Case Report
Volume 2 Issue 2 - 2019
Parathyroid Carcinoma Initially Misdiagnosed As Mild Benign Hypercalcemia
1Juliana J Matthews, MD; Division of Endocrinology, Vanderbilt University Medical Center; Nashville, TN
2Shichun Bao, MD, PhD; Division of Endocrinology, Vanderbilt University Medical Center; Nashville, TN
3Shimon Harary, MD; Baptist Medical Group- The Endocrine Clinic; Memphis, TN
4Joyce E Johnson, MD; Division of Pathology, Vanderbilt University Medical Center; Nashville, TN
5Kathryn McCrystal Dahir, MD; Division of Endocrinology, Vanderbilt University Medical Center; Nashville, TN
2Shichun Bao, MD, PhD; Division of Endocrinology, Vanderbilt University Medical Center; Nashville, TN
3Shimon Harary, MD; Baptist Medical Group- The Endocrine Clinic; Memphis, TN
4Joyce E Johnson, MD; Division of Pathology, Vanderbilt University Medical Center; Nashville, TN
5Kathryn McCrystal Dahir, MD; Division of Endocrinology, Vanderbilt University Medical Center; Nashville, TN
*Corresponding Author: Kathryn McCrystal Dahir MD, Associate Professor of Medicine Vanderbilt University Medical Center Endocrinology and Diabetes 1313 21st Ave. South 444 Oxford House Nashville, TN 37232.
Received: January 29, 2019; Published: February 02, 2019
Abstract
Objective: To describe the case of a 36-year-old man who presented with severe hypercalcemia and hyperparathyroidism after 6 years of mild hypercalcemia and was found to have parathyroid carcinoma.
Methods: We present the clinical, laboratory, exam, and imaging findings, along with a brief review of the literature.
Results: A 36-year-old man lost to follow up six years ago after presenting with mild hypercalcemia but normal PTH presented with fatigue, weight loss, nausea, vomiting, constipation, neck pain, a right neck mass, and bilateral non-healing humerus fractures. Labs on admission revealed elevated calcium of 17.4 mg/dL, PTH 1769 pg/mL and creatinine 2.42 mg/dL. Neck CT and ultrasound showed a 2.9 x 4.4 x 5 cm mass adjacent to right inferior thyroid pole, which matched the intense uptake in Sestamibi parathyroid scan. He underwent urgent en-bloc resection with right thyroid lobectomy and parathyroidectomy. Pathology confirmed a 5 cm parathyroid carcinoma extensively invading into thyroid tissue with multifocal lymphovascular invasion.
Discussion: Primary hyperparathyroidism due to parathyroid carcinoma is rare, usually presenting with neck mass, significantly elevated calcium and PTH, and concomitant renal and bone manifestations- suspicion is especially high in those that present with hypercalcemia crisis. En-bloc surgical resection is the first-line therapy. This case highlights the importance differentiating this condition from benign causes of hypercalcemia when atypical presentations of hypercalcemia occur.
Keywords: Hypercalcemia; Hypercalcemic crisis; Hyperparathyroidism; Parathyroid carcinoma
Introduction
Parathyroid carcinoma is a rare cause of primary hyperparathyroidism, which is most commonly caused by a parathyroid adenoma. While there is overlap in the presentation of these disorders, certain features increase the likelihood of malignancy. Here we present a rare presentation of an indolent course of hypercalcemia in a young man with parathyroid carcinoma.
Case Presentation
A 36-year-old man with a history of hypercalcemia and bilateral non-healing humerus fractures from a fall off a ladder three months prior presented to the Emergency Department with progressively worsening fatigue, abdominal pain, nausea, vomiting, constipation, unintentional 70 lbs weight loss, and a right sided neck mass that developed a few weeks prior. He denied changes to medications or taking supplements, with the exception of ibuprofen which he took regularly for chronic low back pain.
The patient had a history of asymptomatic mild hypercalcemia with baseline calcium of 11.3 mg/dl (ref: 8.5 – 10.5 mg/dl) that was evaluated by an endocrinologist six years prior to presentation, when he was 30 years old. At that time, he had a normal PTH level of 40 pg/ml (ref: 10-65 pg/ml) and negative PTHrP (< 0.2 pmol/L). There was concern for familial hypocalciuric hypercalcemia (FHH), although the 24-hour urinary calcium was found to be slightly higher than expected for FHH at 189 mg/L (ref: 50-150 mg/L) with a total urine volume of 1895 ml. Calcium to creatinine clearance ratio (CCCR) was 0.016. This is less consistent with FHH, in which a CCCR of less than 0.01 is seen in approximately 80 percent of patients [1]. The patient’s CCCR was in the range expected in primary hyperparathyroidism, where CCCR is usually between 0.01 and 0.05, although most often > 0.02 [1]. As the patient also had no known family history of hypercalcemia, FHH was lower on the differential. Unfortunately, he was lost to follow up and the necessary genetic testing for CASR gene to confirm FHH was not obtained, nor was a Sestamibi parathyroid scan.
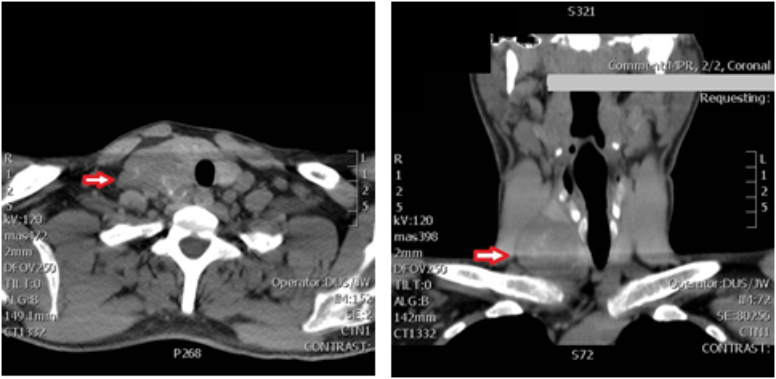
On examination in the ED the patient was alert and fully oriented. Vital signs were within normal range other than a blood pressure of 156/105. BMI was 25.6. Exam was notable for a large 4 cm right sided neck mass which was hard, fixed and nontender. Initial labs were notable for significantly elevated calcium of 17.4 mg/dl (ref: 8.5-10.2 mg/dl), PTH of 1769 pg/ml (ref: 10-65 pg/ml), and creatinine of 2.42 mg/dl (ref: 0.70-1.50 mg/dl), with low-normal 25(OH)D of 26 (ref: 25-80 ng/ml). TSH was within normal range at 0.880 mIU/L (ref: 0.40-4.0 mIU/L). CT of the neck showed a 2.9 x 4.4 x 5.0 cm mass adjacent to the right inferior thyroid pole (Figure 1). This matched a large focus of intense uptake in a Sestamibi parathyroid scan, which showed nearly complete washout from the normal tissue on delayed views; per Radiology impression, “this large right lower cervical parathyroid lesion may represent a large parathyroid adenoma versus parathyroid carcinoma” (Figure 2). X-rays of bilateral forearms showed areas of prior fractures- proximal right radial and ulnar and mid-shaft left radial and ulnar fractures were ununited and angulated, with loose hardware.
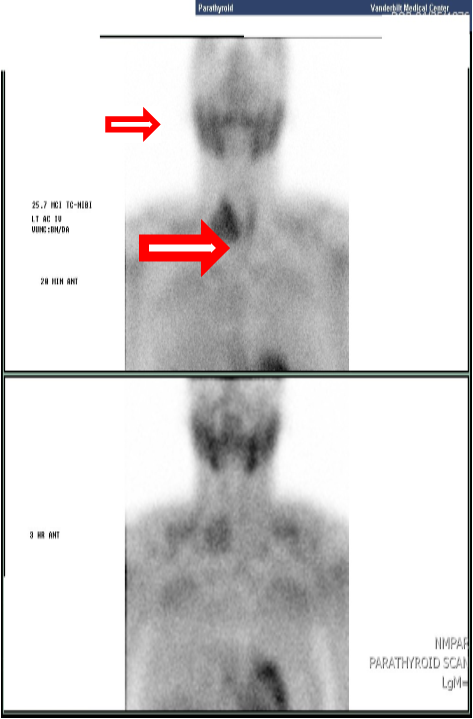
Figure 2: Sestamibi scan: large focus of intense uptake adjacent to thyroid right lobe with nearly complete washout from normal thyroid tissue.
The patient was treated with aggressive IV fluids, calcitonin, and zoledronic acid. He experienced gradual improvement in symptoms and normalization of calcium levels. Endocrine Surgery was consulted, and patient underwent an urgent en-bloc resection with right thyroid lobectomy and parathyroidectomy. Pathology revealed the tumor was 5 cm in greatest dimension and densely adherent to the adjacent thyroid lobe, which was resected en bloc. Multiple calcifications were present throughout the lesion. Microscopically, the tumor was arranged in nests and trabeculae; mitoses were frequent, and occasional foci of necrosis were present. There was multifocal capsular and vascular invasion, and the tumor invaded into the perithyroidal connective tissue. Final peripheral soft tissue margins were negative, although close (0.1 cm). (Figure 3). Final peripheral soft tissue margins and right level IV lymph nodes were negative for malignancy. Genetic testing was performed which showed no complete or partial deletion or duplication of the CDC73 (HRPT2) gene (ExonArrayDx).
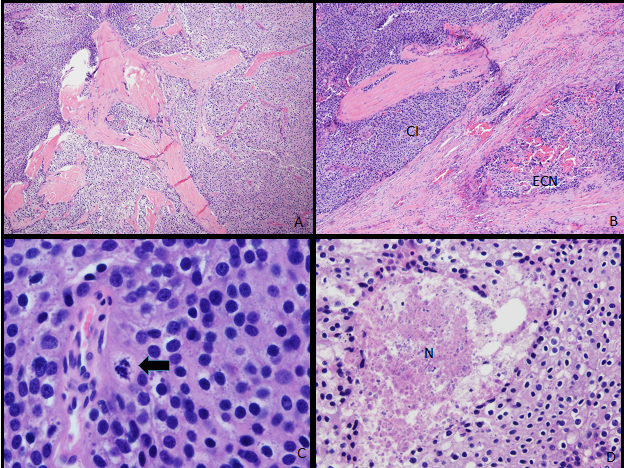
Figure 3: Pathology: Parathyroid carcinoma with lymphovascular invasion and tumor necrosis. (A) The tumor cells are arranged in nests separated by broad fibrous bands (original magnification x25). (B) Capsular invasion is at upper left (CI), with an extracapsular nodule present in adjacent perithyroidal connective tissue (ECN) (original magnification x25). (C) Mitoses (arrow) are frequent (original magnification x125). (D) Necrosis (N) is occasionally present (original magnification x42.5).
Patient’s post-operative course was complicated by hungry bone syndrome, with hypocalcaemia (Ca 7.7 mg/dl), hypophosphatemia (Phos 2.0 mg/dl) and mild hypomagnesaemia (Mg 1.7 mg/dl). He was treated with calcium and calcitriol. Post-operative PTH dropped to 13 pg/ml. At outpatient follow up, PTH continued to be monitored and was found to be increased to 409 pg/ml five months later, with normal calcium of 9.2 mg/dl and elevated creatinine of 2.68 mg/dl. Extensive imaging including ultrasound, CT, 18-F PET, bone scan, and parathyroid scan did not reveal cancer recurrence. In addition to continuing calcium and calcitriol supplementation, the patient was started on cincalcet in order to suppress PTH secretion. His PTH ultimately decreased to 124 pg/ml then 33 pg/ml after two months of cincalcet therapy, and calcium remained within normal range and cinacalcet was discontinued. On subsequent follow ups, patient has shown no evidence of cancer recurrence.
Discussion
Parathyroid carcinoma (PC) is one of the rarest known malignancies, with fewer than 1000 cases reported since its initial oncologic description in 1904 by de Quervain [2]. It is a rare cause of primary hyperparathyroidism (0.1- 5.0%), which is most commonly caused by a parathyroid adenoma [2, 3]. In this case, hyperparathyroidism was initially misdiagnosed as presumptive FHH and ultimately found to be due to parathyroid carcinoma. This rare presentation of an indolent course of hypercalcemia in a young man with parathyroid carcinoma raises questions regarding the approach in diagnosing this disease. While the diagnosis of parathyroid carcinoma can be challenging given its rarity and similar features to parathyroid adenoma and FHH, key factors can help differentiate these diseases. It is imperative to consider PC in the differential diagnosis of hyperparathyroidism as long-term survival is largely dependent of the extent of the primary surgical resection. Counseling patients on the importance of long-term follow-up, particularly in the case of a young patient or atypical presentations, is essential.
The pathogenesis of PC is not fully known, although an inactivating mutation of the HRPT2 (CDC73) tumor suppressor gene has been recognized as playing a central role [2, 4]. Studies have confirmed the presence of HRPT2 mutations in genetic syndromes such as hyperparathyroidism-jaw tumor syndrome (HPT-JT) and in sporadic parathyroid carcinoma [4]. Other than in genetic syndromes, there are no established risk factors for PC [2].
Parathyroid carcinoma can be difficult to diagnose preoperatively as it shares many clinical features with benign causes of hyperparathyroidism. PC is often only diagnosed through a combination of intraoperative recognition of the disease as well as postoperative histological examination. Still, clinical features can increase suspicion for PC versus an adenoma or FHH (Table 1).
| Presentation | Parathyroid carcinoma | Parathyroid adenoma | FHH |
| Symptomatic hypercalcemia | + | - | - |
| Decreased bone density | + | +/- | - |
| Family history of hypercalcemia | - | - | + |
| CCCR** | > 0.2 | > 0.2 | < 0.1 |
| Parathyroid levels | ^^^ | ^ | ^ |
| Average age at presentation | 44-54 | 50-60 | - |
| Sex | 1:1 | 3:1 female: male | - |
| Neck mass | + | - | - |
| Mutation associated | CDC73/ HRPT2 | MEN1 | CaSR, AP2S1, GNA11 |
*FHH = Familial hypocalciuric hypercalcemia
**CCCR = calcium: creatinine clearance ratio
Table 1: Comparing the presentation of parathyroid carcinoma, adenoma, and FHH.
**CCCR = calcium: creatinine clearance ratio
Table 1: Comparing the presentation of parathyroid carcinoma, adenoma, and FHH.
Parathyroid carcinoma should be suspected in a patient with hyperparathyroidism who presents with marked hypercalcemia, very high PTH concentrations, or a neck mass [2]. The hypercalcemic symptoms in cancer patients are often more pronounced than in those cases arising from benign parathyroid tumors. PC patients are also more likely to simultaneously present with renal and skeletal involvement, as was seen in our patient [2,7,8]. Polyuria, nephrocalcinosis, and nephrolithiasis are common renal complications. Skeletal consequences include bone pain, osteopenia, and pathologic fractures. Digestive complications include nausea, abdominal pain, peptic ulcers, and pancreatitis. Psychiatric symptoms include fatigue and depression [7].
Upon physical examination, a palpable neck mass has been reported in 30–76% of parathyroid cancer cases [6]. This is in contrast to benign parathyroid tumors which are almost always no palpable. A comprehensive exam should include further evaluation for metastases, as one-third of patients with PC have metastatic disease, most commonly in the lymph nodes, lungs, liver, and bone [2, 7].
The majority (90%) of parathyroid cancers are hormonally functional, with PTH hyper secretion [2]. Mean serum PTH concentrations may be 5 to 10 fold higher than the upper limit of normal along with severe hypercalcemia (mean serum calcium 14.6 to 15.9 mg/dl) [2]. In contrast, parathyroid adenomas and FHH usually present with mild, asymptomatic hypercalcemia found incidentally. In the rare cases of nonfunctional PC, patients can have normal calcium and PTH levels [8]. These patients typically present with a neck mass and mass-effect symptoms, including hoarseness from recurrent laryngeal nerve invasion [2, 6]. This further attests to the difficult of diagnosing PC pre-operatively, as patients may present with normal serum calcium and PTH levels [8].
Parathyroid carcinomas tend to be larger in size than benign adenomas or hyperplasia, often greater than 4 cm diameter. Although preoperative imaging studies help plan the operative approach, they do not reliably distinguish PC from adenoma. The recommended approach for surgery is to obtain at least two localizing studies, commonly a combination of a Technetium-99 Sestamibi scan and a neck ultrasound [2, 9]. CT scans of the neck may be obtained to assess for local invasion and can be helpful if Sestamibi scan is negative. CT or MRI of the neck, mediastinum, chest, and abdomen are beneficial for determination of metastatic spread [7].
The diagnosis of PC is typically made at the time of surgery performed to correct hyperparathyroidism. Diagnostic criteria rely on pathologically confirmed local invasion of contiguous structures, positive lymph nodes or distant metastases [2]. Fine needle aspiration (FNA) is not recommended prior to initial operation due to inconclusive results and risk of seeding [7]. When the diagnosis of PC is suspected preoperatively, initial surgery should include en-bloc resection of the parathyroid mass and any adjacent tissues that have been invaded by tumor, including ipsilateral thyroid lobe and isthmus, lymph nodes, and soft tissue [10,11]. Histologic features that suggest malignancy include mitotic activity, necrosis, and capsular and vascular invasion.
Surgery is the mainstay of therapy for the initial treatment of parathyroid carcinoma as well as locally recurrent or metastatic disease. Better outcomes are associated with complete resection of the tumor at the time of initial surgery, further emphasizing the importance of considering PC pre-operatively. Under-staging and under-treatment has been shown to contribute to the high recurrence rate and mortality of PC [8, 11]. En bloc resection of the carcinoma and adjacent structures in the neck has been associated with an 8% local recurrence rate and a long-term overall survival rate of 89%, while simple parathyroidectomy results in a 51% local recurrence rate and 53% long-term survival rate [11].
Although resection is the definitive treatment for the hypercalcemic crisis seen in PC, patients should be medically stabilized prior to surgical intervention. The initial treatment of hypercalcemia in patients with parathyroid carcinoma is similar to management of elevated calcium due to other causes, including aggressive hydration with intravenous fluids. Agents that block bone resorption, such as bisphosphonates and calcitonin, can be used adjunctively to stabilize calcium levels. If renal failure is present, hemodialysis may be required. Cincalcet has also been used successfully to control hypercalcemia in some reports [2, 12]. Denosumab is an option for patients who have hypercalcemia refractory to bisphosphonates and cincalcet [13].
Treatment options for inoperable patients are limited. Adjuvant radiation and chemotherapy generally have poor results and are mostly reserved for unrespectable disease [2, 12]. Recent reports have demonstrated radiofrequency ablation to be a promising option [12]. Anti-PTH immunotherapy has also shown promise with refractory disease. Biologic agents based on gene products such as parafibromin, an inhibitor of cell proliferation in parathyroid neoplasia, telomerase inhibitors such as azidothymidine, and immune therapy constitute novel emerging therapies [10]. Further research is necessary to develop nonsurgical treatments for patients who are not surgical candidates or where surgery has failed.
Persistent or recurrent disease occurs in over 50% of patients with parathyroid carcinoma, usually manifested by elevated serum calcium and PTH [2, 9]. Patients thus require lifelong surveillance with careful and frequent biochemical evaluation to differentiate between recurrence and other factors that elevate PTH, including hungry bone syndrome, renal failure, and parathyroid hormone resistance.
The prognosis of parathyroid carcinoma is variable, with reported 5-year survivals ranging between 70-85% [10]. Although it can be aggressive, PC is slow-growing neoplasm in most patients, who ultimately succumb to complications of hypercalcemia rather than from tumor burden. Its natural progression often involves a protracted clinical course plagued by disease recurrence, complications from surgery, and end-organ damage from intractable hypercalcemia [2]. For now, the completeness of the initial surgical resection plays a prominent role in achieving the best therapeutic outcome and prognosis. Long term follow-up to evaluate for worsening hypercalcemia and recurrence is key.
Conclusion
Primary hyperparathyroidism due to parathyroid carcinoma is rare, usually presenting with neck mass, significantly elevated calcium and PTH, renal insufficiency and bone disease. While the diagnosis of parathyroid carcinoma can be challenging given its rarity and similar features to parathyroid adenoma and FHH, key factors can help differentiate these diseases. En-bloc surgical resection is the first-line and best therapy. Treatment options for inoperable patients or those with recurrent disease are limited, with further research needed on nonsurgical therapies. Given high rates of recurrence, surveillance PTH monitoring is critical along with lifelong follow up with an endocrinologist.
Acknowledgements
The authors would like to acknowledge Dr. Simonds and Dr. Welch for their contribution to this report.
The authors would like to acknowledge Dr. Simonds and Dr. Welch for their contribution to this report.
Conflict of Interest
Disclosures: Kathryn Dahir is a clinical trial investigator for Alexion Pharmaceuticals and Mereo BioPharma Group and has received consultancy fees from Ultragenyx Pharmaceuticals.
Disclosures: Kathryn Dahir is a clinical trial investigator for Alexion Pharmaceuticals and Mereo BioPharma Group and has received consultancy fees from Ultragenyx Pharmaceuticals.
References
- Christensen SE., et al. “Discriminative power of three indices of renal calcium excretion for the distinction between familial hypocalciuric hypercalcaemia and primary hyperparathyroidism: a follow-up study on methods”. Clinical Endocrinology 69.5 (2008): 713-720.
- Wei CH and Harari A. “Parathyroid carcinoma: update and guidelines for management”. Current Treatment Options in Oncology 13.1 (2012): 11-23.
- Ozolins A., et al. “Evaluation of malignant parathyroid tumour in two European cohorts of patients with sporadic primary hyperparathyroidism”. Langenbeck's Archives of Surgery 401.7 (2016): 943-951.
- Cetani F., et al. “Genetic analyses of the HRPT2 gene in primary hyperparathyroidism: germline and somatic mutations in familial and sporadic parathyroid tumors”. The Journal of Clinical Endocrinology and Metabolism 89.11 (2004): 5583-5591.
- Schantz A and Castleman B. “Parathyroid carcinoma. A study of 70 cases”. Cancer 31.3 (1973): 600–605.
- Shane E. “Clinical review 122: parathyroid carcinoma”. The Journal of Clinical Endocrinology and Metabolism 86.2 (2001): 485-493.
- Givi B and Shah JP. Parathyroid Carcinoma. Clinical Oncology (Royal College of Radiologists (Great Britain)) 22.6 (2010): 498-507.
- Campenni., et al. “Parathyroid carcinoma as a challenging diagnosis: Report of three cases”. Hormones 11.3 (2012): 368-376.
- Kebebew E., et al. “Localization and reoperation results for persistent and recurrent parathyroid carcinoma”. Archives of Surgery136.8 (2001): 878-885.
- Owen RP., et al. “Parathyroid carcinoma: a review”. Head & Neck 33.3 (2011): 429-436.
- Koea JB and Shaw JH. “Parathyroid cancer: Biology and management”. Surgical Oncology 8.3 (1999): 155-165.
- Sharretts JM., et al. Parathyroid Cancer. Seminars in Oncology 37.6 (2010): 580-590.
- Vellanki P., et al. “Denosumab for Management of Parathyroid Carcinoma-Mediated Hypercalcemia”. The Journal of Clinical Endocrinology and Metabolism 99.2 (2014): 387-390.
Citation:
Kathryn McCrystal Dahir MD., et al. “Parathyroid Carcinoma Initially Misdiagnosed As Mild Benign Hypercalcemia”. Archives of Endocrinology and Diabetes Care 2.2 (2019): 187-193.
Copyright: © 2019 Kathryn McCrystal Dahir MD., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.