Research Article
Volume 1 Issue 2 - 2017
A Crossectional Survey of Shoats G.I.T. Parasites and Lungworm in Lemu Bilbilo District of Arsi Zone Oromia Regional State, Ethiopia
Assela Regional Animal Health Diagnostic Laboratory, Assela, Oromiya, Ethiopia
*Corresponding Author: Belachew Tesfaye, Assela Regional Animal Health Diagnostic Laboratory, Assela, Oromiya, Ethiopia.
Received: March 16, 2017; Published: June 01, 2017
Abstract
The present study was conducted in Lemu bilbilo district in October 2015/16 with the objectives to determine the prevalence of GIT parasites and similarly to identify the species of lungworms involved and assess possible risk factors of GIT and lungworm parasites in sheep. For this purpose 645 fecal samples for GIT and 355 for lungworm parasites were examined for the presence of these worms. The overall prevalence of GIT parasites was 66.8% (431/645), while overall lungworm prevalence was 48.5% (172/355) in the study area. Animal species, sex, body condition, age, treatment status and months of the study period were identified as risk factors for the occurrence of stated parasites. From GIT parasites Strongyle groups 31.9% (206/645), Strongyloid 8.4% (54/645), Ascaris 12.7% (82/645), Trichuris 4.8% (31/645),Fasciola 5.3% (34/645), Paraphistomum 4.3% (28/645), Monezia 5.9% (38/645), Coccidia 33.8% (218/645) and concerning Lungworms Dictyocaulus filaria 29.9% (106/355), Muellerius capillaris 37.7% (134/355), Protostrongylus rufescens 9% (32/355) were identified during this study.
The findings of the current study suggested that there is high prevalence of GIT and lungworm infestations in the studied area were stake holders should think for the proper control and prevention of GIT and lungworm parasites like application of repeated de-worming.
Keywords: Lungworm; Gastro intestinal Tract (GIT); Risk factors
Abbreviations: GIT: Gastro intestinal Tract; CSA: Central Statistical Agency; ILCA: International Livestock Center for Africa; m.a.s.l: meters above sea levels; EPG: egg per gram
Introduction
In Ethiopia there are about 25.9 million sheep and 21.9 million goats 1 (CSA, 2010) which play important role in the rural economy and enable the country to earn substantial amount of foreign currency through export of skins and other by products.
Of the total sheep population, 75% are raised in highlands with altitudes above 1,500 meter above sea level. The rest, 25%, are reared in the lowlands. Sheep require minimal inputs and maintenance costs to live in various conditions, from desert to humid rainforest (Gatenby, 1991; Alemu and Merkel, 2008).
Small ruminants are important contributors to food production in Ethiopia, providing 33% of meat and 14% of milk consumption. In the central highlands of Ethiopia where mixed crop livestock production system is practiced, small ruminants account for 40% of cash income and 19% of the house hold meat consumption (Zelalem, A and I.C. Fletcher, 1993). Sheep and goats contribute a quarter of the domestic meat consumption; about half of the domestic wool requirement; 40% of fresh skins and 92% of the value of semi - proceed skin and hide export trade. It is estimated that 1, 078, 000 sheep and 1, 128, 000 goats are used in Ethiopia for domestic consumption annually. There is also a growing export market for sheep and goats meat in the Middle Eastern Gulf States and some African countries. At optimum off take rates, Ethiopia can export 700, 000 sheep and 2 million goats annually and at the same time supply, 1, 078, 000 sheep and 1, 128, 000 goats for the domestic market (Alemu., et al. 2008). Hence, an increase in small ruminants production could contribute to the attainment of food self sufficiency in the country particularly in response to protein requirement for the growing human population as well as to enhance the export earnings (Teferi M, 2000).
Endoparasites, including lungworms, are the major cause of death and morbidity in the Ethiopian highlands. Up to half of all sheep deaths and morbidity on farms in Ethiopian highlands are caused by pneumonia and endoparasites (ILCA, 1990). Endoparasites, including Dictyocaulus filaria, are major causes of death and morbidity (ILCA, 1990). Disease alone accounts for mortality of 30% in lambs and 20% in adults (Demelash., et al. 2006). A loss of US $81.8 million is reported annually due to helminthes parasite. Furthermore, they render animals more susceptible to other infections (Demelash., et al. 2006).
Prevention and control of these parasites are, therefore, critical to enhance the economic benefit from these species of livestock. However, the incidence of parasitic diseases including respiratory helminthosis varies greatly from place to place depending on the relative importance of factors (Alemu., et al. 2008). Therefore, the objectives of the present study was to determine the prevalence of GIT and lungworm parasites in sheep, identify some of the determinant risk factors involved in infection, to recommend a feasible and sound control scheme for the study area and identify the involved species of lungworms and groups of GIT parasites.
Materials and Methods
Study Area
The study was conducted in the highland altitudes of Lemu bilbilo district of Arsi zone, southeastern part of Ethiopia which is located at 231 Kms. from Addis Ababa and latitudes of about 039016.340N and longitudes of about 07’33.131E as well as at an elevation ranging from 2755-2878 meters above sea levels. The area receives the annual mean minimum and maximum rainfall of about 800 and 1400 mms respectively, which comes from the long and short rainy seasons. The annual mean minimum and maximum temperature is 18°C and 28°C respectively. The small ruminant populations in this area were 291,285 sheep and 28, 864 goats.
The study was conducted in the highland altitudes of Lemu bilbilo district of Arsi zone, southeastern part of Ethiopia which is located at 231 Kms. from Addis Ababa and latitudes of about 039016.340N and longitudes of about 07’33.131E as well as at an elevation ranging from 2755-2878 meters above sea levels. The area receives the annual mean minimum and maximum rainfall of about 800 and 1400 mms respectively, which comes from the long and short rainy seasons. The annual mean minimum and maximum temperature is 18°C and 28°C respectively. The small ruminant populations in this area were 291,285 sheep and 28, 864 goats.
Four PAs (Lemu dima, Bekoji negeso, Sirbo and Inkolo gerjela) having altitudes 2755-2878 m.a.s.l. were randomly selected.
Study Animals and Management
The study animals were indigenous breeds of sheep raised in the highland areas of Lemu bilbilo district. The animals in the study area were kept under extensive traditional management system and maintained in small house hold flocks of mixed age for subsistence and small scale private farms.
The study animals were indigenous breeds of sheep raised in the highland areas of Lemu bilbilo district. The animals in the study area were kept under extensive traditional management system and maintained in small house hold flocks of mixed age for subsistence and small scale private farms.
Study Design and Sample Size Determination
A cross sectional study was conducted to determine the prevalence of Shoats GIT parasite & lung worm infection in the highland areas of Lemu bilbilo district. Study animals were selected based on simple random sampling technique.
A cross sectional study was conducted to determine the prevalence of Shoats GIT parasite & lung worm infection in the highland areas of Lemu bilbilo district. Study animals were selected based on simple random sampling technique.
The sample size was determined using the formula given by Thrusfield (2007) as follows:
Where, N = required sample size, Pexp = expected prevalence, d2 = desired absolute precision
As there was no previous estimated p r e v a l e n c e o f GIT and l u n g w o r m parasites o f s m a l l ruminants in the studied areas and to get the maximum sample size, 50% was considered as expected prevalence at 5% desired absolute precision. Hence the minimum sample size required for the current study was 384, but increased the sample size to 645 for GIT parasites to account for the effect of randomness and representativeness in sampling and 355 samples for lungworms were sampled.
Fecal Sample Collection and Examination
Fecal samples were collected directly from the rectum of each study animals. For GIT parasites collected samples were put into universal bottles, labeled and kept preserved by 2% potassium dichromate prior to transportation to the Asela regional veterinary laboratory where it was immediately examined or kept preserved not to be hatched before processing. The sedimentation and floatation technique as described by Soulsby, (1986)was used to detect the presence of eggs of liver Trematodes (Fasciola), Nematodes, Monezia and Oocysts of Coccidia in the samples. In this study the saturated floatation solution used was NaCl (sodium chloride). Positive fecal samples were subjected to McMaster egg counting technique and the degree of infection was categorized based on Soulsby, (1986). The animals were then categorized as lightly, moderately and severely infected according to their egg per gram of faces (EPG) counts.
Fecal samples were collected directly from the rectum of each study animals. For GIT parasites collected samples were put into universal bottles, labeled and kept preserved by 2% potassium dichromate prior to transportation to the Asela regional veterinary laboratory where it was immediately examined or kept preserved not to be hatched before processing. The sedimentation and floatation technique as described by Soulsby, (1986)was used to detect the presence of eggs of liver Trematodes (Fasciola), Nematodes, Monezia and Oocysts of Coccidia in the samples. In this study the saturated floatation solution used was NaCl (sodium chloride). Positive fecal samples were subjected to McMaster egg counting technique and the degree of infection was categorized based on Soulsby, (1986). The animals were then categorized as lightly, moderately and severely infected according to their egg per gram of faces (EPG) counts.
Egg counts from 50-799, 800-1200 and over 1200 eggs per gram of feces were considered as light, moderate and severe infection respectively (Soulsby, 1982; Urquhart., et al. 1996).
For lungworm parasites examination, collected faeces were kept in screw capped universal bottles and taken to Bekoji veterinary clinics’. Examination for the presence of larvae 1 was conducted using modified Baerman method. About 10 grams of fresh faeces was taken and wrapped with gauzes, fixed on a string-rod on the conical flask and submerged in a conical flask filled with warm water of about 45°c which covers about ¾ of the fecal samples. Then the sample was kept overnight undisturbed for about 24 hours. Then the supernatant was discarded and the samples left at the bottom was mixed well and poured into the watching glass under the stereomicroscope and examined under 10x and 40x magnification powers for species identification lungworms (Taylor., et al. 2007). The L1 of Dictyocaulus filaria is larger in size, brown in color due to food granules in their intestinal cells with cranial cuticular knob and blunt tail, while L1 of Muellerius capillaris is smaller in size, whitish in color with “S” shaped tip and dorsal spine and L1 of Protostrongylus is also whitish in color smaller in size with “S” shaped tip that is similar with L1 of Muellerius capillaries but without dorsal spine.
Statistical Analysis
Proportions of results were calculated
Proportions of results were calculated
Results
The overall prevalence of GITP at the highlands of Lemu bilbilo district was 66.8% (431/645), and GIT parasites infections in descending order of their occurrence were, Stongyle 31.9% (206/645), Strongyloid 8.4% (54/645), Ascaris 12.7% (82/645), Trichuris 4.8% (31/645), Fasciola 5.3% (34/645), Paraphistomum 4.3% (28/645), Monezia 5.9% (38/645), Coccidia 33.8% (218/645). Similarelly prevalence of lungworm parasite was 48.5% (233/355), while lungworm parasite species infections in descending order of their occurrence was D. filaria 29.9% (106/355), M. capillaries 37.7% (134/355)and P. rufescens 9% (32/355)among the total 355 sheep examined. As shown in table 2.
The results of quantitative faecal examination using the McMaster technique for GIT nematodes of 645 infected sheep was 67/645(10.4 %), 179/645(27.8%) and 399/645 (61.8%), for heavy, moderate and light infections, respectively. More of the infected sheep had a faecal egg count in a range of 500-800 e.p.g and more (Table 1).
| Intensity of infection | Examined no of Samples (%) | e.p.g |
| Light | 399/645 (61.8%) | 500-800 |
| Moderate | 179/645(27.8%) | 801-1200 |
| Heavy | 67/645 (10.4%) | More than 1200 |
| Total | 645 (100%) |
Table 1: Intensity of infection based on egg per gram count of examined
positive animals for nematode according to Hansen and Perry (1994).
| Nematodes | Fasciola | Paraphistomum | Monezia | Coccidia | Overall GIT & Lungworm parasite +ve prevalence | |||||||
| Stongyle | Strongyloid | Ascaris | Trichuris | Species of lungworm | ||||||||
| D, filaria | M. capillaries | p. rufescens | Overall Lungworm +ve prevalence | |||||||||
| 31.9% (206/645) | 8.4% (54/645) | 12.7% (82/645) | 4.8% (31/645) | 29.9% (106/355) | 37.7% (134/355) | 9% (32/35) | 48.5% (172/355) | 5.3% (34/645) | 4.3% (28/645) | 5.9% (38/645) | 33.8% (218/645) | 66.8% (431/645) |
Table 2: Prevalence of GIT and lungworm parasites in Lemu bilbilo district.
Discussions
The overall November month prevalence of shoats GIT parasites in the highlands of Lemu bilbilo district wss 66.8% (431/645), while Coccidia 33.8% (218/645), Stongyle 31.9% (206/645), Ascaris 12.7% (82/645), Strongyloid 8.4% (54/645), Monezia 5.9% (38/645), Fasciola 5.3% (34/645), Trichuris 4.8% (31/645), Paraphistomum 4.3% (28/6450.
Also prevalence of lungworm was 48.5% (233/355), while M. capillaries 37.7% (134/355), D. filaria 29.9% (106/355), and P. rufescens 9% (32/355) among the total 355 sheep examined. The current overall lungworm infestation rate finding was relatively similar with those of other researchers worked in different parts of Ethiopia and the prevalence’s reported by Alemu., et al. 2008, in North West Ethiopia, Mezgebu, M. 1995, in Addis Ababa and Mihreteab, B. and A. Aman, 2010 in Tiyo district who reported prevalence of 53.6%, 48% and 57.1% respectively, while Netsanet, B., 1992, in Debrebirhan reported high prevalence of 73.25%.
The differences in the prevalence of the above studies might be associated with differences in the egg/larval detection methods, the difference in the study areas which favors the survival of the egg/larvae of the GIT/lung worm or the snail intermediate host in case of P. rufescens and the different sample sizes used by the researchers. It might also be associated with nutritional status, level of immunity, management practice of the animal (in our case there was deworming intervention practice in the area before the study), rain fall, humidity, temperature differences and season of examination in the respective study areas.
Acknowledgements
First and foremost, I like to thank the heavenly father God for giving my strength and patience during my all life and study program. I would like to express my deep sincerely appreciation and thanks to Dr. Henok Ayalew, for his intellectual guidance, close supervision and devotion of time in criticizing this paper.
First and foremost, I like to thank the heavenly father God for giving my strength and patience during my all life and study program. I would like to express my deep sincerely appreciation and thanks to Dr. Henok Ayalew, for his intellectual guidance, close supervision and devotion of time in criticizing this paper.
Conflict of Interest
I declare that this paper presents the work carried out by myself and does not incorporate with the acknowledgement of any material and finance
I declare that this paper presents the work carried out by myself and does not incorporate with the acknowledgement of any material and finance
References
- Abebe W and Essays G. “Survey of ovine and Caprine gastrointestinal Helminthosis in eastern part of Ethiopia during the dry season of the year”. Revue de Médecine Vétérinaire 152.5 (2001): 379-385.
- Alemu Y and Merkel RC. “Sheep and Goat Production Handbook for Ethiopia”. International Development United States Agency (2008): pp: 3.
- Central Statistical Agency of Ethiopia (CSA) (2010) Livestock and livestock characteristics. In: agricultural sample survey 2009/10 [2002 E.C. (Private peasant holdings), volume 3 bulletin No. 468 Addis Ababa, Ethiopia.
- Demolish Yilma J and Hassen C. “Ovine helminthosis is major health constraints to productivity of sheep in Ethiopia”. Faculty of Veterinary Medicine, Awassa University, Awassa, Ethiopia. (2006): pp: 65-90.
- Gatenby RM (The tropical agriculturalist). “Sheep (Tropical Agriculture Series)” London: Macmillan Education (1991) pp: 154.
- Hansen J and Perry B. “The Epidemiology, Diagnosis and Control of Helminth parasites of Small Ruminants”. A handbook, 2nd Ed. ILRAD (International Laboratory for Research on Animal Diseases), Nairobi, Kenya (1994): pp: 83.
- International Livestock Center for Africa (ILCA). “Proceeding the research planning work shop on resistance to endoparasites in small ruminants”. Annual report, ILCA. Addis Ababa, Ethiopia (1990).
- Kusiluka L and Kambarage DD. “Diseases caused by helminthes”. In: A Hand Book of Diseases of small ruminants. Common diseases of Sheep and Goats in Sub-Saharan Africa, Overseas development administration animal health programme (1996): pp: 8-24.
- Mezgebu M. “Study on bovine fascioliasis and lung worm infection in Addis Ababa”. (1995).
- Mihreteab B and A Aman. “Ovine Lungworms in Tiyo District, South-East Ethiopia: Prevalence, Effect of Altitude and Major Host Related Risk Factors”. Global Veterinaria 7.3 (2010): 219-225.
- Netsanet B. “Study on the prevalence and control of lung worms Dictyocaulus and Muellerius) in local Ethiopia high land sheep in and around Debre Birhan”. DVM Thesis, Addis Ababa University, Debrezeit, Ethiopia (1992).
- Regassa F., et al. “Epidemiology of Gastrointestinal Parasites of Ruminant in Western Oromia, Ethiopia”. The International Journal of Applied Research in Veterinary Medicine 4.1 (2006): 7-11.
- Soulsby EJ. “Helminthes, arthropods and protozoa of domestic animals 7th Edition”. London: Bailliers Tindall (1986): pp.: 247-250.
- Teferi M. “Epidemiological study on ovine pasteurollosis in Arsi, south east Ethiopia Debre Zeit”. Faculty of Veterinary Medicine, Addis Ababa University, DVM Thesis (2000).
- Temesgen T. “Study on prevalence of ovine gastrointestinal parasite in and around Bedele”. DVM Thesis, HU, FVM, Haramaya, Ethiopia (2008).
- Thrusfield M. “Veterinary Epidemiology 3nd Edition”, Black well science Ltd. United kingdom (2007): pp: 182-198.
- Tigist T. “Gastrointestinal parasitosis of small ruminants in and around Debre Zeit”. DVM thesis, HU, FVM, Haramaya, Ethiopia (2008).
- Zelalem A and IC Fletcher. “Small Ruminant Productivity in Ethiopia Mixed Farming System”. In Proceeding of 4th National Livestock Improvement Conference 13-15 November, IAR Addis Ababa, Ethiopia (1993) pp: 193-196.
Citation:
Belachew Tesfaye and Teshome Gunse. “A Crossectional Survey of Shoats G.I.T. Parasites and Lungworm in Lemu Bilbilo District
of Arsi Zone Oromia Regional State, Ethiopia”. Multidisciplinary Advances in Veterinary Science 1.2 (2017): 68-72.
Copyright: © 2017 Belachew Tesfaye and Teshome Gunse. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.





























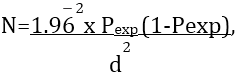
 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.