Research Article
Volume 1 Issue 1 - 2016
Prevalence of Antifungal-Resistance Among Fungal Agents Isolated in Poultry-Feeds from Poultry Farms in Sokoto, Nigeria.
1Department of Veterinary Microbiology, Faculty of Veterinary Medicine, Usmanu Danfodiyo University, Sokoto - Nigeria
2Department of Veterinary Anatomy, Usmanu Danfodiyo University, Sokoto, Nigeria
2Department of Veterinary Anatomy, Usmanu Danfodiyo University, Sokoto, Nigeria
*Corresponding Author: MB Abubakar, Department of Veterinary Microbiology, Faculty of Veterinary Medicine, Usmanu Danfodiyo University, Sokoto – Nigeria.
Received: October 26, 2016; Published: December 28, 2016
Abstract
The aim of this study was to determine the prevalence of fungal agents isolated from poultry feeds for antifungal-resistance. A total of 275 fungal isolates comprising of Aspergillus spp, (n = 135), Penicillium spp (n = 50), Rhizopus spp (n = 35), Fusarium spp (n = 20) and Mucor (n = 35) isolated from poultry feeds in Sokoto state, Nigeria were screened for resistance to Fluconazole, Nystatin and Voriconazole, using disk diffusion method. The result shows that 12.7% of each fungal genera were susceptible to all the antifungal agents tested. Resistance to at least one or more antifungal agent were expressed by 85.2% of the Aspergillus spp., 80% of Penicillium spp. and 75% of Fusarium spp, with 51.9%, 70% and 50% of them showing multiple drug resistance to two or more antifungal agent tested. Antifungal resistance was found to be expressed by all (100%) of Mucor and Rhizopus spp respectively, with > 70% showing multiple drug resistance. The resitance was found to be more for fluconazole than Voriconazole. Regular mycological analysis of poultry feeds shall be imposed to ensure quality and safety of poultry feeds in the study area.
Keywords: Antifungal-Resistance Fungal-agents; Multi-drug resistance; Moulds; Poultry-feeds
Introduction
The indiscriminate use of anti-fungal agents for the prevention of poultry diseases may contribute to the emergence of moulds and yeasts that are resistant to these agents (Reis., et al. 2012). Poultry feed quality is an important pre-requesite for achieving optimal production results and preservation of health condition of the birds (Abo-Shama., et al. 2015). Moulds and mycotoxin contamination of feed and feed ingredients occur worldwide, and because of the ubiquitous nature of these microorganisms they cannot be totally eliminated from feed ingredients (Trenholm., et al. 1988). Presence of mycoflora especially moulds in animal feed indicate a potential threat not only to feed quality but also to the well-being of animals that are being fed with it (Beuchat., et al. 1978).
Although infection with pathogenic yeast such as Candidaalbicans and Cryptococcus neoformans are the most common invasive and opportunistic mycotic diseases (Denning., et al. 1998), filamentous fungi such as Aspergillus fumigatus are emerging as prominent agents causing morbidity and mortality worldwide (Groll & Walsh., et al. 2001). Increase in the rate of antifungal-resitant strains of fungal agents and the rate at which diseases such as invasive aspergillosis were reported in poultry and other animals pose a public health concern. Diagnosis and treatment of diseases caused by these agents in animals and humans remain a challenge, and therefore understanding the efficacy and limitations of the available antifungal agents is essential to select the appropriate agent for treatment (Miceli & Lee., et al. 2011).
In a study of mycotic agents associated with poultry feeds in Sokoto state, a high rate of feed contaminated by fungal agents such as Aspergillus, Rhizopus, Penicillium, Fusarium and Mucor was recorded(Aliyu., et al. 2012), and some of the fungal agents involved might be resistant to commonly used antifungal agents such as triazoles. However, some moulds such as Mucor spp. and Fusarium spp. were reported to be less susceptible to these group of antifungal (Araujo., et al. 2015). These antifungal-resistant strains may infect animals, especially poultry and humans causing serious diseases that responds poorly to treatment. To the best knowledge of the authors, there is no documented report on susceptibility profiles of fungal agents isolated from poultry feeds in Sokoto and its environs. Therefore, this research is aimed at determining the susceptibility of selected fungal agents isolated from poultry feeds to three most commonly used antifungal agents in the area.
Material and Methods
Study area: The study was conducted in Sokoto metropolis and environs. Sokoto township is the capital city of Sokoto State in Nigeria. The state is the second largest livestock producing state in the country (SSIPC 2008). The state is an agric-based with most of the populace (over two million) engaged in rearing of one or more specie(s) of animals especially ruminants and poultry. Poultry production in the state is mostly local with few farmers engaged in medium/large scale production(Maikasuwa and Jabo., et al. 2011). Although commercially prepared feeds are predominantly used in the study area, few farmers compounded their feed.
Organism Tested: A total of 275 fungal isolate comprising of Aspergillusspp. (n = 135), Penicilliumspp. (n = 50), Rhizopusspp. (n = 35), Fusariumspp. (n = 20) and Mucorspp. (n = 35) were used for the sensitivity testing. These fungal agents were previously isolated and identified during a 12 month (May 2010 to April 2011) survey of fungal contaminants in poultry feeds in Sokoto and its environs. The isolates were reactivated on Sabroud dextrose agar (SDA) at room temperature for seven days (Saleemi., et al.2010). An inoculants of each fungus sample was prepared by adding 5 millilitre of sterile saline solution to the surface of cultures to harvest the spores. The suspension of each filamentous fungus was adjusted to contain 1 x 106 conidia/ml through a comparison of the turbidity with that of number six (#6) tube of the McFarland turbidity scale, and confirmed using spectrophotometer at 530 nm (Diogo., et al. 2010).
Antifungal Agents Used: Two triazole and a polyene antifungal medicaments in the form of Drug-impregnated disks were used in this study. This are Fluconazole (25 µg: CT1806B), Nystatin (100 units: CT0073B) and Voriconazole (1 µg: CT1807B) (Oxoid Ltd. Basingstoke; U.K).
Antifungal Susceptibility Testing of Fungal Isolates: The disk-diffusion technique was performed according to the procedure described in the M44-A method (Sheehan., et al. 2004). Mueller Hinton agar (Acumedia Limited; 7101A) was prepared according toinstructions from the manufacturers, and 15-20 ml aliquot dispensed in Petri dishes. The plates were then swabbed evenly with a sterile cotton-wool swab dipped into the standardized inoculum of the fungal suspension, and allowed to stay for 5-10 minutes to get absorbed. The drug-impregnated disks comprising of Fluconazole (25 µg:CT1806B), Nystatin (100 units: CT0073B) and Voriconazole (1 µg: CT1807B) (Oxoid Ltd. U.K.) were aseptically placed on the cultured plates in equidistant points and lightly pressed. The plates were incubated at room temperature (22-25°C) for 4 to 7 days to allow for fungal growth. The sensitivity to antifungal agents of each test-fungal agent was determined by measuring the diameter (mm) of the zone of inhibition, using transparent meter rule. Each isolate was classified into: sensitive or resistant based on the reported standard criteria for interpreting inhibition zones as follows; Nystatin ≤ 10 mm, Fluconazole ≤ 14 mm and Voriconazole ≤ 11 mm as adopted from (Nweze., et al. 2010) and (Diogo., et al. 2010).
Result and Discussions
The frequency of resistance to the antifungal agents tested, as exhibited by the fungal isolates obtained from poultry feed samples is shown in Table 1. Of the 275 fungal isolate tested, 35 (12.7%) were susceptible to all the antifungal agents tested, and 240 (87.3%) had expressed resistance to one or more of the agents. The resistance was observed more with Mucorand Rhizopusspp (100%) followed by Aspergillusspp118 (85.2%), and the least was found in Penicilliumspp37 (74%) respectively. Multi-drug resistance to two or more antifungal agents was observed highest among Rhizopus spp(85.7%) followed by Mucorspp (71.4%) and least was among Fusariumspp (50.0%).
| Isolates | Number of Isolates | No. Resistance | % |
| Aspergillusspp | 135 | 118 | 87.41 |
| Penecilliumspp | 50 | 37 | 74.00 |
| Rhizopusspp | 35 | 35 | 100.00 |
| Fusariumspp | 20 | 15 | 75.00 |
| Mucorspp | 35 | 35 | 100.00 |
| TOTAL | 275 | 240 | 87.27 |
Table 1: Frequency of antifungal resistance expressed by fungal isolates from commercially prepared and self-compounded poultry feeds in Sokoto town and its environs.
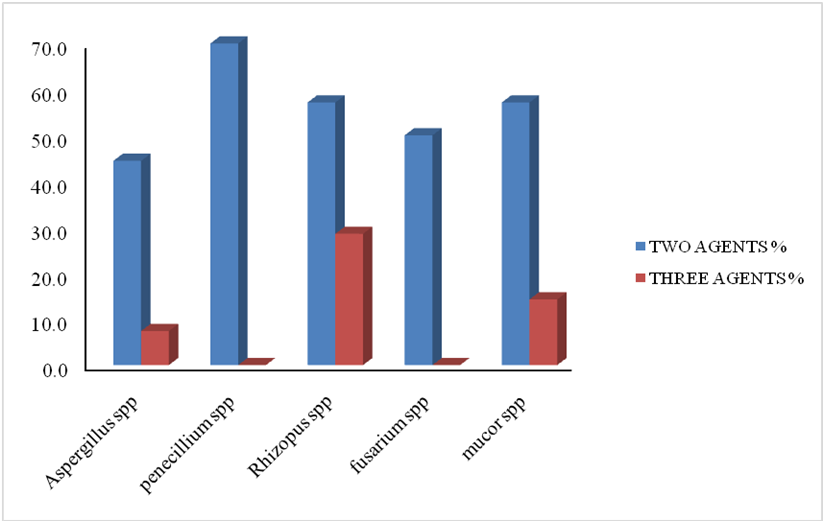
Of the 135 Aspergillusspp tested, 87.3% were resistant to at least one or more antifungal agent, with more than fifty percent (51.9%) showing multiple drug resistance. More than thirty percent were resistant to only one antifungal agent and 44.4% and 7.4% of the isolates were resistant to two and three antifungal agents respectively. Of the 20 isolates Fusariumspptested, resistant to at least one or more antifungal agents was observe in (--%), and 50.0% showed multiple drug resistance to two antifungal agents (Table 1). All 100.0% the Mucorand Rhizopusspptested expressedresistant to at least one or more antifungal agent, with more than 70% of each showing multiple drug resistance. For Penicilliumspp, 80.0% of the isolates were resistant to at least one antifungal agent, with 70.0% showing multiple drug resistance to two antifungal agents (Table 2).
| No. antifungal resisted | Fungal Isolates | ||||
| Aspergillusspp. | Fusariumspp. | Mucorspp. | Penicilliumspp. | Rhizopusspp. | |
| 1 | (n = 135) | (n = 20) | (n = 35) | (n = 50) | (n = 35) |
| 2 | 60 (44.4%) | 10 (50.0%) | 20 (57.1%) | 35 (70.0%) | 20 (57.1%) |
| 3 | 10 (7.4%) | 0 (0.0%) | 5 (14.3%) | 0 (0.0%) | 10 (28.6%) |
| Total MDR | 70 (51.9%) | 10 (50.0%) | 25 (71.4%) | 35 (70.0%) | 30 (85.7%) |
Table 2: Frequency of multidrug resistant fungal isolates in poultry feed samples collected from Sokoto and its environs.
Among the antifungal agents tested, resistance to Fluconazole was the highest as it was resisted by all (100%) of the isolatestested (Figure 1).
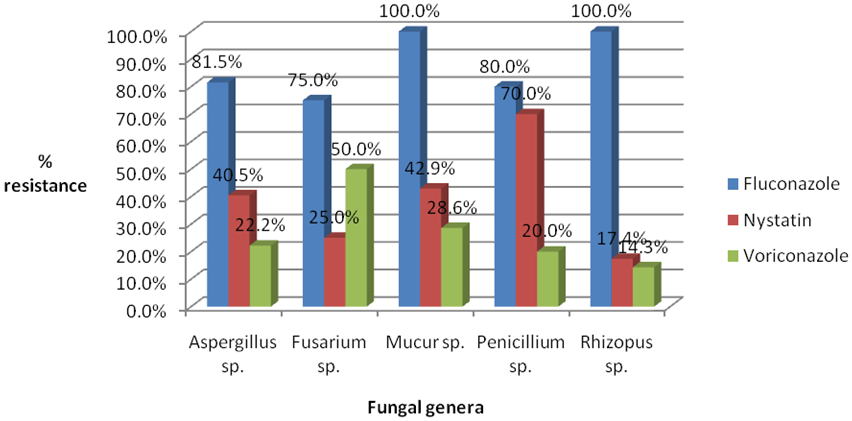
Figure 1: Frequency of antifungal resistance among fungi isolates from poultry feed samples in Sokoto metropolis and its environs.
This study evaluated the in-vitro susceptibility of commonly used antifungals against 275 filamentous fungi isolated from poultry feeds. The results indicate that high percentages of fungal organisms tested in the present study were resistant to Fluconazole (85.0%), but relatively few are resistant to Veruconazole (Figure 2). The resistance could be attributed to indiscriminate use of the drugs in the study area, in contrast to the high susceptibility to Voriconazole (76.4% of the isolates) which are not commonly used drug in both humans and animals in the study area. This finding was in agreement with that of Pfaller., et al. (2002), which reported approximate 91% susceptibility of Aspergillusspptovoriconazole, but Fusariumand Mucor spp ere relatively resistant. In the present study, some moulds such as Mucorspp. and Fusariumspp. were found to be less susceptible than those reported by Arujo., et al. (2015). In USA, the prevalence of Azole resistance in certain fungal species is estimated to be 3-6%. The resistance may be partially attributed to the use of agricultural azoles which protect crops from fungal infections (CDC, 2014). Apart from being resistance to antifungal agents, the fungal species especially Fusarium and Aspergillus may produce mycotoxins in poultry feeds.
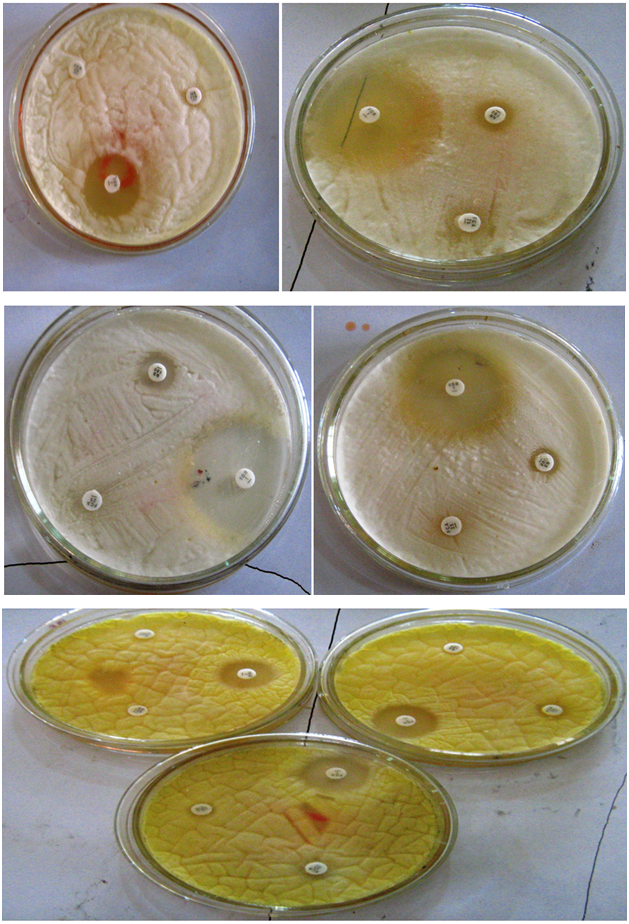
Figure 3: Selected pictures of antifungal susceptibility testing of moulds isolated from poultry feeds in Sokoto metropolis and its environs, Nigeria. The following consistency and trend can be clearly noticed from the pictures: the larger zone of inhibition around Voriconazole disk, followed by a narrow zone of inhibition around Nystatin disk and no zone of inhibition around Fluconazole disk.
Conclussion
In Conclussion, the present study highlighted on the occurrence and prevalence of antifungal-resistance phenotypes of fungal agents in poultry feeds from selected farms in the area of study. Base on the above findings, fluconazole was most resisted to by the fungi tested in contrast to their higher susceptibility to Voriconazole. The increased use of triazoles in prophylactic and treatment of animal and plant diseases in this area may lead to increase antifungal drug-resistant problem, as observed in Aspergillus species elsewhere (Arunaloke., et al. (2011). There presence is of animal health and public health concern, as they may carry the resistant-gene and transfer it to infected animals and humans.
Regular mycological analysis of poultry feeds shall be imposed to ensure quality and safety of poultry feeds in the study area. There is also the need to investigate further the possible association between antifungal resistance and mycotoxin production.
Acknowledgments
We wish to acknowledge the technical support of Mal. Lawali Kanoma and Yakubu Umar Dabai of Veterinary Microbiology laboratory, Usmanu Danfodiyo University, Sokoto.
References
- Aliyu RM., et al. “Mycological quality of commercially prepared and self compounded poultry feeds in Sokoto Metropolis, Sokoto, Nigeria”. African Journal of Microbiology Research 6.46 (2012): 7314-7318.
- Araujo R., et al. “Unpredictable susceptibility of emerging clinical moulds to tri-azoles: review of the literature and upcoming challenges for mould identification”. European Journal of Clinical Microbiology & Infectious Diseases 34.7 ( 2015): 1289-1301.
- Beuchat L. “Microbial alterations of grains, legumes, and oilseeds”. Food Technology 32.5 (1978): 193-198.
- Denning DW., et al. “An EORTC multicentre prospective survey of invasive aspergillosis in haematological patients: diagnosis and therapeutic outcome. EORTC Invasive Fungal Infections Cooperative Group”. Journal of Infection 37.2 (1998): 173–80.
- Diogo HC., et al. “Evaluation of the disk-diffusion method to determine the in vitro efficacy of terbinafine against subcutaneous and superficial mycoses agents.”. Anais Brasileiros de Dermatologia 85.3 (2010): 324-30.
- Gimeno A and Martins ML. “Mycotoxins and Mycotoxicosis in Animals and Humans”. Miami, USA: Special Nutrient, INC. 2003.
- Groll AH and Walsh TJ. “Uncommon opportunistic fungi: new nosocomial threats”. Clinical Microbiology and Infection 7.2 (2001): 8-24.
- Maikasuwa MA and Jabo MSM. “Profitability of Backyard Poultry Farming in Sokoto Metropolis, Sokoto State, North-West, Nigeria”. Nigerian Journal of Basic and Applied Sciences 19.1 (2011): 111-115.
- McNeil MM., et al. “Trends in mortality due to invasive mycotic diseases in the United States, 1980-1997”. Clinical Infectious Diseases 33.5 (2001): 641-647.
- Miceli MH and Lee SA. “Emerging moulds: epidemiological trends and antifungal resistance”. Mycoses 54.6 (2011).
- Nweze EI., et al. “Agar-Based Disk Diffusion Assay for Susceptibility Testing of Dermatophytes”. Journal of Clinical Microbiology 48.10 (2010): 3750-3752.
- Obi CN and Ozugbo IJ. “Microbiological Analyses of Poultry Feeds Sold in Umuahia Main Market, Abia State, Nigeria”. Research Journal of Applied Sciences 2.1 (2007): 22-25.
- Perfect JR and Schell WA. “The new fungal opportunists are coming”. Clinical Infectious Diseases 22.2(1996): 112-8.
- Pfaller MA., et al. “Antifungal activities of posaconazole, ravuconazole, and voriconazole compared to those of itraconazole and amphotericin B against 239 clinical isolates of Aspergillus spp. and other filamentous fungi: report from SENTRY Antimicrobial Surveillance Program, 2000”. Antimicrobial Agents and Chemotherapy 46.4 (2002): 1032-1037.
- Pfaller MA. “The epidemiology of invasive mycoses--narrowing the gap”. Clinical Infectious Diseases 27.5 (1998): 1148-50.
- Saleemi MK., et al. “Mycoflora of poultry feeds and mycotoxins producing potential of Aspergillus species”. Pakistan Journal of Botany 42.1 (2010): 427-434.
- Sheehan DJ., et al. “Method for Antifungal Disk Diffusion Susceptibility Testing of Yeasts; Approved Guideline”. (2004).
- SSIPC. “Beef up Your Profits by Investing in Sokoto State’s Livestock Sector, Sokoto”. (2008).
- Trenholm HL., et al. “Reducing Mycotoxins in Animal Feeds” Ottawa 1827E: Agriculture Canada Publication (1988).
- Zain ME. “Impact of mycotoxins on humans and animals”. Journal of Saudi Chemical Society 15.2 (2011): 129-144.
- Reis EJC., et al. “Observational study of prevalence and antifungal resistance of pathogenic yeast from Broilers”. 18th Congress of the International Society for Human and Animal Mycology. Berlin-Germany (2012).
- Chakrabarti A. “Drug resistance in fungi: an emerging Problem”. Regional health forum 15.1 (2011): 97-103.
- Abo-Sharma and U H. “The investigation of pathogenic fungi in poultry feed in some selected poultry farms in Sohag Governorate, Egypt”. Journal of Microbiology and Biotechnology Research 5.6 (2015): 1-8.
- Krnjaja, V., et al. “The frequency of pathogenic fungi genera in poultry feed”. Macedonian Journal of Animal Science 1.1 (2011): 187-19101.
- Bhanderi BB., et al. “Antifungal Drug Resistance - Concerns for Veterinarians”. Veterinary World 2.5 ( 2009): 204-207.
- CDC. Antifungal resistance (2014).
Citation:
MB Abubakar., et al. “Prevalence of Antifungal-Resistance Among Fungal agents Isolated in Poultry-Feeds from Poultry Farms in Sokoto, Nigeria”. Multidisciplinary Advances in Veterinary Science 1.1 (2016): 21-26.
Copyright: © 2016 MB Abubakar., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.





























 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.