Research Article
Volume 1 Issue 1 - 2017
Evaluation of Urine and Feces for Non-invasive Pregnancy Detection in Mouse (Mus musculus)
1Department of Comparative Medicine, University of Washington, Seattle, WA, USA
2Office of Laboratory Animal Resources, West Virginia University, Morgantown, WV, USA
2Office of Laboratory Animal Resources, West Virginia University, Morgantown, WV, USA
*Corresponding Author: Ida M Washington, DVM, PhD, DACLAM, Director and Attending Veterinarian, West Virginia University, Morgantown, WV, USA.
Received: February 23, 2017; Published: March 08, 2017
Abstract
Background: Current methods for determining pregnancy in mice during early gestation are unreliable, invasive, or require anesthesia and sophisticated equipment and training. We hypothesized that a reliable and non-invasive murine pregnancy detection method could be developed using urine or feces from the pregnant dam that would ultimately reduce unnecessary sacrifice of non-pregnant laboratory mice.
Methods: Urine and fecal samples were collected from time-mated female mice of CD-1 or C57BL/6 background strains at gestational days E0.5 to E5.5 (pre-implantation), E6.5 to E10.5 (organogenesis), E11.5 to E15.5 (early fetal), and E16.5 to E18.5 (late fetal) and compared with samples from non-pregnant controls. Urine samples were stored frozen, then assayed for protein and creatinine, and ratios of protein to creatinine were calculated to correct for urine concentration. Proteins in each sample were separated by gel electrophoresis, and resulting bands were stained with Coomassie blue. Bands were imaged and densities calculated for samples from mice at different stages of pregnancy and compared with controls. Bands were then excised and proteins identified by mass spectrometry. Fecal samples were stored frozen, then lyophilized, pulse-vortexed in ethanol, and extracts frozen until assayed. A progesterone enzyme immunoassay was optimized with fecal samples from non-pregnant mice, then used to assay fecal samples from mice at different stages of gestation compared to non-pregnant controls.
Results: Our results using urine samples showed increased urinary proteins only at the pre-implantation stage compared to non-pregnant controls. Mass spectrometry on excised bands identified major urinary proteins, centrosomal protein, and keratin, but no pregnancy-specific proteins. Our results using fecal samples demonstrated increased progesterone at all stages of pregnancy compared with non-pregnant controls, with the greatest elevation at the organogenesis stage (E6.5-E10.5).
Conclusion: We conclude that enzyme immunoassays of fecal samples for progesterone metabolites may be a promising approach for reliable and non-invasive pregnancy detection in mice.
Keywords: Pregnancy detection; Non-invasive; Mouse; Urine; Feces
Abbreviations: E (embryonic days)
Introduction
Current methods for determining pregnancy in mice during early gestation are unreliable, invasive, or require anesthesia and sophisticated imaging equipment and training. A reliable and non-invasive detection method for early murine pregnancy would have many benefits. Such a test would improve the efficiency of developmental biology research based on timed pregnancies and help satisfy the Three R’s (reduction, refinement, replacement) of laboratory animal science [1] by reducing the unnecessary sacrifice of non-pregnant laboratory mice for research purposes.
Mating in mice usually occurs during the dark cycle, followed by implantation of the early embryo in the uterine mucosa beginning at approximately 5 days of gestation [2]. The widely-studied embryonic period of organogenesis, when most organs form and are particularly sensitive to teratogens, occurs during embryonic days (E) 6.5 to E10.5 in the mouse. The first visible sign of pregnancy in the mouse is abdominal swelling that appears at approximately E11.5 to E12.5, during the early fetal period. Pregnancy duration is approximately 20 days in the mouse and varies by environment and strain [3]. If a mating does not result in pregnancy, approximately 11 days elapse until the next estrus [4]. The short gestation, large litter size, and presence of a post-partum estrus in the mouse, has allowed researchers and production facilities to breed this species relatively quickly for use in academic research.
Several approaches are currently used to indicate pregnancy in mice, including the presence of a vaginal plug [5], increased maternal body weight [6,7], and visualization of conceptuses using diagnostic ultrasound [8,9]. Each of these methods has limitations that detract from its applicability as a reliable, non-invasive, and rapid method of pregnancy detection. A solidified plug of seminal fluid is observed at the vaginal opening after mating, but the presence of a plug does not guarantee pregnancy. Conversely, a plug may be missed in an animal that is actually pregnant [5]. The ability to predict pregnancy from the presence of a vaginal plug also varies significantly with strain [6]. Maternal body weight has been used to determine pregnancy in mice after E7.0 but it is not completely reliable until approximately 10.5 days of gestation, after the period when many embryonic studies are performed. Ultrasound imaging can be used to detect pregnancy post-implantation (E6.5 and later) but requires expensive equipment and training [8,9] and requires immobilization of the dam by anesthesia, which may negatively affect the embryo [10].
Human chorionic gonadotropin is a 36.7 kDa glycoprotein hormone that is excreted in pregnant female urine and can be used to detect human pregnancy after approximately 10 days of gestation [11]. This diagnostic test has revolutionized early pregnancy detection in humans [12]. However, human chorionic gonadotropin is not present in mice, and mouse urinary proteins have not been well evaluated for their potential use in pregnancy detection.
Plasma progesterone levels in mice increase sharply in early pregnancy and remain elevated throughout gestation [13]. Fecal progesterone has been measured and used to determine reproductive status in a wide range of species [14-21], but there is little information on fecal progesterone levels in the pregnant mouse.
We hypothesized that a reliable and non-invasive murine pregnancy detection method could be developed using factor(s) excreted in the urine or feces of the pregnant dam during the early stages of gestation, before pregnancy is outwardly visible. An optimal test would produce minimal stress to the dam, yield rapid results, and ultimately reduce the unnecessary sacrifice of non-pregnant laboratory mice to improve the efficiency of developmental biology research and satisfy the Three R’s of laboratory animal science [1]. The studies described here evaluate the use of urine and fecal samples for non-invasive pregnancy detection in mice.
Material and Methods
Animals
Healthy adult female mice (Mus musculus) of CD-1 or C57BL/6 background strains were used for these studies. Mice were obtained either from approved vendors or from in-house breeding. Mice were time-mated by placing one male with 2 or 3 females overnight and checked for vaginal plugs the following morning. Plug detection was considered E0.5. All animal work was performed in accordance with Institutional Animal Care and Use Committee approved protocols at the University of Washington and Seattle Children’s Research Institute and was consistent with standards described in the Guide for the Care and Use of Laboratory Animals [22].
Healthy adult female mice (Mus musculus) of CD-1 or C57BL/6 background strains were used for these studies. Mice were obtained either from approved vendors or from in-house breeding. Mice were time-mated by placing one male with 2 or 3 females overnight and checked for vaginal plugs the following morning. Plug detection was considered E0.5. All animal work was performed in accordance with Institutional Animal Care and Use Committee approved protocols at the University of Washington and Seattle Children’s Research Institute and was consistent with standards described in the Guide for the Care and Use of Laboratory Animals [22].
Housing
All mice used in this study were housed in individually-ventilated Allentown® (Allentown, PA) or Thoren® (Hazelton, PA) cages supplied with reverse osmosis and chlorine-treated water provided in bottles or by automatic watering system (Edstrom Industries, Waterford WI). Air exposed to high-efficiency particulate filtration was circulated actively through cages at 65 to 70 changes per hour. Corncob bedding of ¼ inch diameter (Bed-O’cobs®, The Andersons Inc., Maumee OH) was added at approximately ½ inch deep to all clean cages. Rodent chow (Purina LabDiet 5053®, PharmaServ, Framingham, MA) was provided ad libitum to all animals.
All mice used in this study were housed in individually-ventilated Allentown® (Allentown, PA) or Thoren® (Hazelton, PA) cages supplied with reverse osmosis and chlorine-treated water provided in bottles or by automatic watering system (Edstrom Industries, Waterford WI). Air exposed to high-efficiency particulate filtration was circulated actively through cages at 65 to 70 changes per hour. Corncob bedding of ¼ inch diameter (Bed-O’cobs®, The Andersons Inc., Maumee OH) was added at approximately ½ inch deep to all clean cages. Rodent chow (Purina LabDiet 5053®, PharmaServ, Framingham, MA) was provided ad libitum to all animals.
Mice were housed in barrier-maintained specific pathogen-free rooms from which sentinel mouse serum was tested every three or four months by the Clinical Standard Panel® and annually by the Basic Standard Panel® (IDEXX Laboratories, Columbia, MO) and confirmed free of mouse hepatitis virus, minute virus of mice, generic parvovirus (NS1), mouse parvovirus (MPV1-5), Theiler’s murine encephalomyelitis virus, epizootic diarrhea of infant mice, Sendai virus, Mycoplasma pulmonis, pneumonia virus of mice, reovirus-3, lymphocytic choriomeningitis virus, and ectromelia virus. Feces and tape tests of sentinel mice were confirmed negative for pinworms and fur mites by in-house testing. Cages were manipulated only in vertical laminar flow change stations located within these rooms. Environmental parameters of the housing rooms were maintained at temperatures of 73 ±2°F, relative humidity of 30 to 70 percent, light cycles of 12 hours on and 12 hours off, and 12 to 15 air changes per hour.
Urine Collection
Urine samples were obtained from female mice that were not mated and not pregnant (N = 14), or at embryonic days of gestation representing pre-implantation (E0.5 to E5.5, N = 2), organogenesis (E6.5 to E10.5, N = 8), early fetal (E11.5 to E15.5, N = 7), or late fetal (E16.5 to E18.5, N = 7) stages. Pregnancy was confirmed in each test mouse by the subsequent birth of litters or euthanasia of the dam to remove embryos or fetuses for other studies. Mice with vaginal plugs that were later found not to be pregnant were not included in this study.
Urine samples were obtained from female mice that were not mated and not pregnant (N = 14), or at embryonic days of gestation representing pre-implantation (E0.5 to E5.5, N = 2), organogenesis (E6.5 to E10.5, N = 8), early fetal (E11.5 to E15.5, N = 7), or late fetal (E16.5 to E18.5, N = 7) stages. Pregnancy was confirmed in each test mouse by the subsequent birth of litters or euthanasia of the dam to remove embryos or fetuses for other studies. Mice with vaginal plugs that were later found not to be pregnant were not included in this study.
For urine collection, each mouse was manually restrained for less than 30 seconds and positioned such that the urethral opening was directly above a piece of plastic film, weigh boat, or micro-centrifuge tube. The sample volume from each collection varied from 0 to 20 µL. Samples of less than 5 µL were excluded from analysis. Samples were coded numerically and stored at -4°C until thawed for protein analysis.
Protein to Creatinine Ratios in Urine Samples
The ratio of protein to creatinine levels in urine samples was used to correct for differences in urine concentration and allow comparison of protein levels in different urine samples. Methods used were essentially those previously described [23].
The ratio of protein to creatinine levels in urine samples was used to correct for differences in urine concentration and allow comparison of protein levels in different urine samples. Methods used were essentially those previously described [23].
The concentration of protein present in each sample was determined using the DC Protein Assay® (Bio-Rad Laboratories, Hercules CA). A standard curve was generated from a stock solution of 2 mg/mL bovine serum albumin in water diluted from 0 to 2 mg/mL in duplicate. Urine samples were diluted 1:10 in water and 5 µL added to 25 µL Reagent A and 200 µL Reagent S on a 96-well plate. Each sample was assayed in duplicate. Absorbance was read at 620 nm after 15 min. The mean standard absorbance was plotted against the known protein concentrations, and a linear fit in Excel® (Microsoft, Redmond WA) was used to generate the standard curve of the line. Protein concentration was determined by solving the equation of the line for each sample.
The concentration of creatinine present in each sample was determined using the Creatinine Assay Kit® (Cayman Chemical Company, Ann Arbor MI). A standard curve was generated from a stock solution of 20 mg/dL creatinine in water diluted from 0 to 15 mg/dL in duplicate. Urine samples were diluted 1:10 in water and 15 µL added to 150 µL alkaline picrate solution on a 96-well plate. Each sample was assayed in duplicate. Initial absorbance was read at 492 nm after 10 min. Acid solution was added to all wells and incubated for 20 min on a shaker before the final absorbance was read at 492 nm. For all wells, final absorbance was subtracted from initial absorbance, and absorbance of the 0 mg/dL standard was subtracted from this adjusted absorbance. The mean standard absorbance was plotted against the known creatinine concentrations, and a linear fit in Excel® (Microsoft, Redmond WA) was used to generate the standard curve of the line. Creatinine concentration was determined by solving the equation of the line for each sample.
SDS-PAGE Gels of Urine Samples
Urine samples were adjusted by dilution to produce consistent protein to creatinine ratios for SDS-PAGE analysis. Samples were centrifuged for 4 min at 6800 relative centrifugal force. Laemmli Sample Buffer® (Sigma-Aldrich, St Louis MO) was added to the diluted sample in a 1:1 ratio to produce 20 µL total volume. Samples were simultaneously run on 4-20% Mini–Protean Precast Gels® (Bio-Rad Laboratories, Hercules CA). A PageRuler Prestained Protein Ladder® with molecular weights ranging from 10 to 170 kDa (Thermo Fisher Scientific Inc., Waltham MA) was run in the first and last lanes of each gel. Samples were randomized according to a number code to prevent bias during image analysis. Electrophoresis was run at 35 volts as samples moved through the loading gel and at 100 volts for approximately two additional hours as samples moved through the resolving gel.
Urine samples were adjusted by dilution to produce consistent protein to creatinine ratios for SDS-PAGE analysis. Samples were centrifuged for 4 min at 6800 relative centrifugal force. Laemmli Sample Buffer® (Sigma-Aldrich, St Louis MO) was added to the diluted sample in a 1:1 ratio to produce 20 µL total volume. Samples were simultaneously run on 4-20% Mini–Protean Precast Gels® (Bio-Rad Laboratories, Hercules CA). A PageRuler Prestained Protein Ladder® with molecular weights ranging from 10 to 170 kDa (Thermo Fisher Scientific Inc., Waltham MA) was run in the first and last lanes of each gel. Samples were randomized according to a number code to prevent bias during image analysis. Electrophoresis was run at 35 volts as samples moved through the loading gel and at 100 volts for approximately two additional hours as samples moved through the resolving gel.
Following electrophoresis, the gels were soaked for 1h in 500 mL protein fixing solution (50% ethanol and 10% acetic acid in water), then stored with gentle agitation overnight in gel-washing solution (50% methanol and 10% acetic acid in water). Gels were stained with Coomassie Brilliant Blue (0.4g Coomassie Brilliant Blue R350, 20% methanol, 20% acetic acid) for approximately 2.5h. Excess stain was removed by applying de-stain solution (50% methanol and 10% acetic acid in water) on a slow shaker until the background color was visibly reduced. Gel images were obtained using an AlphaImager system® (Proteinsimple, Santa Clara CA).
The density of distinct bands in each experimental sample was calculated as a ratio to the average density of the 15 kDa molecular weight standard bands on the corresponding gel. The molecular weight markers were used as standards because they contain known quantities of reference proteins. The sample numbers were then decoded and identified to allow statistical analysis and band excision for mass spectrometry.
Mass Spectrometry of Urine Samples
Representative distinct bands from six different samples were excised from SDS gels and analyzed by mass spectrometry using standard methods at the Proteomics Laboratory in the Center for Developmental Therapeutics at Seattle Children’s Research Institute. Representative band excised for analysis is demonstrated in Figure 1.
Representative distinct bands from six different samples were excised from SDS gels and analyzed by mass spectrometry using standard methods at the Proteomics Laboratory in the Center for Developmental Therapeutics at Seattle Children’s Research Institute. Representative band excised for analysis is demonstrated in Figure 1.
Fecal Collection
Fecal samples were obtained from female mice that were not mated or pregnant (N = 34), or at embryonic days of gestation representing pre-implantation (E0.5 to E5.5, N = 12), organogenesis (E6.5 to E10.5, N = 11), early fetal (E11.5 to E15.5, N = 12), or late fetal (E16.5 to E18.5, N = 9) stages. Pregnancy was confirmed in each test mouse by the subsequent birth of litters or euthanasia of the dam to remove embryos or fetuses for other studies. Mice with vaginal plugs that were later found not to be pregnant were not included in this study.
Fecal samples were obtained from female mice that were not mated or pregnant (N = 34), or at embryonic days of gestation representing pre-implantation (E0.5 to E5.5, N = 12), organogenesis (E6.5 to E10.5, N = 11), early fetal (E11.5 to E15.5, N = 12), or late fetal (E16.5 to E18.5, N = 9) stages. Pregnancy was confirmed in each test mouse by the subsequent birth of litters or euthanasia of the dam to remove embryos or fetuses for other studies. Mice with vaginal plugs that were later found not to be pregnant were not included in this study.
For fecal collection, each mouse was manually restrained for less than 30 seconds and positioned such that fecal pellets fell directly into a micro-centrifuge tube, or mice were placed in a clean cage top until feces were produced. The number of fecal pellets varied from 1 to 4 per mouse. Tubes were numerically coded to avoid investigator bias and stored at -4°C until analyzed.
Progesterone Assay of Fecal Samples
Fecal progesterone assays were performed in the laboratory of Dr Samuel Wasser in the Center for Conservation Biology at the University of Washington, Seattle.
Fecal progesterone assays were performed in the laboratory of Dr Samuel Wasser in the Center for Conservation Biology at the University of Washington, Seattle.
Fecal samples were lyophilized in a FreeZone Freeze Dry System® (Labconco, Kansas City, MO) for at least 48h to allow hormone concentrations to be expressed per gram dry weight. The freeze-dried samples were then mixed thoroughly, weighed, and pulse-vortexed in 70% ethanol for 30 min as previously described [24]. Sample extracts were then stored at -20°C prior to progesterone assay of all samples.
The Progesterone Enzyme Immunoassay Kit #K025-H5® (Arbor Assays, Ann Arbor, MI) was used to measure fecal progesterone metabolites. The broad-spectrum antibodies in this kit have been used to detect fecal progesterone metabolites in a wide range of mammalian and avian species [24]. The assay was optimized for the mouse by testing feces from 17 non-pregnant female mice to validate the parallelism and accuracy of the enzyme immunoassay and to ensure that the antibodies adequately recognized murine fecal progesterone metabolites.
Once the assay validation was complete, 78 test fecal samples from pregnant and non-pregnant mice were analyzed for progesterone content. The sample identities were then decoded and the results analyzed.
Statistical Analysis
Numerical values were analyzed for statistical significance with an ANOVA, Tukey’s honestly significant difference (HSD) test, and t-test, using a significance level of p < 0.05.
Numerical values were analyzed for statistical significance with an ANOVA, Tukey’s honestly significant difference (HSD) test, and t-test, using a significance level of p < 0.05.
Results
Urine Protein Assay
Calculated ratios of protein to creatinine varied from 1.02 to 2.86 (mean 2.17 ± 0.55 SD) in mouse urine samples collected for analysis. The predominant bands produced after separation of urine samples by gel electrophoresis appeared at approximately 20 kDa (Figure 1). Protein band densities, when calculated as ratios to the average density of the 15 kDa MW marker bands, were statistically higher (p < 0.05) in mouse urine at E0.5-E5.5 (pre-implantation) but not different from non-pregnant mouse urine at E6.5-E10.5 (organogenesis), E11.5-E15.5 (early fetal), or E16.5-E18.5 (late fetal) stages (Figure 2).
Calculated ratios of protein to creatinine varied from 1.02 to 2.86 (mean 2.17 ± 0.55 SD) in mouse urine samples collected for analysis. The predominant bands produced after separation of urine samples by gel electrophoresis appeared at approximately 20 kDa (Figure 1). Protein band densities, when calculated as ratios to the average density of the 15 kDa MW marker bands, were statistically higher (p < 0.05) in mouse urine at E0.5-E5.5 (pre-implantation) but not different from non-pregnant mouse urine at E6.5-E10.5 (organogenesis), E11.5-E15.5 (early fetal), or E16.5-E18.5 (late fetal) stages (Figure 2).
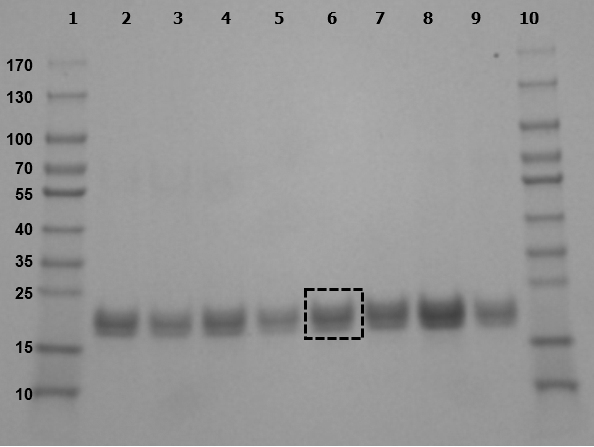
Figure 1: Representative SDS-PAGE gel of mouse urine samples.
Urine samples separated by gel electrophoresis produced bands at approximately 20 kDa.
Lanes: 1, 10: MW markers
2, 4, 5, 9: urine from non-pregnant mice
3, 6: urine from E9.5 pregnant mice
7: urine from E18.5 pregnant mouse
8: urine from E7.5 pregnant mouse
Box in lane 6 demonstrates representative band excised and analyzed by mass spectrometry.
Urine samples separated by gel electrophoresis produced bands at approximately 20 kDa.
Lanes: 1, 10: MW markers
2, 4, 5, 9: urine from non-pregnant mice
3, 6: urine from E9.5 pregnant mice
7: urine from E18.5 pregnant mouse
8: urine from E7.5 pregnant mouse
Box in lane 6 demonstrates representative band excised and analyzed by mass spectrometry.
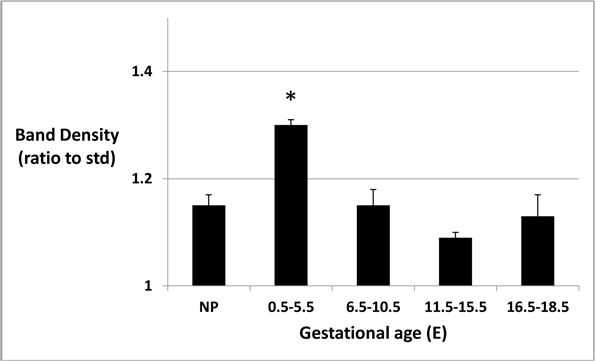
Figure 2: Urinary protein band density at different gestational ages.
Band densities, as ratios to average density of 15 kDa MW marker band, are higher at E0.5-E5.5 (p < 0.05).
Band densities, as ratios to average density of 15 kDa MW marker band, are higher at E0.5-E5.5 (p < 0.05).
Mass spectrometry was identified as a valid method to determine the identity of unknown protein bands separated by gel electrophoresis. Analysis of six representative protein bands excised from Coomassie-stained gels identified five different proteins (Table 1). The three major urinary proteins (MUP-1, -2, and -3) were the predominant constituents, with MUP-1 the most abundant. A centrosomal protein (CEP57L1) and skin protein (keratin 5) were minor constituents of these protein bands. Thus, there were apparently no pregnancy-specific proteins identified by mass spectrometry.
| Protein ID (Uniprot ID) | Annotation | Spectral counts (abundance) | Unique peptides | Sequence coverage % | False discovery rate |
| P11588 | Major urinary protein 1 | 554 | 11 | 61.1 | 0.0 |
| P11589 | Major urinary protein 2 | 147 | 3 | 16.11 | 0.0 |
| P04939 | Major urinary protein 3 | 18 | 2 | 12.5 | 0.0 |
| Q8VDS7 | Centrosomal protein CEP57L1 | 6 | 1 | 2.5 | 0.0 |
| Q32P04 | Keratin 5 | 4 | 1 | 2.24 | 0.011 |
Table 1: Proteins identified by mass spectrometry in excised bands from mouse urine samples separated by SDS-PAGE.
Fecal Progesterone Assay
Validation of the progesterone assay using mouse fecal samples was demonstrated by parallelism (Figure 3A) and accuracy (Figure 3B) of the enzyme immunoassay kit. These findings suggested good recognition of the murine fecal progesterone metabolites by the broad-spectrum progesterone antibodies used. Progesterone concentrations in fecal samples of non-pregnant mice used for optimization studies varied from 54 to 408 (mean 183.2 ± 14.5 SD) ng per gram of feces.
Validation of the progesterone assay using mouse fecal samples was demonstrated by parallelism (Figure 3A) and accuracy (Figure 3B) of the enzyme immunoassay kit. These findings suggested good recognition of the murine fecal progesterone metabolites by the broad-spectrum progesterone antibodies used. Progesterone concentrations in fecal samples of non-pregnant mice used for optimization studies varied from 54 to 408 (mean 183.2 ± 14.5 SD) ng per gram of feces.
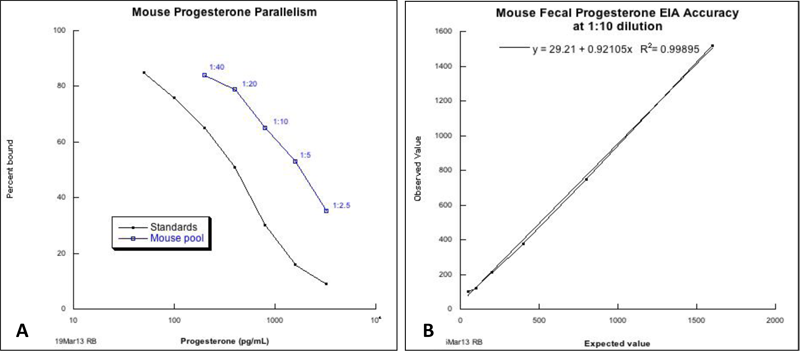
Figure 3: Validation of fecal progesterone assay.
A. Mouse progesterone parallelism
B. Mouse fecal progesterone EIA accuracy
Both assays demonstrate good recognition of murine fecal metabolites by progesterone antibodies in EIA kit.
A. Mouse progesterone parallelism
B. Mouse fecal progesterone EIA accuracy
Both assays demonstrate good recognition of murine fecal metabolites by progesterone antibodies in EIA kit.
Progesterone levels detected by enzyme immunoassay varied from 89 to 1599 ng per gram of feces in the 78 fecal samples collected for analysis. Mean (± SEM) progesterone concentration (ng per gram feces) was lowest (233.2 ± 17.7) in non-pregnant mouse feces and highest (724.7 ± 139.6) in feces from pregnant mice at E6.5 to E10.5 (Figure 4). An analysis of variance demonstrated overall significance at P < 0.0001. Tukey’s HSD and t-tests showed statistically elevated (P < 0.05) fecal progesterone in pregnant mice at E0.5-E5.5 (pre-implantation), E6.5-E10.5 (organogenesis), and E11.5-E15.5 (early fetal) compared to non-pregnant mouse feces, while progesterone concentration was higher at E16.5-E18.5 (late fetal) compared to non-pregnant mice by t-test only (p < 0.05).
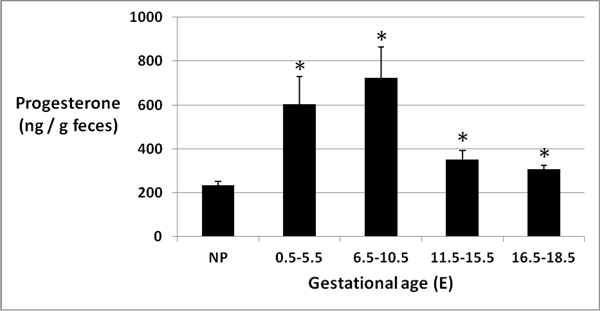
Figure 4: Fecal progesterone at different gestational ages
Mean (± SEM) progesterone concentration (ng/g feces) was highest in feces from pregnant mice at E6.5-E10.5 (p < 0.05).
Mean (± SEM) progesterone concentration (ng/g feces) was highest in feces from pregnant mice at E6.5-E10.5 (p < 0.05).
Discussion
This project was undertaken to explore the possibility of developing a reliable and non-invasive murine pregnancy detection method using factor(s) excreted in urine or feces of the pregnant dam during the early stages of gestation. Such a test would benefit developmental biology researchers and reduce the unnecessary sacrifice of non-pregnant laboratory mice during early gestation and before pregnancy is reliably detected or outwardly visible. Our results suggest that mouse urine may not provide pregnancy-specific proteins at readily-detectable levels, whereas fecal progesterone, as determined by enzyme immunoassay, may provide a viable early pregnancy detection method in the mouse.
Current methods of pregnancy detection in mice are generally unreliable, causing potential wastage of non-pregnant mice, or invasive, adding stress and its potential effect on the pregnancy. Most researchers use the presence of a vaginal (or copulation) plug to indicate that mating has occurred and to aid with pregnancy timing. This plug forms in the female vagina from the coagulation of male vesicular and coagulating gland secretions after mating [5]. The day of plug detection is considered either embryonic day 0.5 or 1, depending on researcher convention and the time of detection. The presence of a plug does not guarantee that fertilization has occurred or that pregnancy is sustained, resulting in a rate of false positive identification of pregnant mice that varies considerably by strain [6]. Failure to find a plug may result in false negative identification of pregnancy if the plug was incompletely formed or coagulated, if it formed too deep in the vagina to be easily detected, or if it dislodged from the vagina prior to checking [5].
Maternal body weight of mice, measured before mating and 1 week or later after mating, has been shown to indicate pregnancy more consistently than plug detection in two genetically engineered strains [6]. However, this method cannot be used effectively at gestational days less than 7.0 and is only minimally reliable at 7.5 to 10.5 days of gestation [IMW, personal observation], which is during organogenesis when many embryonic studies are performed. There is also potential variation in weight gain according to the age of the dam, number of previous pregnancies, and number of embryos or fetuses in utero.
Ultrasonography is an effective method to detect the presence of gestational sacs in pregnant mice at implantation and later and can be used for morphometric analysis of late embryonic and fetal development [8,9]. However, this method involves expensive equipment that requires a significant investment of resources and training. In addition, the anesthesia required to immobilize the dam for adequate imaging may negatively impact fetal development [10].
We initially sought to identify pregnancy-specific urinary protein(s) in female mice, similar to human chorionic gonadotropin present in human urine, which could lead to a rapid urine-based assay for early pregnancy detection. Urinary proteins in mice are primarily identified as major urinary proteins (MUPs), which are known to function in communication and scent marking in mice and are produced at three to four-fold higher concentrations in adult male mice than in adult female mice [25]. Previous studies have identified pregnancy-associated proteins in mouse plasma, including the 21 kDa early pregnancy factor present in early gestation [26]; the 70 kDa alpha-fetoprotein present in mid- to late-gestation [27]; and the 140 kDa pregnancy-associated murine protein present at E10-12 [28]. The detection of these proteins in plasma requires blood collection and thus invasive methods for assay. It was our hope that one or more of these proteins are excreted and detectable in the urine, although pregnancy-specific proteins in mouse urine have not previously been reported.
The degree of urine concentration varies between individual mice and with environmental conditions. The amount of urinary protein varies with urine concentration, whereas creatinine, which is a by-product of protein metabolism, is excreted at a constant rate [23]. Thus, the ratio of protein to creatinine is standardly used to correct for differences in urine concentration in order to compare protein levels in different urine samples. The ratios of protein to creatinine identified in this study (mean of 2.17) were similar to those previously reported [23].
The protein bands we identified by SDS-PAGE separation and analyzed by mass spectrometry were primarily the major urinary proteins present in all mouse urine of both sexes. These proteins are more abundant in the male, and it is unclear why these proteins are increased in the pregnant female at the earliest stages of pregnancy. Their presence may reflect residual male urine in the female urogenital tract but is unlikely specific to pregnancy.
The results of this work suggest that a pregnancy-specific urinary protein, if present, is not produced in sufficient concentration in the urine to be detected by Coomassie staining of SDS-PAGE gels. In addition, acquiring urine samples in mice proved to be more challenging than we anticipated, suggesting that samples could easily be missed. The variation in urine concentration between samples also makes assay development more complicated, even if a candidate protein is identified in the future.
Plasma progesterone is increased in early murine pregnancy [13] and it has been identified in the urine of pregnant mice [29]. However, progesterone metabolites are primarily excreted in the feces of mice [30]. We therefore sought to measure fecal progesterone levels at different stages of pregnancy and compare these with levels in non-pregnant feces. Fecal progesterone metabolites have been evaluated by radioimmunoassay and found to be elevated in ovulating hamsters [14]. Fecal progesterone has also been measured and used to determine reproductive status in a wide variety of species, including the baboon, elephant, wolf, sun bear, sea otter, right whale, and elk [15-21], but there is little information on fecal progesterone levels in the pregnant mouse.
Our results demonstrate a significant elevation in fecal progesterone in mice at all stages of pregnancy tested compared to non-pregnant mice using the enzyme immunoassay method, with highest progesterone levels during the organogenesis stage (E6.5 to E10.5). This finding makes the fecal progesterone assay a viable method of pregnancy detection, because the organogenesis stage is a major focus of developmental biology research and represents a stage of gestation before the mouse is visibly pregnant.
Conclusion
Our results thus suggest that enzyme immunoassays of fecal samples for progesterone metabolites may be a promising approach for a reliable and non-invasive pregnancy detection method in mice. Fecal pellets are easier to obtain than urine samples in a mouse, and as few as one single fecal pellet is sufficient to detect progesterone levels by an enzyme immunoassay. Future work will focus on creating an assay that is readily usable by researchers for cage-side pregnancy detection, with the ultimate goal of satisfying the Three R’s by reducing the unnecessary sacrifice of non-pregnant laboratory mice.
Acknowledgements
Technical assistance provided by staff in the Office of Animal Care, CIBR, and Proteomics Lab at Seattle Children’s Research Institute, and the Transgenic Core and Wasser lab at the University of Washington, is much appreciated. This project was funded by an ACLAM Foundation Award to IMW.
Conflict of interest
The authors have no financial interest or other conflict of interest.
The authors have no financial interest or other conflict of interest.
References
- Russell WMS and Burch RL. “The Principles of Humane Experimental Technique”. Methuen London (1959).
- Carson DD., et al. “Embryo implantation”. Developmental Biology 223 (2000): 217-237.
- Murray SA., et al. “Mouse gestation length is genetically determined”. Plos One 5.8 (2010): 12418.
- Dewar AD. “Observations on pseudopregnancy in the mouse”. Journal of Endocrinology 18.2 (1959): 186-190.
- Suckow MA., et al. “The Laboratory Mouse”. CRC: Boca Raton FL (2001).
- Mader SL., et al. “Refining timed pregnancies in two strains of genetically engineered mice”. Lab Animal 38.9 (2009): 305-310.
- Onojafe FI. “Vaginal mucous plug and weight gain in mice”. Tech Talk 10 (2005): 4.
- Brown SD., et al. “Ultrasound diagnosis of mouse pregnancy and gestational staging”. Comp Med 56.4 (2006): 262-271.
- Mu JW., et al. “In vivo quantification of embryonic and placental growth during gestation in mice using micro-ultrasound”. Reproductive Biology and Endocrinology 6 (2008): 34-51.
- Warren JR., et al. “Inhibition of preimplantation mouse embryo development by isoflurane”. American Journal of Obstetrics & Gynecology166.2 (1992): 693–698.
- Wilcox AJ., et al. “Time of implantation of the conceptus and loss of pregnancy”. The New England Journal of Medicine 340.23 (1999): 1796-1799.
- Cole LA. “New discoveries on the biology and detection of human chorionic gonadotropin”. Reproductive Biology and Endocrinology7 (2009): 8-85.
- Murr SM., et al. “Plasma progesterone during pregnancy in the mouse”. Endocrinology94.4 (1974): 1209-12111.
- Chelini MOM., et al. “Quantification of fecal estradiol and progesterone metabolites in Syrian hamsters (Mesocricetus auratus)”. Brazilian Journal of Medical and Biological Research 38.11 (2005):1711-1717.
- Frederick C., et al. “Methods of estrus detection and correlates of the reproductive cycle in the sun bear (Helarctos malayanus)”. Theriogenology 74.7 (2010): 1121-1135.
- Larson S., et al. “Noninvasive reproductive steroid hormone estimates from fecal samples of captive female sea otters (Enhydra lutris)”. General and Comparative Endocrinology 134.1 (2003): 18-25.
- Rolland RM., et al. “Assessing reproductive status of right whales (Eubalaena glacialis) using fecal hormone metabolites.” General and Comparative Endocrinology 142.3 (2005): 308-317.
- Wasser SK., et al. “Excretion rates and metabolites of oestradiol and progesterone in baboon (Papio cynocephalus cynocephalus) faeces”. Journal of Reproduction & Infertility 101.1 (1994): 213-220.
- Wasser SK., et al. “Using Fecal Steroids to Evaluate Reproductive Function in Female Maned Wolves”. The Journal of Wildlife Management59.4 (1995): 889-894.
- Wasser SK., et al. “Excretory fate of estradiol and progesterone in the African elephant (Loxodonta africana) and patterns of fecal steroid concentrations throughout the estrous cycle”. General and Comparative Endocrinology102.2 (1996): 255-262.
- White PJ., et al. “Diagnosing pregnancy in free-ranging elk using fecal steroid metabolites”. Journal of Wildlife Diseases31.4 (1995): 514-522.
- National Research Council. Guide for the Care and Use of Laboratory Animals. National Academy Press: Washington DC (2011).
- Cheetham SA., et al. “Limited variation in the major urinary proteins of laboratory mice”. Physiology & Behavior96.2 (2009): 253-261.
- Wasser SK., et al. “Non-invasive measurement of thyroid hormone in feces of a diverse array of avian and mammalian species”. General and Comparative Endocrinology 168.1 (2010): 1-7.
- Beynon RJ and Hurst JL. “Urinary proteins and the modulation of chemical scents in mice and rats”. Peptides 25.9 (2004): 1553-1563.
- Morton H. “Early pregnancy factor: an extracellular chaperonin 10 homologue”. Immunology and Cell Biology 76.6 (1998): 483-496.
- Anderson LLI., et al. “Maternal plasma levels of alpha-fetoprotein during oestrous cycles and pregnancy in mice”. Animal Reproduction 23 (1990): 157-167.
- Hau J., et al. “Studies of pregnancy-associated murine serum proteins”. Journal of Reproduction & Infertility 54.2 (1978): 239-243.
- deCatanzaro D., et al. “Enzymeimmunoassay of oestradiol, testosterone and progesterone in urine samples from female mice before and after insemination”. Reproduction 126.3 (2003): 407-414.
- Busso JM and Ruiz RD. “Excretion of Steroid Hormones in Rodents: An Overview on Species Differences for New Biomedical Animal Research Models”. Contemporary Aspects of Endocrinology Chapter 16, (2011).
Citation:
Vivian R Pauley and Ida M Washington. “Evaluation of Urine and Feces for Non-invasive Pregnancy Detection in Mouse (Mus musculus)”. Multidisciplinary Advances in Veterinary Science 1.1 (2017): 31-40.
Copyright: © 2017 Vivian R Pauley and Ida M Washington. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.





























 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.