Research Article
Volume 2 Issue 1 - 2018
Evaluation of Hepatic Fibrosis Using Acoustic Radiation Force Impulse Among Obese Children.
1,3Dept of Radio diagnosis, S.P. Medical College, Bikaner, Rajasthan
2Dept. of clinical nutrition and dietetics, SMT.V.H.D central institute of home sciences, Bengaluru
2Dept. of clinical nutrition and dietetics, SMT.V.H.D central institute of home sciences, Bengaluru
*Corresponding Author: GL Meena, senior professor & h.o.d., dept of radio diagnosis, sardar patel medical college, Bikaner, Rajasthan.
Received: August 02, 2018; Published: August 18, 2018
Abstract
Objectives: To investigate the potential usefulness of acoustic radiation force impulse ARFI for detecting LF in overweight and obese children.
Material and Methods: A cross-sectional study was conducted in 148 schoolchildren. A diagnosis of non-alcoholic fatty liver disease (NAFLD) and LF was based on ultrasound (US) and ARFI shear wave velocity (SWV).
Results: The laboratory parameters were normal in all the children. NAFLD was observed in 50 children (33.8%). The median SWV was 1.18 0.28 m/s. Differences between ARFI categories and hepatic steatosis grades were observed (X2 = 43.38, P = 0.0005). No fibrosis or insignificant fibrosis (SWV 1.60 m/s) was detected in 137 children (92.5%), and significant fibrosis (SWV > 1.60 m/s) in 11 children (7.5%), nine of whom had normal US or mild steatosis.
Conclusion: The present study is the first to evaluate the utility of the ARFI technique for detecting LF in overweight and obese children. The results of the study suggest that children with normal laboratory parameters such as normal liver ultrasound or mild steatosis may present with significant LF.
Keywords: Imaging; Steatosis; Obesity; Children; Elastography
Introduction
Childhood obesity is a major public health problem in all countries in the industrialized world [1,2]. Nonalcoholic fatty liver disease (NAFLD) is increasing at alarming rates in obese children [3,4]. Childhood NAFLD has become a significantly common liver disease [5,6]. NAFLD is a progressive disease that encompasses a spectrum of liver diseases, ranging from simple steatosis to non-alcoholic steatohepatitis (NASH) and cirrhosis [3,4,7]. NASH, which is more common in obese children, has the potential to advance to liver fibrosis and liver failure [7]. Liver biopsy (LB) is considered to be the most accurate method for diagnosing NAFLD but is not practical in population-wide studies [8,9].
Thus, there is a clear need for noninvasive alternatives to liver biopsy. Recent years have witnessed the development of alternative noninvasive techniques such as elasticity imaging methods, which include acoustic radiation force impulse (ARFI) imaging. ARFI imaging is a new non-invasive technique, integrated into a conventional ultrasound (US) system, which provides information on the localized mechanical properties of soft tissue using high-intensity, short-duration acoustic pulses to generate localized displacements in tissue [10,11]. ARFI shear wave velocity (SWV) is proportional to the square root of tissue elasticity [11,12]. SWV, expressed in m/s, is directly related to the stiffness of the tissue. The ARFI technique is a reliable and rapid method for the assessment of liver fibrosis (LF).
ARFI is a method for differentiating patients with NASH from patients with simple steatosis and it can also predict significant liver fibrosis (LF) [13]. There are a number of ARFI imaging studies of the liver in adults [12–18], especially for estimating the degree of LF. However, only a few published studies have evaluated liver fibrosis with ARFI in children [19–22], and they include healthy children and children with liver disease. To our knowledge, no studies have been reported in the literature on the ARFI technique for evaluation of the liver in obese children. Early detection of NASHassociated fibrosis is crucial for the prognosis of disease progression. The purpose of this study was to investigate whether ARFI imaging is potentially useful for detecting LF in overweight and obese children.
Material and Methods
Study population
A cross-sectional study was conducted in 148 schoolchildren (77 boys, 71 girls) from Gov. children’s school, Bikaner (Raj, INDIA), selected at random from 303 eligible children out of the 750 who were recruited. Of the 750 children, 447 were excluded for the following reasons: errors in filling out the application (n = 98); a body mass index (BMI) categorized as normal weight or underweight (n = 302); and presence of a chronic disease (n = 47). The present study is part of the health program, which was introduced in the 2013–2014 school year aimed at establishing strategies for preventing obesity. The criteria for inclusion were: (i) overweight or obese children aged 5–10 years; (ii) not presenting with diseases or physical restrictions that would prevent physical activity; and (iii) not following any type of diet or medical treatment that might condition progress in the intervention program. The criteria for exclusion were: (i) presenting with chronic diseases; (ii) not attending any of the assessments; and (iii) not signing the informed consent form.
A cross-sectional study was conducted in 148 schoolchildren (77 boys, 71 girls) from Gov. children’s school, Bikaner (Raj, INDIA), selected at random from 303 eligible children out of the 750 who were recruited. Of the 750 children, 447 were excluded for the following reasons: errors in filling out the application (n = 98); a body mass index (BMI) categorized as normal weight or underweight (n = 302); and presence of a chronic disease (n = 47). The present study is part of the health program, which was introduced in the 2013–2014 school year aimed at establishing strategies for preventing obesity. The criteria for inclusion were: (i) overweight or obese children aged 5–10 years; (ii) not presenting with diseases or physical restrictions that would prevent physical activity; and (iii) not following any type of diet or medical treatment that might condition progress in the intervention program. The criteria for exclusion were: (i) presenting with chronic diseases; (ii) not attending any of the assessments; and (iii) not signing the informed consent form.
The study was conducted according to the guidelines of the Helsinki Declaration. The study was approved by the Research Ethics Committee of the attached college Sardar Patel medical college, Bikaner. Signed informed consent forms were obtained from the children’s parents.
Anthropometric and clinical characteristics
All the measurements were taken in schools by an experienced, well-trained technician following a standard protocol and using calibrated instruments. Height and weight were measured. BMI was calculated as weight/height 2 (kg/m2) and the BMI z-score was calculated using the World Health Organization (WHO) Anthro Plus software. The child’s nutritional status was classified as follows: overweight (BMI z-score > 1 to 2), obesity (BMI z-score > 2) based on the WHO.
All the measurements were taken in schools by an experienced, well-trained technician following a standard protocol and using calibrated instruments. Height and weight were measured. BMI was calculated as weight/height 2 (kg/m2) and the BMI z-score was calculated using the World Health Organization (WHO) Anthro Plus software. The child’s nutritional status was classified as follows: overweight (BMI z-score > 1 to 2), obesity (BMI z-score > 2) based on the WHO.
Biochemical measurements were taken in the P.B.M, Hospital. Venous blood samples were obtained from all the schoolchildren after a 10-h overnight fast and glucose, insulin, total cholesterol (CHOL), low-density lipoproteins (LDL), high-density lipoproteins (HDL), low-density lipoproteins (LDL), triglycerides (TG), glutamate oxaloacetate transaminase (GOT), glutamate pyruvate transaminase (GPT), and gamma glutamyl transferase (GGT) were measured.
NAFLD ultrasound and liver ARFI
US examinations were performed in the respective schools by one of the six radiologists, with 22 years of experience in US and 3years of experience in the ARFI technique, independently and with an agreed study protocol. Each radiologist performed 24 or 25 examinations. US studies were performed with Acuson S2000 Virtual Touch Tissue Quantification (Siemens, Erlangen, Germany) using a 4 MHz conventional convex transducer. The examinations were performed with a fasting interval of > 3h. The children were assessed in the supine position with their right arm in maximum abduction and the study was conducted between the 6th and 7th intercostal spaces for the right lobe of the liver and subcostal for the left lobe of the liver.
US examinations were performed in the respective schools by one of the six radiologists, with 22 years of experience in US and 3years of experience in the ARFI technique, independently and with an agreed study protocol. Each radiologist performed 24 or 25 examinations. US studies were performed with Acuson S2000 Virtual Touch Tissue Quantification (Siemens, Erlangen, Germany) using a 4 MHz conventional convex transducer. The examinations were performed with a fasting interval of > 3h. The children were assessed in the supine position with their right arm in maximum abduction and the study was conducted between the 6th and 7th intercostal spaces for the right lobe of the liver and subcostal for the left lobe of the liver.
A diagnosis of NAFLD, or hepatic steatosis (HS), was based on the US scan. The severity of HS was graded as follows: Grade 0 = normal steatosis, defined as normal liver echotexture; Grade 1 = mild steatosis, as a slight and diffuse increase in fine parenchymal echoes with normal visualization of the diaphragm and portal vein borders; Grade 2 = moderate steatosis, as a moderate and diffuse increase in fine echoes with slightly impaired visualization of the portal vein borders and diaphragm; and Grade 3 = severe steatosis, defined as fine echoes with poor or no visualization of the portal vein borders, diaphragm, and posterior portion of the right lobe[23].
ARFI measurement of SWV (m/s) was performed during free soft breathing of the child. The region of interest (ROI) was positioned 1–2 cm from the liver capsule at a maximum depth of 8cm, in a homogeneous parenchyma that did not include vessels or surrounding structures. Ten valid measurements were taken as follows: (i) right liver lobe (RLL): the average of six measurements in segments VI, VII, and VIII (two measurements in each) was obtained; (ii) left liver lobe (LLL): the average of four measurements in segments II and III was obtained. SWVs obtained from the RLL were included in the different ARFI categories [12] according to the following cutoffs: ARFI 0 (1.90 m/s). Moreover, the ARFI categories were used as a basis to establish the following fibrosis classification: no fibrosis includes ARFI category 0; insignificant fibrosis includes ARFI categories 1 and 2; and significant fibrosis includes ARFI categories 3 and 4.
ARFI measurement of SWV (m/s) was performed during free soft breathing of the child. The region of interest (ROI) was positioned 1–2 cm from the liver capsule at a maximum depth of 8cm, in a homogeneous parenchyma that did not include vessels or surrounding structures. Ten valid measurements were taken as follows: (i) right liver lobe (RLL): the average of six measurements in segments VI, VII, and VIII (two measurements in each) was obtained; (ii) left liver lobe (LLL): the average of four measurements in segments II and III was obtained. SWVs obtained from the RLL were included in the different ARFI categories [12] according to the following cutoffs: ARFI 0 (1.90 m/s). Moreover, the ARFI categories were used as a basis to establish the following fibrosis classification: no fibrosis includes ARFI category 0; insignificant fibrosis includes ARFI categories 1 and 2; and significant fibrosis includes ARFI categories 3 and 4.
Statistical analysis
The statistical analysis was conducted with the SPSS statistical software package version 19.0 (SPSS Inc., Chicago, IL, USA). Data were expressed as mean standard deviation (SD). The quantitative variables with a normal distribution were analyzed using Student’s t test and otherwise the Mann–Whitney U test. The 2 test was also used to compare the qualitative variables. Differences among multiple means were assessed by 1-way ANOVA and post hoc test analysis. Correlations between the SWV of the right liver lobe and the different variables were analyzed with Pearson correlation coefficients. The relation between ARFI categories and HS grades was analyzed using the 2 test and contingency tables. Differences were deemed significant at P < 0.05.
The statistical analysis was conducted with the SPSS statistical software package version 19.0 (SPSS Inc., Chicago, IL, USA). Data were expressed as mean standard deviation (SD). The quantitative variables with a normal distribution were analyzed using Student’s t test and otherwise the Mann–Whitney U test. The 2 test was also used to compare the qualitative variables. Differences among multiple means were assessed by 1-way ANOVA and post hoc test analysis. Correlations between the SWV of the right liver lobe and the different variables were analyzed with Pearson correlation coefficients. The relation between ARFI categories and HS grades was analyzed using the 2 test and contingency tables. Differences were deemed significant at P < 0.05.
Results
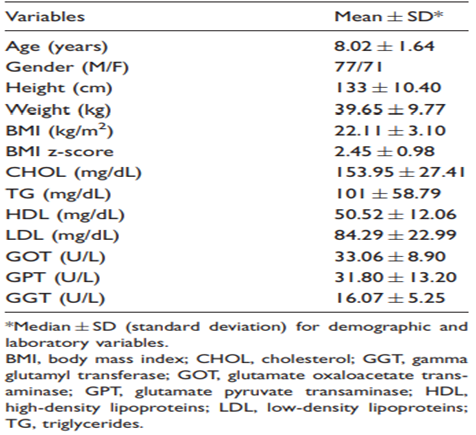
The main anthropometric and clinical features of the subjects included in the analysis are shown in Table 1. Of the 148 participating children, 95 (64.2%) were obese and 53 (35%) were overweight. A positive correlation was observed between SWV and BMI (r = 0.179; P < 0.029) and GPT (r = 0.279; P < 0.001), while no significant differences were observed between SWV and the remaining parameters evaluated in this study.
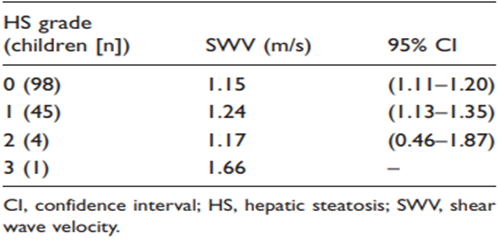
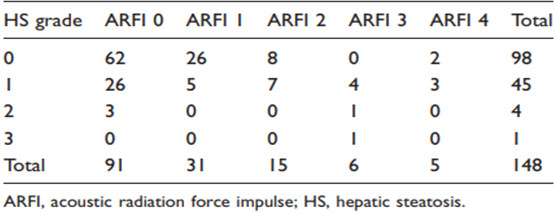
Of the total number of children (n = 148) US did not detect NAFLD in 98 (66.2%) but did observe NAFLD in 50 (33.8%). HS distribution was grade 0 in 98 (66.2%), grade 1 in 45 (30.4%), grade 2 in four (2.7%), and grade 3 in one (0.7%). Table 2 shows the relationship between HS grades and SWV, with no significant differences observed (P= 0.130). Table 3 shows the relationship between ARFI categories and HS grades, with significant differences observed (2 = 43.38, P = 0.0005).
The mean SWV of all the children (n = 148) was 1.18 0.28 m/s in the RLL and 1.46 0.25 m/s in the LLL, with significant differences (P < 0.001). Significant differences were also observed for gender (P < 0.001), with higher SWVs in girls (1.26 0.35 m/s) than in boys (1.12 0.18 m/s). The distribution of mean SWVs between ARFI categories was as follows: ARFI 0 (n = 91) 1.03 0.13 m/s; ARFI 1 (n = 31) 1.24 0.04 m/s; ARFI 2 (n = 15) 1.40 0.07 m/s; ARFI 3 (n = 6) 1.75 0.08 m/s; and ARFI 4 (n = 5) 2.21 0.28 m/s. The ARFI 0, ARFI 1, and ARFI 2 categories considered together included 76 girls and 61 boys, and the ARFI 3 (Figure 1 and 2) and ARFI 4 categories accounted for ten boys and one girl, with significant differences (X2 = 8.77, P= 0.0003). No fibrosis (ARFI 0) was observed in 91 children (61.5%), insignificant fibrosis (ARFI 1 and ARFI 2) was detected in 46 children (31%), and significant fibrosis (ARFI 3 and ARFI 4) was detected in 11 children (7.5%).
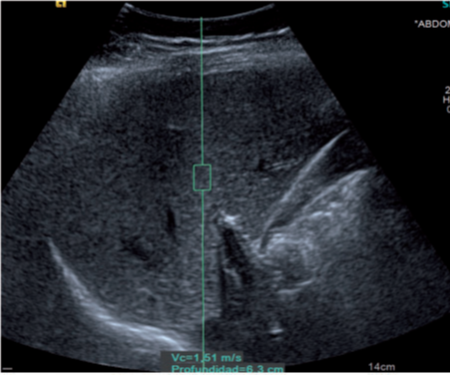
Figure 1: A 6-year-old obese boy. Ultrasonogram showing normal liver echotexture
(Grade 0 hepatic steatosis) and shear wave velocity of 1.62 m/s (ARFI category 3).
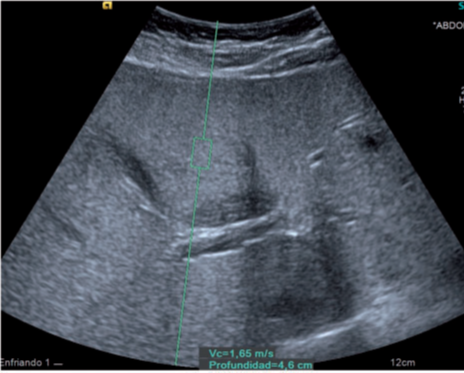
Figure 2: A 9-year-old obese boy. Ultrasonogram showing moderate increase in liver echotexture
(Grade 2 hepatic steatosis) and shear wave velocity of 1.65 m/s (ARFI category 3).
Discussion
This is the first study to use the ARFI technique for detecting LF in overweight and obese children aged 5–10 years. LB is currently considered the gold standard for assessment of hepatic fibrosis [3,15]. However, there are now non-invasive imaging methods that allow identification of steatosis and LF, such as the ARFI technique. ARFI is integrated into a conventional US system and the procedure can be performed during a routine US examination. NAFLD is increasing at alarming rates in obese children [3,4]. Childhood NAFLD has become a major common liver disease [5,6]. NAFLD is a progressive disease that encompasses a spectrum of liver diseases, ranging from simple steatosis to non-alcoholic steatohepatitis (NASH) and cirrhosis [3,4,7]. The prevalence of US-detected NAFLD is estimated at 30–60% in obese children [4,7,24]. In our study the prevalence of US-detected NAFLD was 33.8%.
NAFLD is a common liver disease worldwide and US is widely used in screening [7,24]. Moreover, US has a reasonable accuracy for detecting moderate-to-severe HS, although it is less accurate for detecting mild HS [25,26] and it cannot exclude fibrosis (6). Also, the evaluation of NAFLD by US has important interobserver variability and the reproducibility of results is limited [25,27]. However, US has a high negative predictive value for excluding NAFLD in subjects with a normal or slightly increased liver echogenicity, with an accuracy of more than 80% [24] and US examination of the liver is recommended as an initial screening procedure.
Early detection of LF is crucial for the prognosis of disease progression. However, identification of patients with fibrosis has been difficult because they can remain asymptomatic for a long time, since no single clinical or laboratory parameter can reflect the presence of LF. In the present study, the laboratory parameters were normal in all the children (n = 148). There are many studies that use the ARFI method for evaluating LF in adults [12–18]. However, few studies have been performed with the ARFI technique to assess LF in children [19–22]. The mean SWV in our study was 1.18 0.28 m/s, which is close to the upper range of 1.07–1.19 m/s reported in children with a healthy liver [28–30]. With respect to gender, the SWV values were significantly higher in girls (1.26 0.35 m/s) than in boys (1.12 0.18 m/s). Published studies are not consistent regarding the effect of gender on SWV. A few studies in adults [31–33] and a study conducted with ARFI in children [34] show no relationship to this parameter, whereas other studies using transient elastography do show an influence of sex, with lower SWV values in girls [35,36]. Further studies are needed, therefore, to be able to establish whether sex affects SWV values. Moreover, a positive correlation was also observed in our study between SWV and BMI and GPT. However, in a study of children with cystic fibrosis [37] a negative correlation was found between SWV and BMI, and no significant correlation was found with GPT.
The SWV value was also seen to be higher in the LLL than the RLL. Similar differences have been encountered in other studies, in both adults [14,15] and children [20,21]. This may be because the LLL is more liable to compression and closer to the cardiovascular system and, therefore, the heartbeat, or also because it is smaller than the right liver lobe. It is therefore suggested that the velocities be measured in different segments of the right liver lobe (no less than six measurements), although no common protocol has been established for performing the ARFI technique, which means more studies are needed to be able to unify criteria [38–40]. Using the ARFI categories (12) as a basis in the present study we detected no fibrosis or insignificant fibrosis in 92.5% of the children (n = 137) and significant fibrosis in 7.5% (n = 11).
It was observed that children with a normal liver ultrasound or mild steatosis (n = 9) had significant fibrosis. This is probably because steatosis is gradually replaced by the extracellular matrix deposition that leads to fibrosis [41]. It should also be noted that US examinations have a subjective operator-related component, which is why we do not consider this study to be sufficient as a screening method for the assessment of children with NAFLD, because US examinations of normal livers might have a certain degree of fibrosis. Moreover, as the possibility of performing a liver biopsy is not contemplated as a screening method in overweight or obese children, we consider that the combination of conventional US and ARFI technique is fundamental for detecting NAFLD and fibrosis. We also propose the following liver fibrosis classification: No Fibrosis = SWV < 1.20 m/s; Insignificant Fibrosis = SWV of 1.20–1.60 m/s; and Significant Fibrosis = SWV > 1.60 m/s.
A limitation of the present study was that the NAFLD or LF stage was not determined in our children by LB. Although, LB remains the ‘‘imperfect’’ reference standard for NAFLD diagnosis, it represents an impractical screening procedure because it is both expensive and invasive [42,43], especially in young children. We do not consider LB to be justified in children with significant fibrosis detected using the ARFI technique and with normal laboratory parameters. For these reasons, surrogate markers such as US or the ARFI technique are usually used to detect NAFLD and fibrosis. ARFI is a valuable non-invasive method, which is both safe and reproducible (12). Our hypothesis is that NFALD or fibrosis in children may be reversible with the implementation of physical activity programs.
Conclusion
In conclusion, the present study is the first to evaluate the utility of the ARFI technique for detecting LF in overweight and obese children aged 5–10 years. The results of the study show that children with normal laboratory parameters such as normal liver ultrasound or mild steatosis may present with significant LF.
References
- Loomba R., et al. “Advances in pediatric nonalcoholic fatty liver disease”. Hepatology 50.4 (2009): 1282-1293.
- Ogden CL., et al. “Prevalence of overweight and obesity in the United States, 1999-2004”. JAMA 295.13 (2006): 1549-1555.
- Palmeri ML., et al. “Noninvasive evaluation of hepatic fibrosis using acoustic radiation force-based shear stiffness in patients with nonalcoholic fatty liver disease”. Journal of Hepatology 55.3 (2011): 666-672.
- Awai HI., et al. “Evidence and recommendations for imaging liver fat in children, based on systematic review”. Clinical Gastroenterology and Hepatology 12.5 (2014): 765-773.
- Angulo P. “Nonalcoholic fatty liver disease”. The New England Journal of Medicine 346 (2002): 1221-1231.
- Berardis S and Sokal E. “Pediatric non-alcoholic fatty liver disease: an increasing public health issue”. European Journal of Pediatrics 173.2 (2014): 131-139.
- AlKhater SA. “Pediatric non-alcoholic fatty liver disease: an overview”. Obesity Reviews 16.5 (2015): 393-405.
- Barshop NJ., et al. “Epidemiology, pathogenesis and potential treatments of pediatric non-alcoholic fatty liver disease”. Alimentary Pharmacology & Therapeutics 28.1 (2008): 13-24.
- Widhalm K and Ghods E. “Nonalcoholic fatty liver disease: a challenge for pediatricians”. International Journal of Obesity 34.10 (2010): 1451-1467.
- Sarvazyan AP., et al. “Shear wave elasticity imaging: a new ultrasonic technology of medical diagnostics”. Ultrasound in Medicine & Biology 24.9 (1998): 1419-1435.
- Zhai L., et al. “An integrated indenter-ARFI imaging system for tissue stiffness quantification”. Ultrasonic Imaging 30.2 (2008): 95-111.
- Guzma´n-Aroca F., et al. “Reproducibility of shear wave velocity measurements by acoustic radiation force impulse imaging of the liver: a study in healthy volunteers”. Journal of Ultrasound in Medicine 30.7 (2011): 975-979.
- Fierbinteanu Braticevici C., et al. “Value of acoustic radiation force impulse imaging Elastography for non-invasive evaluation of patients with nonalcoholic fatty liver disease”. Ultrasound in Medicine & Biology 39.11 (2013): 1942-1950.
- Toshima T., et al. “New method for assessing liver fibrosis based on acoustic radiation force impulse: a special reference to the difference between right and left liver”. Journal of Gastroenterology 46.5 (2011): 705-711.
- Karlas T., et al. “Acoustic radiation force impulse imaging (ARFI) for non-invasive detection of fibrosis: examination standards and evaluation of interlobe differences in the healthy subjects and chronic liver disease”. Scandinavian Journal of Gastroenterology 46.12 (2011): 1458-1467.
- Friedrich-Rust M., et al. “Performance of Acoustic Radiation Force Impulse imaging for the staging of liver fibrosis: a pooled meta-analysis”. Journal of Viral Hepatitis 19.2 (2012): 212-219.
- Nierhoff J., et al. “The efficiency of acoustic radiation force impulse imaging for the staging of liver fibrosis: a met analysis”. European Radiology 23.11 (2013): 3040-3053.
- Li C., et al. “Diagnostic accuracy of real-time shear wave Elastography for staging of liver fibrosis: a meta-analysis”. Medical Science Monitor 22 (2016): 1349-1359.
- Noruegas MJ., et al. “Acoustic radiation force impulse imaging in the assessment of liver fibrosis in children”. Pediatric Radiology Journal 42.2 (2012): 201-214.
- Hanquinet S., et al. “Acoustic radiation force impulse (ARFI) Elastography for the noninvasive diagnosis of liver fibrosis in children”. Pediatric Radiology Journal 43.5 (2013): 545-551.
- Pico´ Aliaga SD., et al. “Acoustic radiation force impulse imaging Elastography is efficacious in detecting hepatic fibrosis in children”. Radiología 57.4 (2015): 314-320.
- Dillman JR., et al. “Ultrasound shear wave speed measurements correlate with liver fibrosis in children”. Pediatric Radiology 45.10 (2015): 1480-1488.
- Kim SH., et al. “Appropriateness of a donor liver with respect to macrosteatosis: application of artificial neural networks to US images–initial experience”. Radiology 234.3 (2005): 793-803.
- El-Koofy N., et al. “Ultrasonography as a non-invasive tool for detection of nonalcoholic fatty liver disease in overweight/obese Egyptian children”. European Journal of Radiology 81.11 (2012): 3120-3123.
- Strauss S., et al. “Interobserver and Interobserver variability in the sonographic assessment of fatty liver”. American Journal of Roentgenology 189.6 (2007): 320-323.
- Hernaez R., et al. “Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis”. Hepatology 54.3 (2011): 1082-1090.
- Cengiz M., et al. “Sonographic assessment of fatty liver: Interobserver and Interobserver variability”. International Journal of Clinical and Experimental Medicine 7.12 (2014): 5453-5460.
- Hanquinet S., et al. “Acoustic radiation force impulse imaging-normal values of liver stiffness in healthy children”. Pediatric Radiology 435. (2013): 539-544.
- Matos H., et al. “Acoustic radiation force impulse imaging in pediatric patients: normal liver values”. Journal of Pediatric Gastroenterology and Nutrition 59.6 (2014): 684-688.
- Fontanilla T., et al. “Normal values of liver shear wave velocity in healthy children assessed by acoustic radiation force impulse imaging using a convex probe and a linear probe”. Ultrasound in Medicine & Biology 40.3 (2014): 470-477.
- Son CY., et al. “Normal liver elasticity values using acoustic radiation force impulse imaging: a prospective study in healthy living liver and kidney donors”. Journal of Gastroenterology and Hepatology 27.1 (2012): 130-136.
- Popescu A., et al. “The mean values of liver stiffness assessed by Acoustic Radiation Force Impulse Elastography in normal subjects”. Medical Ultrasonography 13.1 (2011): 33-37.
- Horster S., et al. “Comparing acoustic radiation force impulse imaging to transient Elastography to assess liver stiffness in healthy volunteers with and without Valsalva manoeuvre”. Clinical Hemorheology and Microcirculation 46.2.3 (2010): 159-168.
- Eiler J., et al. “Standard value of ultrasound Elastography using acoustic radiation force impulse imaging (ARFI) in healthy liver tissue of children and adolescents”. Ultraschall Med 33.5 (2012): 474-479.
- Roulot D., et al. “Liver stiffness values in apparently healthy subjects: influence of gender and metabolic syndrome”. Journal of Hepatology 48.4 (2008): 606-613.
- Sirli R., et al. “Transient electrographic evaluation of subjects without known hepatic pathology: does age change the liver stiffness?” Journal of Gastrointestinal and Liver Diseases 18.1 (2009): 57-60.
- Can˜as T., et al. “Hepatic and splenic acoustic radiation force impulse shear wave velocity Elastography in children with liver disease 6 Acta Radiologica 0(0) associated with cystic fibrosis”. BioMed Research International 2015 (2015): 517369.
- Piscaglia F., et al. “Accuracy of Virtual Touch Acoustic Radiation Force Impulse (ARFI) imaging for the diagnosis of cirrhosis during liver ultrasonography”. Ultraschall Med 32.2 (2011): 167-175.
- Karlas T., et al. “Acoustic radiation force impulse imaging (ARFI) for non-invasive detection of liver fibrosis: examination standards and evaluation of interlobe differences in healthy subjects and chronic liver disease”. Scandinavian Journal of Gastroenterology 46.12 (2011): 1458-1467.
- Toshima T., et al. “New method for assessing liver fibrosis based on acoustic radiation force impulse: a special reference to the difference between right and left liver”. Journal of Gastroenterology 46.5 (2011): 705-711.
- Guzma´n-Aroca F., et al. “Detection of non-alcoholic steatohepatitis in patients with morbid obesity before bariatric surgery: preliminary evaluation with acoustic radiation force impulse imaging”. European Radiology 22 (2012): 2525-2532.
- Ratziu V., et al. “Sampling variability of liver biopsy in nonalcoholic fatty liver disease”. Gastroenterology 128.7 (2005): 1898-1906.
- Vajro P., et al. “Diagnosis of nonalcoholic fatty liver disease in children and adolescents: position paper of the ESPGHAN Hepatology Committee”. Journal of Pediatric Gastroenterology and Nutrition 54.5 (2012): 700-713.
Citation:
GL Meena., et al. “Evaluation of Hepatic Fibrosis Using Acoustic Radiation Force Impulse Among Obese Children.” Archives of
Endocrinology and Diabetes Care 2.1 (2018): 139-146.
Copyright: © 2018 GL Meena., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.