was -1.4 ± 1.0 g/cm2 and in T2DM group was -0.95 ± 0.6 g/cm2 (P = 0.08. There were no specific patterns of differences of BMD of the lumbar spine, femur neck and hip in between T2DM and T2DM cases according to age, HbA1c and duration of diabetes.
Conclusion: Our findings demonstrate high frequency of osteoporosis in patients with T1DM and T2DM. Although bone density testing is not routinely performed in patients with diabetes, these data suggest that screening may be important particularly in young women with type 1 diabetes. In addition, these women should be counseled regarding lifestyle interventions that may improve bone health, including adequate intake of calcium and vitamin D, and exercise.
Keywords: Osteoporosis; Diabetes Mellitus
Introduction
Decreased bone density and reduction in the bone strength are most common metabolic bone problems, which are usually diagnosed after bone fractures, especially in the lumbar spine and femoral neck [1]. Osteoporosis has become an alarming health problem throughout the entire world, and approximately 200 million people in the world are threatened by this deleterious disease [2,3]. Osteoporosis is often described as a silent disease because it is typically asymptomatic until a fracture occurs [4]. More than 50 years ago, it was shown that diabetes mellitus could be associated with a loss of bone mass leading to osteoporosis [5]. This finding has since received a great deal of attention and been investigated by a number of researchers [5-14]. As osteoporosis is a major health problem, its occurrence among patients who have diabetes further increases their burden of disease. However, in spite of numerous studies, the relationship between diabetes and osteoporosis remains controversial [15-17]. Several reports indicated that T1DM has a strong relationship with osteoporosis; whereas in the case of the relationship between type 2 diabetes and osteoporosis, there has been inconsistency in the studies [14, 18-21]. These observations suggest that adverse effects on bone health may occur early after the diabetes diagnosis. This study was undertaken to estimate the frequency of osteoporosis among Saudi patients with T1DM and T2DM, living in the western province, attending the diabetes centre at King Fahd Armed Forces Hospital, Jeddah, KSA.
Methods
Eligible patients were patients with T1DM and T2DM. All patients were recruited from the population of the Diabetic Centre at King Fahad Armed Forces Hospital. All the patients gave their or their guardian informed consent prior to their inclusion. Eligible patients met with investigators for a complete history and physical examination, to have baseline laboratory assessments including glycosylated hemglobin (HbA1c). HbA1c was expressed as percentage and measured using the high performance liquid chromatography. All measurements in BMD were measured T-score, converted to Z - scores using a data bank for age-matched speed of sound values supplied by the manufacturer. Osteopenia was defined as a Z-score of less than -1 (Z - score < - 1). T-scores at the lumbar spine, neck of femur or hip of < - 2.5 defining osteoporosis. Severe osteopenia was defined as Z - score −2.0 and lower (Z - score ≤ −2.0). Mild osteopenia was defined as a Z score greater than -2 and less than −1 (−2 < Z-score < − 1). Normal mineralization was a Z score of -1 and higher (Z - score ≥ − 1). We excluded patients with a history of fractures of less than 1 year and those with associated bone/joint problems, liver disease or patients on long-term steroid therapy.
Statistical Analysis
Univariate analysis of baseline and follow up demography and clinical laboratory endpoints were accomplished using unpaired t-test and one-way Anova with post-hoc between groups where appropriate. Chi square(X2) test were used for categorical data comparison. All statistical analyses were performed using SPSS Version 22.0. All P values were based on two-sided tests. The difference between groups was considered significant when P < 0.05.
Univariate analysis of baseline and follow up demography and clinical laboratory endpoints were accomplished using unpaired t-test and one-way Anova with post-hoc between groups where appropriate. Chi square(X2) test were used for categorical data comparison. All statistical analyses were performed using SPSS Version 22.0. All P values were based on two-sided tests. The difference between groups was considered significant when P < 0.05.
Results
Among 53 cases of individuals with diabetes who participated in this study, 27 cases (50.9%) were T1DM and 26 (49.1%) were T2DM, (table 1). The mean age difference between T1DM and T2DM was -8. 2 (95% confidence interval (CI); -13.4, -3.1), p = 0.002. Mean HbA1c and diabetes duration difference between T1DM and T2DM was -1.1 (95% CI; -2.3, 0.008), p =0.05 and 6.7 (95% CI; 1.6, 11.8), p = 0.01 subsequently.
| Parameters | Total | T1DM | T2DM | P value | |||
| Osteoporosis | Osteoporosis | ||||||
| Yes | No | Yes | No | ||||
| Number | 53 | 17 (63.0) | 10 (37.0) | 9 (34.6) | 17(65.4) | < 0.0001 | |
| Age (years) | 27.6 ± 8.2 | 22.7 ± 4.3 | 21.4 ± 3.3 | 30.9 ± 5.6 | 34.2±8.8 | < 0.0001 | |
| Gender | Male | 28 (52.8) | 9 (32.1) | 4 (14.3) | 5 (17.9) | 10(35.7) | 0.8 |
| Female | 25 (47.2) | 8 (32.0) | 6 (24.0) | 4 (16.0) | 7 (28.0) | ||
| Duration of diabetes (years) | 8.4 ± 6.2 | 11.4 ± .2£ | 8.7 ± 2.6 | 4.7 ± 4.4 | 7.6 ± 6.2 | 0.07 | |
| Body mass index (kg/m²) | 24.7 ± 4.8 | 22.8 ± 3.1 | 25.2 ± 2.9 | 24.8 ± 5.4 | 25.9 ± 3.4 | 0.1 | |
| Height (cm) | 163.2 ± 10.3 | 160.7 ± 1.3 | 159.2 ± 6.2 | 162.5 ± 8.6 | 168.1 ± 10.8 | 0.09 | |
| HbA1c | 8.3 ± 1.5 | 7.8 ± 0.9§ | 7.5 ± 1.3 | 8.9 ± 1.9 | 8.9 ± 1.4 | 0.02 | |
| 25-hydroxyvitamin D (nmol/l) | 35.9 ± 11.9 | 34.4 ± 10.7 | 31.6 ± 11.9 | 27.1 ± 5.4 | 42.5 ± 12.2 | 0.06 | |
| Total serum calcium (mmol/l) [2.15 - 2.55] | 2.3 ± 0.1 | 2.3 ± 0.1 | 2.3 ± 0.1 | 2.4 ± 0.1 | 2.4 ± 0.1 | 0.4 | |
| Serum albumin (g/l) [34 - 52] | 44.1 ± 3.5 | 43.6 ± 4.2 | 43.8 ± 4.0 | 44.3 ± 1.6 | 44.6 ± 3.3 | 0.5 | |
| Phosphate(mmol/L) [0.74 - 1.2] | 1.2 ± 0.3 | 1.2 ± 0.3 | 1.3 ± 0.2 | 1.3 ± 0.3 | 1.1 ± 0.3 | 0.7 | |
| Alkaline phosphotase (U/L) | 90.3 ± 54.6 | 114.7 ± 83.3 | 79.1 ± 13.7 | 76.2 ± 20.6 | 79.2 ± 37.0 | 0.2 | |
| Parathyroid hormome (pmol/L) [1.3 - 7.4] | 6.4 ± 6.8 | 9.3 ± 11.1 | 5.0 ± 1.4 | 5.6 ± 3.0 | 4.7 ± 1.9 | 0.2 | |
| Magnesium (mmol/L) [0.6 - 1.1] | 0.8 ± 0.1 | 0.8 ± 0.2 | 0.8 ± 0.2 | 0.9 ± 0.1 | 0.8 ± 0.1 | 0.2 | |
| 24-hours urine calcium | 2.8 ± 3.9 | 3.7 ± 6.9 | 1.6 ± 1.1 | 2.0 ± 0.8 | 2.9 ± 1.3 | 0.6 | |
Table 1: Characteristics of patients with type 1 diabetes mellitus stratified by presence of Osteoporosis
Data are number (%) and mean ± SD. £: P = 0.01. §: P = 0.05
Data are number (%) and mean ± SD. £: P = 0.01. §: P = 0.05
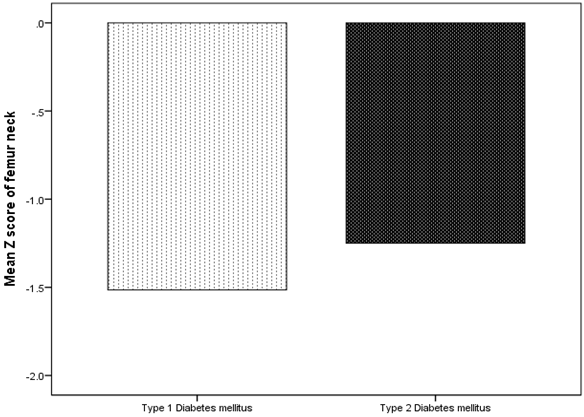
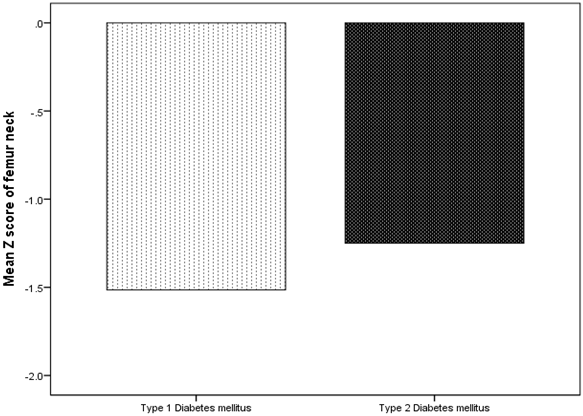
BMD showed that Osteoporosis was found in 26 cases (49.1%). 17 cases (65.4%) of T1DM has osteoporosis compared to 9 cases (34.6%) of T2DM, p = 0.01, (figure 1). Male were more frequent with osteoporosis as compared to more frequent osteopenia in female, (figure 2). The mean lumbar spine BMD in the T1DM group was -1.9 ± 0. 9 g/cm2 and in T2DM group was -1. 5 ± 1.0 g/cm2 (P = 0.2), (figure 3). The mean femur neck BMD in T1DM group was -1.5 ± 1.0 g/cm2 and in T2DM group was -1.3 ± 0.7 g/cm2, the difference was not statistically significant (P = 0.4) (figure 4). The mean hip BMD in the T1DM group was -1.4 ± 1.0 g/cm2 and in T2DM group was -0.95 ± 0.6 g/cm2 (P = 0.08) (figure 5). As shown in (figures 6, 7 and 8) there were no specific patterns of differences of BMD of the spine, femur neck and hip in between T1DM and T2DM cases according to age, HbA1c and duration of diabetes.
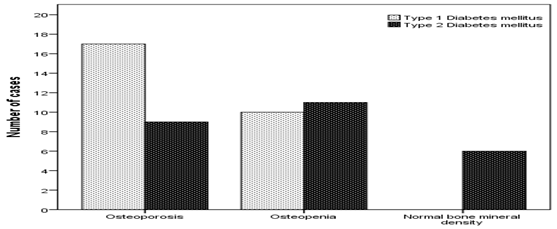
Figure 1: Number of cases with type 1 and type 2 diabetes
mellitus according to bone mineral density.
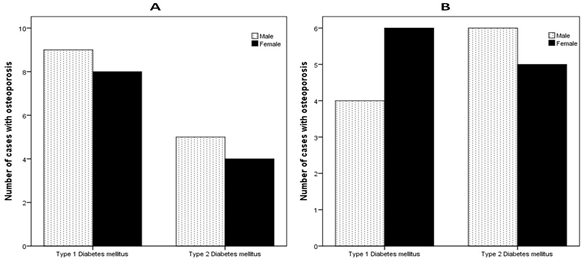
Figure 2: Number of cases with type 1 and type 2 diabetes mellitus
according to bone mineral density (A and B) stratified by gender.

Figure 6: Distribution of Spine, femur neck and hip bone mineral density
subgrouped according to type of diabetes and age in comparison.
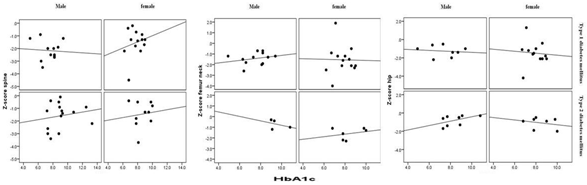
Figure 7: Distribution of Spine, femur neck and hip bone mineral density
subgrouped according to type of diabetes and HbA1c in comparison.

Figure 8: Distribution of Spine, femur neck and hip bone mineral density subgrouped
according to type of diabetes and diabetes duration in comparison.
Discussion
Diabetes is one of the most common chronic disorders worldwide and its many complications can severely affect quality of life. In addition, diabetes has many long term complications affecting almost all tissues. Bone involvement is one of the complications of diabetes. Several lines of evidence indicate that low bone mass at the hip, femoral neck and spine in both male and female patients with T1DM, may eventually lead to an increased risk of bone fracture. In contrast, in T2DM, investigations appear conflicting, and the exact mechanism of this is still unknown [22,24]. These conflicting results may be due to the different methods used to measure the bone density, the difference in duration, severity and treatment of diabetes. Our study shows that the frequency of low BMD was higher in T1DM than T2DM, although patients with T1DM were about 8 years younger than those with type 2 diabetes. In agreement with many studies, the present study has confirmed that osteoporosis is associated with T1DM [25,26]. However, it was unclear until recently whether this translated into increased fracture rates [26,27].
In contrast to our finding, a number of studies have shown that poor glycemic control in patients with T1DM is associated with osteopenia and osteoporosis [27,28]. However, Higher HbA1c was associated with osteopenia and osteoporosis in patients with T2DM. Poor glycemic control for long period of time can result into microvascular complications and can aggravate the loss of bone density by various mechanisms. Nephropathy can predispose patients with diabetes to hypercalciuria and can alter the vitamin D metabolism causing vitamin D deficiency [29-30]. Hyperglycemia may lead to an increased loss of calcium in the urine, which can in turn cause a negative calcium balance [31]. Furthermore, use of loop diuretics in patients with diabetic nephropathy is associated with low bone BMD as these drugs cause the renal excretion of calcium [32]. Insulin-like growth factors and other cytokines may influence diabetic bone metabolism [30]. Moreover, insulin resistance and hyperinsulinemia can result in high rate of bone mineral mass in type 2 diabetic patients. Insulin is an anabolic hormone that increases bone mass through bone formation by insulin receptor substrate 1 and 2 on osteoblasts and by lowering sex hormone binding globulin concentration, which results in increased concentrations of estradiol and testosterone [33,34].
In concordance with our results, T1DM and T2DM have frequent low BMD particularly male. The decrease bone formation and inadequate accrual peak bone mass in children with prepubertal onset T1DM has been proposed as a major contributing factor for low bone strength and osteoporosis in later life [35]. Men with T1DM tend to be particularly prone to osteopenia or osteoporosis compared to women of similar ages. [36,37] Estrogen adequacy and/or use of estrogen‑based oral contraceptive pills might be the reason for higher bone mass in women compared to men [37,38]. Furthermore, hypogonadism is quite common in men with T1DM and that may contribute to osteoporosis in males [39,40]. Another factor which may influence the BMD is body mass index (BMI). Studies have shown that lower BMI is associated with higher incidence of osteoporosis. The adipose tissue, apart from providing mechanical loading, also increases BMD through the activity of adipocytokines. Patients with TIDM also have a negative calcium balance as a result of hypercalciuria during periods of hyperglycemia, functional hypoparathyroidism, vitamin D deficiency and alterations in vitamin D metabolism [41-43].
Cases of both T1DM and T2DM with osteoporosis have lower significantly BMI as compared for cases who have not, 23.6 ± 4.1 vs. 25.6 ± 3.2, p = 0.046. A low BMI as shown in our study is associated the increased possibility of osteoporosis [44]. Overweight and obesity are believed to be protective factors of BMD [45,46]. Obesity is strongly associated with higher BMD probably through mechanical loading and hormonal factors including insulin, estrogen, and leptin [47].
Our finding of lower femur neck-BMD than Lumbar spine-BMD in patients with diabetes is in agreement with some studies, while others did not find such a difference [48-51]. Most studies focus on either T1DM or T2DM, or do not precisely define the diabetes type at all and, thus, the assessment of a cohort of consecutive patients of both diabetes types is unique to our study. The increased bone loss at the femur neck-BMD compared with the Lumbar spine-BMD suggests that osteoporosis in diabetic patients preferentially develops within the appendicular skeleton with predominantly cortical bone. One possible explanation could be the presence of secondary hyperparathyroidism due to vitamin D deficiency, as parathyroid hormone causes predominantly cortical bone loss. However, this needs further investigation. Another explanation for increased LS-BMD values in patients with type 2 diabetes could be an artificially high determination of BMD due to degenerative changes and diffuse idiopathic skeletal hyperostosis, frequently found in such patients [52-55]. Many factors such as exposure to sunlight, vitamin D, number of pregnancies, exercise and dairy consumption can affect the prevalence of osteoporosis. Although the reasons for the divergent distribution of bone mass at the Lumbar spine-BMD and femur neck-BMD cannot be clarified by our study, this finding is of practical relevance and suggests that evaluation of osteoporosis risk in diabetic patients should include both measurement sites and not rely on one.
In conclusion, our findings demonstrate high frequency of osteoporosis in patients with T1DM and T2DM. Although bone density testing is not routinely performed in patients with diabetes, these data suggest that screening may be important particularly in young women with type 1 diabetes. In addition, these women should be counseled regarding lifestyle interventions that may improve bone health, including adequate intake of calcium and vitamin D, and exercise.
Acknowledgments
We are grateful to the staffs from the diabetic centre at King Fahad Armed Forces Hospital for his valuable contributions in data collection.
We are grateful to the staffs from the diabetic centre at King Fahad Armed Forces Hospital for his valuable contributions in data collection.
Conflict of interest
The authors have no conflict of interest to disclose.
The authors have no conflict of interest to disclose.
References
- Delaney MF and LeBoff MS. Metabolic bone disease. In: Ruddy S, Harris ED Jr, Sledge CD, eds. Kelley’s Textbook of Rheumatology. 6th ed. Philadelphia: WB Saunders Co; 2001:1635-52.
- Lampropoulos CE., et al. “Osteoporosis a risk factor for cardiovascular disease?”. Nature Reviews Rheumatology 8.10 (2012): 587-598.
- Chen HL., et al. “Prevalence of osteoporosis and its associated factors among older men with type 2 diabetes”. International Journal of Endocrinology (2013).
- Khoshhal KI. “Childhood osteoporosis”. Journal of Taibah University Medical Sciences 6.2 (2011): 61-66.
- Albright F and Reifenstein EC. “Bone development in diabetic children: a roentgen study”. The American Journal of the Medical Sciences 174.1 (948): 313-319.
- Christensen JO and Svendsen OL. “Bone mineral in pre- and postmenopausal women with insulin-dependent and non-insulin-dependent diabetes mel- litus”. Osteoporosis International 10.4 (1999): 307-311.
- Gunczler P., et al. “Decreased bone mineral density and bone formation markers shortly after diagnosis of clinical type 1 diabetes mellitus”. Journal of Pediatric Endocrinology and Metabolism 14.5 (2001): 525-528.
- Hampson G., et al. “Bone mineral density, collagen type 1 alpha 1 genotypes and bone turnover in premenopausal women with diabetes mellitus”. Diabetologia 41.11 (1998): 1314-1320.
- Kao WH., et al. “Type 2 diabetes is associated with increased bone mineral density in Mexican- American women”. Archives of Medical Research 34.5 (2003): 399-406.
- Kayath MJ., et al. “Prevalence and magnitude of osteopenia associated with insulin-dependent diabetes mellitus”. Journal of Diabetes and its Complications 8.2 (1994): 97-104.
- Krakauer JC., et al. “Bone loss and bone turnover in diabetes”. Diabetes 44.7 (1995): 775–782.
- Miazgowski T and Czekalski S. “A 2-year follow-up study on bone min- eral density and markers of bone turnover in patients with long-standing insulin-dependent diabetes mellitus”. Osteoporosis International 8.5 (1998): 399-403.
- Strotmeyer ES., et al. “Diabetes is associated independently of body composition with BMD and bone volume in older white and black men and women: The Health, Aging, and Body Composition Study”. Journal of Bone and Mineral Research 19.7 (2004): 1084-1091.
- Van Daele PL., et al. “Bone Density in Non-Insulin-Dependent Diabetes Mellitus: The Rotterdam Study”. Annals of Internal Medicine 122.6 (1995): 409-414.
- Leidig-Bruckner G and Ziegler R. “Diabetes mellitus a risk for osteoporosis?”. Experimental and Clinical Endocrinology & Diabetes 109 (2001): S493-514.
- Schwartz AV. “Diabetes mellitus: does it affect bone?”. Calcified Tissue International 73.6 (2003): 515-9.
- Thrailkill KM., et al. “Is insulin an anabolic agent in bone? Dissecting the diabetic bone for clues”. American Journal of Physiology-Endocrinology and Metabolism 289.5 (2005): E735-745.
- Holecki M and Wiecek A. “Relationship between body fat mass and bone metabolism”. Polskie Archiwum Medycyny Wewnetrznej 120.9 (2010): 361-367.
- Espallargues M., et al. “Identifying bone-mass-related risk factors for fracture to guide bone densitometry measurements: a systematic review of the literature”. Osteoporosis International 12 (2001): 811-822.
- Nicodemus KK and Folsom AR. “Iowa Women's Health S. Type 1 and type 2 diabetes and incident hip fractures in postmenopausal women”. Diabetes Care 24.7 (2001): 1192-1197.
- Barrett-Connor E and Holbrook TL. “Sex differences in osteoporosis in older adults with non-insulin dependent diabetes mellitus”. JAMA 268.33 (1992): 3333-3337.
- Petit MA., et al. “Bone mass and strength in older men with type 2 diabetes: the Osteoporotic Fractures in Men Study”. Journal of Bone and Mineral Research 25.2 (2010): 285-291.
- Bridges M J., et al. “Influence of diabetes on peripheral bone mineral density in men: a controlled study”. Acta Diabetologica 42.2 (2005): 82-86.
- Zhou Y., et al. “Prevalence and predictors of osteopenia and osteoporosis in postmenopausal Chinese women with type 2 diabetes”. Diabetes Research and Clinical Practice 90.3 (2010): 261-269.
- Bonds DE., et al. “Risk of fracture in women with type 2 diabetes: the Women’s Health Initiative Observational Study”. The Journal of Clinical Endocrinology & Metabolism 91.9 (2006): 3404-3410.
- Dobnig H., et al. “Type 2 diabetes mellitus in nursing home patients: effects on bone turnover, bone mass, and fracture risk”. The Journal of Clinical Endocrinology & Metabolism 91.9 (2006): 3355–3363.
- Vestergaard P. “Discrepancies in bone mineral density and fracture risk in patients with type 1 and type 2 diabetes–a meta-analysis”. Osteoporosis International 18.4 (2007): 427-444.
- Joshi A., et al. “A study of bone mineral density and its determinants in type 1 diabetes”. Journal of Osteoporosis (2013): 397-814.
- Munoz‑Torres M., et al. “Bone mineral density measured by dual X‑ray absorptiometry in Spanish patients with insulin dependent diabetes mellitus”. Calcified Tissue International 58.5 (1996): 31-39.
- Bouillon R. “Diabetic bone disease”. Calcified Tissue International 6.3 (1991): 155-160.
- McNair P., et al. “Bone loss in diabetes: effects of metabolic state”. Diabetologia 17.5 (1979): 283-286.
- Rejnmark L., et al. “Loop diuretics increase bone turnover and decrease BMD in osteopenic postmenopausal women: Results from a randomized controlled study with bumetanide”. Journal of Bone and Mineral Research 21.1 (2006): 163‑170.
- NAnaforoglu I., et al. “Prevalence of osteoporosis and factors affecting bone mineral density among postmenopausal Turkish women with type 2 diabetes”. Journal of Diabetes and its Complications 23.1 (2009): 12-17.
- Thrailkill K M., et al. “Is insulin an anabolic agent in bone? Dissecting the diabetic bone for clues”. American Journal of Physiology-Endocrinology and Metabolism 289.5 (2005): E735-745.
- Roggen I., et al. “Trabecular bone mineral density and bone geometry of the distal radius at completion of pubertal growth in childhood type 1 diabetes”. Hormone Research in Paediatrics 79.2 (2013): 68-74.
- Hamilton EJ., et al. “A five‑year prospective study of bone mineral densityin men and women with diabetes: The Fremantle Diabetes Study”. Acta Diabetologica 49.2 (2012): 153-158.
- Hadjidakis DJ., et al. “Bone mineral density of both genders in Type 1 diabetes according to bone composition”. Journal of Diabetes and its Complications 20.5 (2006): 302-307.
- Lunt H., et al. “A population‑based study of bone mineral density in women with longstanding type 1 (insulin dependent) diabetes”. Diabetes Research and Clinical Practice 40.1 (1998): 31-38.
- Lopez‑Alvarenga JC., et al. “Poorly controlled type I diabetes mellitus in young men selectively suppresses luteinizing hormone secretory burst mass”. The Journal of Clinical Endocrinology and Metabolism 87.12 (2002): 5507-5515.
- Orwoll ES and Klein RF. “Osteoporosis in men”. Endocrine Reviews 16 (1995): 87-116.
- Thalassinos NC., et al. “Calcium metabolism in diabetes mellitus: Effect of improved blood glucose control”. Diabetic Medicine 10.4 (1993): 341-344.
- Daga RA., et al. “High prevalence of vitamin D deficiency among newly diagnosed youth‑onset diabetes mellitus in north India”. Arq Bras Endocrinol Metab 56.7 (2012): 423‑428.
- Borkar VV., et al. “Low levels of vitamin D in North Indian children with newly diagnosed type 1 diabetes”. Pediatric Diabetes 11.5 (2010): 345-350.
- Espallargues M., et al. “Identifying bone-mass-related risk factors for fracture to guide bone densitometry measurements: a systematic review of the literature”. Osteoporosis International 12.10 (2001): 811-822.
- Wang ML., et al. “The relative contributions of lean tissue mass and fat mass to bone density in young women”. Bone 37.4 (2005): 474-481.
- Barrera G., et al. “A high body mass index protects against femoral neck osteoporosis in healthy elderly subjects.” Nutrition 20.4 (2004): 769-771.
- Felson D., et al. “Effects of weight and body mass index on bone mineral density in men and women: the Framingham Study.” Journal of Bone and Mineral Research 8.5 (1993): 567-573.
- Isaia GC., et al. “Bone metabolism in type 2 diabetes mellitus”. Acta Diabetologica 36.1 (1999): 35-38.
- Compston JE., et al. “Whole body composition and regional bone mass in women with insulin-dependent diabetes mellitus”. Clinical Endocrinology 41.3 (1994): 289-293.
- Tuominen JT., et al. “Bone mineral density in patients with type 1 and type 2 diabetes”. Diabetes Care 22.7 (1999): 1196-1200.
- Majima T., et al. “Decreased bone mineral density at the distal radius, but not at the lumbar spine or the femoral neck, in Japanese type 2 diabetic patients”. Osteoporosis International 16 (2005): 907-913.
- Forestier J and Lagier R. “Ankylosing hyperostosis of the spine”. Clinical Orthopaedics and Related Research 74 (1971): 65-83.
- Resnick D., et al. “Diffuse idiopathic skeletal hyperostosis (DISH) ankylosing hyperostosis of Forestier and Rotes-Querol]”. Seminars in Arthritis and Rheumatism 7.3 (1978): 153-187.
- Spagnola AM., et al. “Vertebral ankylosing hyperostosis (Forestier’s disease) and HLA antigens in Pima Indians”. Arthritis & Rheumatology 21.4 (1978): 467-472.
- Sencan D., et al. “The prevalence of diffuse idiopathic skeletal hyperostosis in patients with diabetes mellitus”. Rheumatology International 25.7 (2005): 518-521.
Citation:
Khalid S Aljabri., et al. “Osteoporosis among Patients with Type 1 and 2 Diabetes Mellitus”. Archives of Endocrinology and
Diabetes Care 1.2 (2018): 71-80.
Copyright: © 2018 Khalid S Aljabri., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.