Research Article
Volume 1 Issue 3 - 2018
Hypertension in Saudi Adults with Type 1 And 2 Diabetes Mellitus
1Department of Endocrinology, King Fahad Armed Forces Hospital, Jeddah, Kingdom of Saudi Arabia
2College of medicine, Um Al Qura University, Makkah, Kingdom of Saudi Arabia
2College of medicine, Um Al Qura University, Makkah, Kingdom of Saudi Arabia
*Corresponding Author: Khalid SJ Aljabri, Department of Endocrinology, King Fahad Armed Forces Hospital, Jeddah, Kingdom of
Saudi Arabia, PO Box 9862, Jeddah 21159, Kingdom of Saudi Arabia.
Received: April 14, 2018; Published: May 24, 2018
Abstract
Background: Diabetes and hypertension (HTN) are among the most common chronic non-communicable diseases.
Methods: A cross sectional study was conducted at the Primary Health Care Clinics at King Fahad Armed Forces Hospital, Jeddah, Saudi Arabia. A total of 1546 Saudi with type 1 diabetes (T1DM) and type 2 diabetes (T2DM) were randomly selected.
Results: A total of 1546 patients attending the Primary Health Care Clinics were reviewed. There were 313 (20.2 %) diagnosed with T1DM and 1233 (79.8%) with T2DM. Out of the total 1546 patients, HTN was present in 124 (8%) of T1DM patients and 777 (50.3%) of T2DM patients. Total of 901 (58.2%) patients with diabetes and HTN included in this study; 365 (40.5%) male and 536 (59.5%) female. HTN was diagnosed in 13.8% of T1DM and 86.2% of T2DM patients. A female predominance (sex ratio male: female) 1:2.8 for T1DM+HTN and 1:3 for T2DM + HTN. T2DM + HTN were significantly older and have higher HbA1c than patients with T1DM+HTN. Significant female predominance across the body mass index groups between T1DM + HTN compared to T2DM+HTN particularly female T2DM + HTN. Significant frequent cases of T2DM+HTN compared to T1DM + HTN across the HbA1c and 24 hours Urine micro albumin groups. No significant gender differences were found in the HbA1c and 24 hours Urine micro albumin groups.
Conclusion: The frequency of hypertension in patients with diabetes in this study is high. It is mandatory to have adequate diagnostic, therapeutic and educational resources in addition to competent physicians who can manage hypertension in diabetic patients by using a continuing, comprehensive and coordinated approach.
Introduction
Diabetes mellitus and hypertension (HTN) are two of the most common diseases affecting both developed and developing countries and occur at a higher prevalence in the older age group and result from both genetic and environmental etiological factors [1-3]. HTN is an extremely common comorbidity in patients with diabetes, affecting approximately 20-60% of patients, depending on age, ethnicity, and body weight [4]. The prevalence of HTN in diabetic individuals appears to be approximately twofold that in the non-diabetic population. This is clearly the case for type I diabetes (T1DM) and is probably valid for type 2 diabetes (T2DM) as well, although the relation is somewhat more controversial with regard to the latter.
Patients with T1DM currently make up about 6% to 8% of the total diabetes population. Among those with T1DM, the incidence of HTN rises from 5 percent at 10 years, to 33 percent at 20 years, and 70 percent at 40 years [5]. HTN is extremely common in patients with T2DM, affecting up to 60% [6]. In contrast to patients with T2DM, those with T1DM typically develop renal disease before developing hypertension [7-9]. The presence of HTN in patients with diabetes markedly enhances development of microvascular and microvascular disease in these individuals. Diabetic individuals with coexisting hypertension have a much greater prevalence of stroke and transient ischemic episodes than do normotensive diabetics. Both hypertension and diabetes mellitus are major independent risk factors for accelerated atherosclerosis and ischemic heart disease and peripheral vascular disease [10-14]. Therefore, the aim of the present study is to determine the frequency of HTN in cases with T1DM and T2DM among the patients who have attended the primary health care center in a Saudi community.
Methods
A cross sectional study was conducted at Primary Health Care Clinics at King Fahad Armed Forces Hospital. A total of 1546 Saudi diabetic patients were randomly selected. The demographic data and medical history were documented. Blood Pressure readings were within a gap of 15 minutes using a mercury sphygmomanometer by palpation and auscultation method in right arm in sitting position. Two readings were taken 15 min apart and the average of both the readings was taken for analysis. HTN was also diagnosed based on anti HTN medications or having a prescription of antihypertensive drugs and were classified as Hypertensive irrespective of their current blood pressure reading or if the blood pressure was greater than 140/90 mmHg i, e systolic BP more than 140 and diastolic BP more than 90 mm of Hg–Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. [15] The Body mass index (BMI) was considered normal if it was below 25 kg/m2, 25-29.9 kg/m2 overweight and 30 kg/m2 or greater was obese. The HbA1c was divided into three groups; < 7.0, 7.0 - 8.9 and ≥ 9.0. 24 hours Urine micro albumin (mg/24 h) was divided into three groups; < 15.0, 15.0 - 45.0 and > 45.0. The study was approved by the ethical board of King Fahad Armed Forces Hospital.
Statistical Analysis
Univariate analysis of baseline and follow up demography and clinical laboratory endpoints were accomplished using unpaired t-test. Chi square(X2) test were used for categorical data comparison. All statistical analyses. Were performed using SPSS Version 22.0. All P values were based on two-sided tests. P < 0.05 was considered significant.
Univariate analysis of baseline and follow up demography and clinical laboratory endpoints were accomplished using unpaired t-test. Chi square(X2) test were used for categorical data comparison. All statistical analyses. Were performed using SPSS Version 22.0. All P values were based on two-sided tests. P < 0.05 was considered significant.
Results
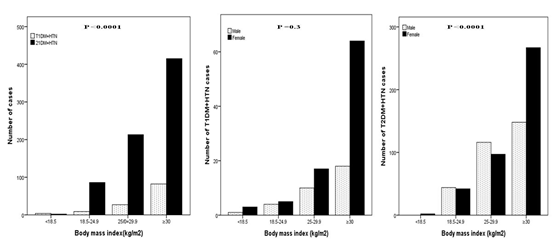
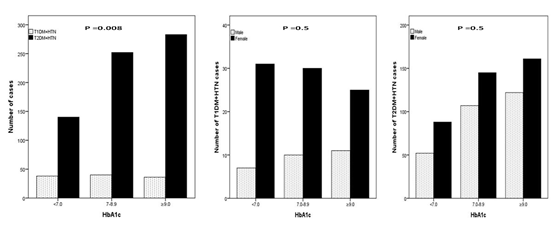
A total of 1546 patients attending the Primary Health Care Clinics were reviewed. There were 313 (20.2%) diagnosed with T1DM and 1233 (79.8%) with T2DM. Out of the total 1546 patients, HTN was present in 124(8%) of T1DM patients and 777(50.3%) of T2DM patients. Total of 901 (58.2%) patients with diabetes and HTN included in this study; 365 (40.5%) male and 536 (59.5%) female, (Table 1). HTN was diagnosed in 13.8% of T1DM and 86.2% of T2DM patients. A female predominance (sex ratio male: female) 1:2.8 for T1DM + HTN and 1:3 for T2DM + HTN. T2DM + HTN were significantly older and have higher HbA1c than patients with T1DM + HTN. (Figure 1) shows significant female predominance across the body mass index groups between T1DM + HTN compared to T2DM + HTN particularly female T2DM + HTN. Significant frequent cases of T2DM + HTN compared to T1DM + HTN across the HbA1c and 24 hours Urine micro albumin groups, Figure 2 and 3. No significant gender differences were found in the HbA1c and 24 hours Urine micro albumin groups.
| Parameters | T1DM + HTN 124 (13.8) | T2DM + HTN 777 (86.2) | P value | |
| Gender | Male | 33(9) | 332 (91) | 0.001 |
| Female | 91 (17) | 445 (83) | ||
| Age(years) | 26.5 ± 3.9 | 40.6 ± 6.5 | < 0.0001 | |
| Body mass index | 33.0 ± 7.4 | 31.4 ± 7.8 | 0.009 | |
| HbA1c | 8.2 ± 2.2 | 8.7 ± 2.2 | 0.04 | |
| Serum creatinine | 65.1 ± 15.5 | 75.0 ± 28.2 | < 0.0001 | |
| 24 hours Urine micro albumin (mg/24h) | 66.0 ± 143.0 | 131.3 ± 312.0 | 0.04 | |
Table 1: Demographic patient’s parameters and Comparison of features between diabetics and hypertension.
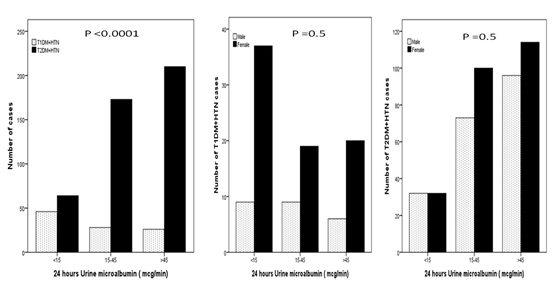
Figure 3: Frequency of T1DM and T2DM and HTN according
to 24 hours Urine micro albumin groups and gender.
Discussion
There is many evidence for an increased prevalence of hypertension in diabetic persons [16]. The coexistence of HTN and T2DM is a major risk factors to the development and progression of microvascular and microvascular complications in people with diabetes compared to the general population [17-19]. Both HTN and T2DM increase the risks of cardiovascular disease, stroke, nephropathy and retinopathy [7,20,21]. Indeed when HTN coexists with diabetes, the risk of cardiovascular disease is increased by 75%, which further contributes to the overall morbidity and mortality of an already high risk population.
The prevalence rate of HTN among T2DM is ranging between 32% and 82% which translates up to 3 times greater than in age- and sex-matched patients with diabetes [7,9,21]. In discordance with our finding where female was more predominance, male sex has been associated with an increased incidence of hypertension in some studies but not in all [22,23]. Compared to Arab population, the prevalence rate of HTN reported in this study (50.3%) among patients with T2DM is lower to the 64.5% rate reported in Qatari diabetics and 72.4% rate reported in Jordanian diabetics. In other Arab populations, the prevalence rate of hypertension is moderate: 53% in Saudi diabetics, 44% in Omani diabetics and 38% in Bahraini diabetics. Compared to other populations, the rate of hypertension among diabetics in our study is lower to the 74%, 74.4% and 73% rates of hypertension reported in UK Caucasians, Italian and Spanish populations, respectively. This prevalence is lower than the 82% prevalence rate reported about Afro-Caribbean individuals living in UK and much higher than the 32% and 39% rates reported among diabetics in the Turkish and Taiwanese populations, respectively. The explanation for differences in frequency by each country could be due to different methods of surveillance, differences in definitions of hypertension, population characteristics and ethnic variations. Hyperglycemia and increase in total body exchangeable sodium leading to extracellular fluid accumulation and expansion of the plasma volume contributes to the pathogenesis of hypertension in DM [22, 24-33].
The close association of diabetes with HTN is commonly thought to be due to underlying obesity, insulin resistance, and/or hyperinsulinemia [20, 34-36]. Hyperinsulinemia induces HTN through increased renal tubular reabsorption of sodium and water, increased sympathetic nervous system activity, proliferation of vascular smooth muscle cells, and alterations of transmembrane cation transport. At physiological concentrations, insulin decreases urinary sodium excretion, an action mediated by binding to specific high-affinity receptors [34-38]. Recent data suggest that cellular insulin resistance, rather than hyperinsulinemia may lead to HTN [39]. In addition, impaired cellular response to insulin predisposes to increased vascular smooth muscle tone which is the hallmark of hypertension in the diabetic patients [39].
Few studies of HTN in T1DM patients have been conducted; the majority of these studies analyzed HTN in adult diabetic patients and reported a prevalence of 24 to 43% which is lower to those observed in the current study [40-47]. Most non-glycemic risk factors for incident HTN identified herein are consistent with those described elsewhere, including older age and greater BMI [22,23,48,49]. BMI merits specific note because their associations with HTN add to growing evidence that obesity is a clinically relevant health problem for people with T1DM [50]. HbA1c was significantly associated with more frequent HTN in T2DM than in T1DM. Extensive literature describes effects of hyperglycemia on the vascular wall. Through increased quantities of advanced glycation end products, reactive oxygen species, and sorbitol, hyperglycemia can lead to vasoconstriction (via alterations of endothelin and nitric oxide) and to extracellular matrix deposition. Activation of protein kinase C may play a central role in these pathways. Changes leading to vascular remodeling may progress over long periods on the development of HTN [51, 52]. However, the long-term effect of hyperglycemia on blood pressure is not known.
Diabetic nephropathy will develop in as many as 40% of IDDM patients [16]. HTN often contributes to the development of nephropathy in many diabetic individuals [53,54]. Diabetic nephropathy, which occurs after 15 years of diabetes in one-third of people with T1DM and 20% of those with T2DM, is an important contributing factor to the development of HTN in the diabetic individual [55]. The HTN associated with diabetic nephropathy is usually characterized by sodium and fluid retention and increased peripheral vascular resistance [16].
The objective of HTN and diabetes care is to reduce its mortality and complications and to improve the quality of life for patients suffering from this chronic health problem [49]. To achieve these aims, it is mandatory to have adequate diagnostic, therapeutic and educational resources in addition to competent physicians who can manage HTN and diabetes by using a continuing, comprehensive and coordinated approach. Many essential resources for the care of patients with HTN and T2DM are not available at primary health care settings [56]. Urgent provision of these resources is essential to introduce good health care for hypertensive diabetic patients.
Several limitations of the current study must be addressed. We used a clinical definition of T1DM that was assigned by physicians and was applicable to all patients, which is similar to previous studies [57]. However, autoantibody and C-peptide levels were not measured. Therefore, some patients with other types of diabetes may have been included. Nevertheless, it is important to emphasize that 93.1% of our patients were diagnosed before the age of 30, which supports the high probability that these patients had T1DM. Additionally, the prevalence of hypertension may have been overestimated because diagnosis was based on the measurement of a blood pressure in one day rather than two separate measurements on two separate days. Due to the crosssectional nature of this study, the observed population reflects a selected yet comprehensive group of patients. Our study could be limited by the question of clustering of cases within the study region and the effect that might have on our estimates, in addition, the current study population may appear limited in size and therefore may underestimate the true frequency of T1DM and T2DM and HTN in the general population. Despite this limitation, our study is one of the Saudi study done on a large sample, which was specifically interested in the problem of hypertension in diabetic patients and reported very important information on the epidemiology of hypertension in Saudi diabetics
In conclusion, the frequency of HTN in patients with T1DM and T2DM in this study is high. It is mandatory to have adequate diagnostic, therapeutic and educational resources in addition to competent physicians who can manage HTN in diabetic patients by using a continuing, comprehensive and coordinated approach.
References
- Fraser FC. “Evolution of a palatable multifactorial threshold model”. The American Journal of Human Genetics 32.6 (1980): 796-813.
- Mueller RF and Young ID. “Emery’s Elements of Medical Genetics. 9th edition. London: Churchill Livingston”. (1995).
- Rotter JI and Motulsky AG. “The genetic basis of common diseases”. Oxford University Press 34.5 (1992): 628-638.
- Arauz-Pacheco C., et al. “Hypertension management in adults with diabetes”. Diabetes Care 27.1 (2004): S65-S67.
- Sowers JR, “Treatment of hypertension in patients with diabetes”. Archives of internal medicine 164.24 (2004): 1850-7.
- Bethesda MD. “Diabetes in America. 2nd edition”. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases (1995).
- Bakris G., et al. “Preserving renal function in adults with hypertension and diabetes: A consensus approach”.American journal of kidney diseases 36.3 (2000): 646-661.
- Sowers JR., et al. “Hypertension in patients with diabetes: strategies for drug therapy to reduce complications”. Postgraduate medicine 10.7 (2000): 47-54.
- Sowers JR., et al. “Diabetes, hypertension, and cardiovascular disease: an update”. Hypertension 37.4 (2001): 1053-1059.
- Winer N and Sowers JR. “Epidemiology of diabetes”. The Journal of Clinical Pharmacology 44.12 (2004): 397-405.
- Lim HS and Lip GY. “Diabetes, the renin-angiotensin system and heart disease”. Current Vascular Pharmacology 1.2 (2003): 225-238.
- El-Atat F., et al. “Diabetes, hypertension, and cardiovascular derangements: pathophysiology and management”. Current Hypertension Reports 6.3 (2004): 215-223.
- Chobanian AV., et al. “The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure: the JNC-7 report”. JAMA 289.19 (2003): 2560-2572.
- Sowers JR. “Recommendations for special populations: diabetes mellitus and the metabolic syndrome”. American Journal of Hypertension 16.11 (2003): 41S-45S.
- “ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults”. Journal of the American College of Cardiology (2017).
- “National High Blood Pressure Education Program Working Group report on hypertension in diabetes”. Hypertension 23.2 (1994): 145-158.
- Libby P., et al. “Report of the National Heart, Lung and Blood Institute National Institute of Diabetes and Digestive and Kidney Diseases Working Group on Cardiovascular Complications of DM”. Circulation 111.25 (2005): 3489-3493.
- Fong DS., et al. “Diabetic retinopathy”. Diabetes Care 27 (2004): 2540-2553.
- Tesfaye S., et al. “EURODIAB Prospective Complications Study Group. Vascular risk factors and diabetic neuropathy”. The New England Journal of Medicine 352.4 (2005): 341-50.
- Sowers JR and Epstein M. “Diabetes mellitus and associated hypertension, vascular disease, and nephropathy: an update”. Hypertension 26.1 (1995): 869-879.
- David M Nathan. “The Diabetes Control and Complications Trial/Epidemiology of diabetes interventions and complications”. The New England Journal of Medicine 348 (2003): 2294-2303.
- Dyer AR., et al. “Ten-year incidence of elevated blood pressure and its predictors: the CARDIA study”. Journal of Human Hypertension 13.1 (1999): 13-21.
- Wilson PW., et al. “Overweight and obesity as determinants of cardiovascular risk: the Framingham experience”. Archives of Internal Medicine 162.16 (2002): 1867-1872.
- Baskar V., et al. “The prevalence of hypertension and utilization of antihypertensive therapy in a district Diabetes Population”. Diabetes Care 25.11 (2002): 2107-2108.
- Bener A., et al. “Prevalence of diagnosed and undiagnosed diabetes mellitus and its risk factors in a population-based study of Qatar”. Diabetes Research and Clinical Practice 84.1 (2009): 99-100.
- Mubarak FM., et al. “Hypertension among 1000 patients with type 2 diabetes attending a national diabetes center in Jordan”. Annals of Saudi medicine 28.5 (2008): 346-351.
- Akbar DH., et al. “Cardiovascular risk factors in Saudi and non-Saudi diabetics”. Saudi Medical Journal 24.6 (2003): 686-687.
- Al-Moosa S., et al. “Diabetes and urbanization in the Omani population: an analysis of national survey data”. Population Health Metrics 24 (2006): 4-5.
- Al-Mahroos F., et al. “Relation of high blood pressure to glucose intolerance, plasma lipids and educational status in an Arabian Gulf population”. International journal of epidemiology 29.1 (2000): 71-76.
- Comaschi M., et al. “SFIDA Study Group--Italian Association of Diabetologists (AMD). Cardiovascular risk factors and metabolic control in type 2 diabetic subjects attending outpatient clinics in Italy: the SFIDA (survey of risk factors in Italian diabetic subjects by AMD) study”. Nutrition, Metabolism & Cardiovascular Diseases 15.4 (2005): 204-211.
- Del Cañizo-Gómez FJ and Moreira-Andrés MN. “Cardiovascular risk factors in patients with type 2 diabetes. Do we follow the guidelines?”. Diabetes Research and Clinical Practice 65.2 (2004): 125-133.
- Baskar V., et al. “Does ethnic origin have an independent impact on hypertension and diabetic complications?”. Diabetes, Obesity and Metabolism 8.2 (2006): 214-219.
- Tseng CH. “Higher risk of hypertension in indigenous type 2 diabetic patients in Taiwan”. Journal of Hypertension 24.9 (2006): 1817-1821.
- DeChatel R., et al. “sodium, renin, aldosterone, catecholamine's and blood pressure in diabetes mellitus”. Kidney International 12.6 (1977): 412-421.
- Anderson EA., et al. “Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans”. The Journal of Clinical Investigation 87.6 (1991): 2246-2252.
- Sechi LA and Bartoli E. “Molecular mechanisms of insulin resistance in arterial hypertension”. Blood Pressure Supplement 1 (1996): 47-54.
- Perlstein TS., et al. “Insulin induces renal vasodilation, increases plasma renin activity, and sensitizes the renal vasculature to angiotensin receptor blockade in healthy subjects”. Journal of the American Society of Nephrology 18.3 (2007): 944-951.
- Purnell JQ., et al. “Effect of excessive weight gain with intensive therapy of type 1 diabetes on lipid levels and blood pressure: results from the DCCT”. JAMA 280.2 (1998): 140-146.
- Lender D., et al. “A double blind comparison of the effects of amlodipine and enalapril on insulin sensitivity in hypertensive patients”. American Journal of Hypertension 12.3 (1999): 298-303.
- D M Maahs., et al. “Hypertension prevalence, awareness, treatment, and control in an adult type 1 diabetes population and a comparable general population”. Diabetes Care 28.2 (2005): 301-305.
- F Collado-Mesa., et al., “Prevalence and management of hypertension in type 1 diabetes mellitus in Europe: the Eurodiab IDDM Complications study”. Diabetic Medicine 16.1 (1999): 41-49.
- MS Roy., et al. “Medical and psychological risk factors for incident hypertension in type 1 diabetic africanamericans”. International Journal of Hypertension (2011): 10.
- K Dahl-Jørgensen., et al. “Atherosclerosis in childhood and adolescent type 1 diabetes: early disease, early treatment?”. Diabetologia 48.8 (2005): 1445-1453.
- BL Rodriguez., et al. “Prevalence and correlates of elevated blood pressure in youth with diabetes mellitus: the search for diabetes in youth study”. Journal of Pediatrics 157.2 (2010): 245-251.
- KO Schwab., et al. “Characterization of 33488 children and adolescents with type 1 diabetes based on the gender-specific increase of cardiovascular risk factors”. Pediatric Diabetes 11.5 (2010): 357-363.
- M Van Vliet., et al. “Overweight is highly prevalent in children with type 1 diabetes and associates with cardio metabolic risk”. Journal of Pediatrics 156.6 (2010): 923-929.
- HD Margeirsdottir., et al. “High prevalence of cardiovascular risk factors in children and adolescents with type 1 diabetes: a population-based study”. Diabetologia 51.4 (2008): 554-561.
- Lauer RM., et al. “Childhood predictors of future blood pressure”. Hypertension 18.3 (1991): 174-181.
- Sonne-Holm S., et al. “Independent effects of weight change and attained body weight on prevalence of arterial hypertension in obese and non-obese men". British Medical Journal 299.6702 (1989): 767-770.
- De Boer IH., et al. “Central obesity, incident micro albuminuria, and change in creatinine clearance in the Epidemiology of Diabetes Interventions and Complications Study”. Journal of the American Society of Nephrology 18.3 (2007): 235–243.
- Brownlee M. “The pathobiology of diabetic complications: a unifying mechanism”. Diabetes 54.6 (2005): 1615-1625.
- Ceriello A. “Controlling oxidative stress as a novel molecular approach to protecting the vascular wallin diabetes”. Current Opinion in Lipidology 17.5 (2006): 510-518.
- Mogensen CE. “Prevention and treatment of renal disease in insulin-dependent diabetes mellitus”. Seminars in Nephrology 10 (1990): 260-273.
- Mykkänen L., et al. “Micro albuminuria precedes the development of NIDDM”. Diabetes 43.4 (1994): 552-557.
- Epstein M and Sowers JR. “Diabetes mellitus and hypertension”. Hypertension 19 (1992): 403-418.
- Al-Sharif AI and Al-Khaldi YM. “Resource availability for care of hypertensives at primary health settings in Southwestern Saudi Arabia”. Saudi Medical Journal 24.5 (2003): 466-471.
- FA McAlister., et al. “Changes in the rates of awareness, treatment and control of hypertension in Canada over the past two decades”. Canadian Medical Association Journal 183.9 (2011): 1007-1013.
Citation:
Khalid S Aljabri., et al. “Hypertension in Saudi Adults with Type 1 And 2 Diabetes Mellitus”. Archives of Endocrinology and
Diabetes Care 1.3 (2018): 99-106.
Copyright: © 2018 Khalid S Aljabri., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.