Research Article
Volume 1 Issue 3 - 2017
Comparison of Virulence Factors and Genetic Relationships of Enterococcus Faecalis Strains Isolated from Clinical, Food and Poultry Samples
1Programa de Pós-Graduação em Microbiologia Agrícola e do Ambiente, Universidade Federal do Rio Grande do Sul, Porto Alegre, RS, Brasil
2Departamento de Ciências Básicas da Saúde, Microbiologia, Universidade Federal de Ciências da Saúde de Porto Alegre, Porto Alegre, RS, Brasil
3Instituto de Ciências Básicas da Saúde, Departamento de Microbiologia, Universidade Federal do Rio Grande do Sul, Porto Alegre, RS, Brasil
4Curso de Medicina Veterinária, Sociedade Educacional de Santa Catarina, Florianópolis, SC, Brasil
2Departamento de Ciências Básicas da Saúde, Microbiologia, Universidade Federal de Ciências da Saúde de Porto Alegre, Porto Alegre, RS, Brasil
3Instituto de Ciências Básicas da Saúde, Departamento de Microbiologia, Universidade Federal do Rio Grande do Sul, Porto Alegre, RS, Brasil
4Curso de Medicina Veterinária, Sociedade Educacional de Santa Catarina, Florianópolis, SC, Brasil
*Corresponding Author: Camila O. Arent, Curso de Medicina Veterinária, Sociedade Educacional de Santa Catarina, Florianópolis, SC, Brasil.
Received: August 17, 2017; Published: September 16, 2017
Abstract
Enterococcus faecalis do not only inhabit the intestines of many animals, but also food and the environment. These microorganisms have intrinsic ability which enables them to persist in different environments. The aims of our study were (i) to carry out a comparative analysis of tetracycline resistance and virulence factor genes of Enterococcus faecalis isolates from food, poultry and clinical samples and (ii) to determine the genetic relationships of these factors among these isolates.
A total of 182 E. faecalis were studied; 70, 52, and 60 strains were isolated from clinical samples, broiler cloacal swabs and food, respectively. Enterococcus faecalis isolates were submitted to research genes for virulence factors tet (L), tet (M), (bop ABCD, ace and agg) by PCR and grouped into clusters according to their genotype. The prevalence among all the genes studied could be considered high, ranging from 61.5 to 99.4% of the virulence factors of genes and 19.2 to 70.3% of the antimicrobial resistance genes, tet (L) and tet (M), respectively, where it was possible to obtain different genetic profiles. The enterococci isolated from food, humans and broiler cloacal swabs showed high genetic diversity, although some strains seemed to be closely related. The 182 isolates formed twelve different clusters independent of the origin of the samples or the diets used in the feeding of broilers, with the similarity index value ranging from 0.16 to 1.0, similarity coefficient, 0.70. In conclusion, enterococci isolated from food, humans and broiler cloacal swabs are genetically different. In addition, the analysis of virulence factors genes and tet genes by PCR proved to be an effective methodology for determining the microbial diversity of Enterococcus faecalis isolates of different environmental sources.
Keywords: Enterococcus faecalis; Antimicrobial resistance; Virulence traits; Diversity
Introduction
Enterococcuscomprises a group of Gram-positive bacteria, which have fewer requirements for growth, to be able to grow at temperatures from 10 to 45°C, pH 9.6 in 6.5% saline and surviving 60°C for 30 minutes. Due to their ability to grow and survive in harsh environmental conditions, enterococci can be found in many different environments, such as the gastrointestinal tract of humans and warm-blooded animals (Nallaparedy., et al. 2000), soil, liquid surfaces and plants or vegetables (Riboldi., et al. 2008; Cassenego., et al. 2011; Castillo-Rojas., et al. 2013). However, the performance of enterococcal species as the etiologic agent of human infections is known.
In recent years, some species have acquired greater importance in nosocomial frames as opportunistic pathogens (Giraffa, 2002). The main species of enterococci that because human infections are Enterococcus faecalis (80 to 90%) and Enterococcus faecium (5 to 15%). In these species associated with the virulence of bacterial pathogenicity and virulence factors was gender (Mannu., et al. 2003; Qin., et al. 2000; Riboldi., et al. 2008; Semedo., et al. 2003).
One bacterium to be pathogenic, it must essentially cling to tissues, invade them, multiply and survive the host defense mechanisms and other bacteria in competition, producing tissue damage. Among the virulence factors most frequently cited in the literature in strains of E. faecalis are the production of aggregation substance (agg), the surface adhesins (ace) and biofilm formation (bop ABCD operon) (Duprè., et al. 2003; Hufnagel., et al. 2004; Lebreton., et al. 2005). These genes, alone or in combination, may play an important role in cell adhesion and biofilm formation (Donlan and Costerton, 2012). Besides this characteristic virulence, one reason for the increase of enterococci infections is related to its ability to develop resistance to a wide variety of antimicrobials.
Enterococcus faecalis reservoirs and vehicles of antibiotic resistance are known and many studies have dealt with over the distribution of antimicrobial resistance genes in strains isolated from enteric habitat, and food samples collected at various stages of the food chain (Rice and Carias, 1998, Aarestrup., et al. 2000; Hummel., et al. 2007; Frazzon., et al. 2009). The tetracycline resistance phenotype is a highly prevalent among enterococci, and resistant to many classes of antimicrobials such genes have been identified. Different genetic mechanisms are responsible for resistance to tetracycline, the most studied are the ribosome protection encoded by tet (M) gene and efflux systems encoded by the tet (L) gene (Clewell., et al. 1995; Huys., et al. 2004; Hummel., et al. 2007).
Several techniques can be performed to determine phylogenetic group in enterococcus, such as Pulsed-field Gel Electrophoresis (PFGE) (Jackson., et al. 2012), random amplified polymorphic DNA (RAPD) (Rossetti and Giraffa, 2007), multilocus sequence type (MLST) (Castillo-Rojas., et al. 2013). PFGE is the “gold standard” technique, but it is costly and time-consuming.
The epidemiological importance of Enterococcus faecalis is associated with the fact that species not only provide intrinsic susceptibility to multiple antimicrobials, but also the presence of virulence factors. This study aimed to evaluate the distribution and diversity of virulence factors in E. faecalis isolates from broilers cloacal swabs, foods of various origins and clinical specimens from infected patients.
Material and Methods
Origin of strains of Enterococcus faecalis
A total of 182 E. faecalis were studied; 70 were isolated from broiler cloacal swabs in 2009, 52 clinical samples such as blood, urine and body secretions collected during the years 2005 to 2009 and 60 isolated from food samples, collected from vegetables, dairy and meat during the years 2006 to 2007 (Riboldi., et al. 2008; Frazzon., et al. 2009, Cassenego., et al. 2011). The isolates were selected from bacterioteca Department of Microbiology (ICBS/UFRGS) and Gram-positive Cocos laboratory UFCSPA. All isolates were confirmed at genus and specie by the technique of polymerase chain reaction (PCR). The primers sequences of tuf and dll genes are shown in Table 1.
A total of 182 E. faecalis were studied; 70 were isolated from broiler cloacal swabs in 2009, 52 clinical samples such as blood, urine and body secretions collected during the years 2005 to 2009 and 60 isolated from food samples, collected from vegetables, dairy and meat during the years 2006 to 2007 (Riboldi., et al. 2008; Frazzon., et al. 2009, Cassenego., et al. 2011). The isolates were selected from bacterioteca Department of Microbiology (ICBS/UFRGS) and Gram-positive Cocos laboratory UFCSPA. All isolates were confirmed at genus and specie by the technique of polymerase chain reaction (PCR). The primers sequences of tuf and dll genes are shown in Table 1.
| Virulence genes | Nucleotide sequence (5’-3’) | Amplicon size (pb)* | Annealing temperature (°C) | Reference |
| tuf | TACTGACAAACCATTCATGATG AACTTCGTCACCAACGCGAAC |
112 | 54 | (Koch et al., 2004) |
| ddl | CACCTGAAGAAACAGGC | 475 | 52 | (d’Azevedo., et al. 2006) |
| ATGGCTACTTCAATTTCACG | ||||
| agg | AAGAAAAAGTAGACCAAC | 1553 | 54 | (Duprè., et al. 2003) |
| AACGGCAAGACAAGTAAATA | ||||
| ace | AAAGTAGAATTAGATCACAC | 320 | 52 | (Eaton and Gasson, 2001) |
| TCTATCACATTCGGTTGCG | ||||
| bopA | CAGCGACATGGACAGCCTAC | 108 | 60 | (Cassenego., et al. 2013) |
| TTGCAGGACCGTCGAGTAAA | ||||
| bopB | ATGACAGAATCCAAAACTGC | 687 | 56 | (Cassenego., et al. 2013) |
| TTACGAAGGGGTTGATTCAC | ||||
| bopC | TTATAGAAGGTTAAATTGAT | 1010 | 48 | (Cassenego., et al. 2013) |
| ATGAAGGATAATCGTATCAC | ||||
| bopD | GGCTTCCTCGTTGATGGCTTC | 126 | 66 | (Hufnagel., et al. 2004) |
| ACGGCACGGAATTTGGGTAAAC | ||||
| tet(M) | GTTAAATAGTGTTCTTGGAG | 406 | 54 | (Frazzon., et al. 2009) |
| CTAAGATATGGCTCTAACAA | ||||
| tet(L) | ACTCGTAATGGTGTAGTTGC | 625 | 58 | (Frazzon., et al. 2009) |
| TGTAACTCCGATGTTTAACACG |
*base pairs
Table 1: Oligonucleotide primers used in the PCR reactions.
Table 1: Oligonucleotide primers used in the PCR reactions.
Total DNA extraction, amplification of virulence genes by PCR and phylogenetic grouping
Strains were grown on BHI liquid medium at 35°C for 24h. Total DNA was extracted using the protocol described by Cassenego., et al. (2011). The presence of bopABCD operon agg, ace tet M) and tet (L) genes was performed by PCR (Frazzon., et al. 2009; Eaton and Gasson, 2001; Duprè., et al. 2003;Cassenego., et al. 2013). The primers sequences and their annealing temperatures are shown in Table 1.
Strains were grown on BHI liquid medium at 35°C for 24h. Total DNA was extracted using the protocol described by Cassenego., et al. (2011). The presence of bopABCD operon agg, ace tet M) and tet (L) genes was performed by PCR (Frazzon., et al. 2009; Eaton and Gasson, 2001; Duprè., et al. 2003;Cassenego., et al. 2013). The primers sequences and their annealing temperatures are shown in Table 1.
The PCR was carried out in a total volume of 25 μL containing: 200 μM of each dNTP, 0.4 μM of each primer, 1.5 mM MgCl2, 1X buffer supplied with Taq polymerase, 1.25 U of Taq DNA polymerase (Gibco BRL, France) and 100 ng of template DNA. PCR was performed with an Omnigene DNA thermal cycler (Hybaid, UK). Cycles used were as follows: 1 cycle at 94°C for 4 min; 40 cycles at 94°C for 1 min, at temperature annealing (see table 1) for 1 min, at 72°C for 2 min; 1 cycle at 72°C for 15 min. Phylogenetic grouping was done based on the presence or absence of the virulence factors genes.
Statistical Analysis
The results were subjected to statistical analysis using the program Paleontological statistics software package for education and data analysis (PAST) version 2.17ce followed normality. The dendrograms was performed using the Jaccard similarity coefficient.
The results were subjected to statistical analysis using the program Paleontological statistics software package for education and data analysis (PAST) version 2.17ce followed normality. The dendrograms was performed using the Jaccard similarity coefficient.
Results
Frequency of genes of virulence factors and antibiotic resistance among samples
To evaluate the distribution of virulence factors genes, 182 E. faecalis strains isolated from humans, food and animals in South Brazil between 2005 and 2009 were chosen. In Table 2, the results for the prevalence of the genes of the virulence factors in all isolates studied.
To evaluate the distribution of virulence factors genes, 182 E. faecalis strains isolated from humans, food and animals in South Brazil between 2005 and 2009 were chosen. In Table 2, the results for the prevalence of the genes of the virulence factors in all isolates studied.
| Percentual of PCR positive | ||||
| Genes | Chicken | Clinical | Food | Total |
| ace | 94.2 | 75 | 86.6 | 86.2 |
| agg | 78.5 | 57.7 | 45 | 61.5 |
| bopA | 98.5 | 96.1 | 90 | 95 |
| bopB | 85.7 | 88.4 | 95 | 89.5 |
| bopC | 98.5 | 96.1 | 95 | 96.7 |
| bopD | 100 | 100 | 98.3 | 99.4 |
| tet(L) | 18.5 | 15.4 | 23.3 | 19.2 |
| tet(M) | 87.1 | 57.7 | 61.6 | 70.3 |
*base pairs
Table 2: Prevalence of virulence and antimicrobial resistance among Enterococcus faecalis isolates from food and clinical samples broilers factors.
Table 2: Prevalence of virulence and antimicrobial resistance among Enterococcus faecalis isolates from food and clinical samples broilers factors.
Enterococcus faecalis isolated from broiler cloacal swabs showed the elevated number of virulence genes compared to food and clinical isolates. In this study, the ace gene was detected in 94.2% and 86.6% of isolates from broiler cloacal swabs and food, respectively, and 75% of the clinical isolates. The agg gene was more frequently detected among isolates from broiler cloacal swabs (78.5%), followed by clinical (57.7%) and food (45%) isolates. The bopA gene was detected more frequently (98.5%) in broiler cloacal isolates, followed immediately by clinical (96.1%) and food (90%) isolates. Like bopA gene, bopC gene was also more prevalent among broiler cloacal swabs (98.5%). The tet (M) gene, in turn, was found in 87.1% of samples from cloacal swabs chicken and 61.6% food and 57.7% of the clinical samples.
Isolates presenting the bopB gene, comprising 89.5% of the total, with the food isolates (95%), then humans and broilers sample, 88.4% and 85.7%, respectively. The bopD gene showed very similar frequencies (98.3% - 100%) among the isolates. Among the genes evaluated in this study, the tet (L) gene showed the lower frequencies among the isolates (23.3 to 15.4%).
Regarding the genes of the operon bopABCD, the bopD gene showed the highest frequency (99.4%) among the isolates, followed immediately by bopC (96.7%), bopA (95%) and bopB (89.5%).
Phylogenetic E. faecalis divergence of virulence and resitant genes content
Phylogenetic grouping was done based on the PCR method using primers targeted at virulence factors genes, bopABCD operon agg, ace tet (M) and tet (L). The phylogenetic tree was constructed from the analysis of the presence of the genes of the virulence factors of isolated from various environments to evaluate the genetic variability of isolates (Figure 1). A total of 182 E. faecalis strains isolated from humans, broilers and food were assigned to four phylogenetic groups (i.e. A, B, C and D) and four subgroups (i.e. A1, A2, B1 and B2).
Phylogenetic grouping was done based on the PCR method using primers targeted at virulence factors genes, bopABCD operon agg, ace tet (M) and tet (L). The phylogenetic tree was constructed from the analysis of the presence of the genes of the virulence factors of isolated from various environments to evaluate the genetic variability of isolates (Figure 1). A total of 182 E. faecalis strains isolated from humans, broilers and food were assigned to four phylogenetic groups (i.e. A, B, C and D) and four subgroups (i.e. A1, A2, B1 and B2).
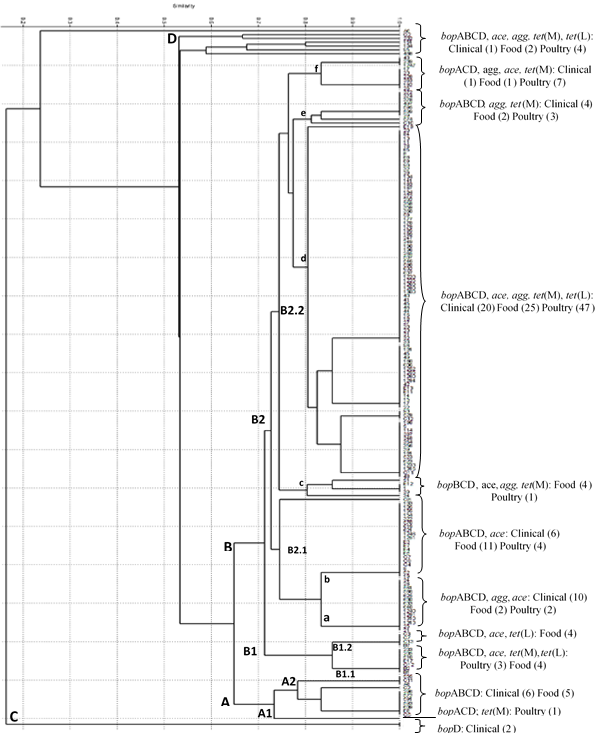
Figure 1: Dendrogram based on results of PCR with primers for genes of virulence
and antimicrobial resistance factors. The similarity was calculated using
the correlation coefficient of Jaccard and clusters were built with PAST.
Groups A and B showed 65% similarity and comprised 173 of the 182 isolates tested. The group B contained most of the collected isolates (161 isolates, 87.36%), followed by group a (12 isolates, 6.59%). Nine isolates (4.94%) were not grouped in clusters described above, because they had similarity coefficients above 0.16 to 0.52. These isolates were grouped into two groups to facilitate the understanding, being named C and D, respectively.
The group A was divided into two subgroups (A1 and A2) with a similarity coefficient above 0.70, indicating genetic proximity. In A1, was included only one E. faecalis isolate of broiler cloaca swabs that was positive to all genes from the bopACD operon and tet (M) gene. The subgroup A2 comprised strains with the complete bop operon and six isolates from clinical (blood and urine) and five food (dairy, vegetables and meat) isolates belonged to this subgroup.
The differences between the strains of cluster B generated two subgroups, the B1 and B2 with similarity coefficients 0.73, indicating genetic proximity and suggesting that these isolates could represent a genetic pool of E. faecalis strains. The B1 was subdivided into two, generating B1.1 and B1.2 subgroups with similarity coefficients 1.0 each other.
The B1.1 was composed by 7 isolates (3 cloacal swabs from chickens and 4 different foods such as meat, dairy and vegetables) which were positive form all bop operon genes, ace, tet (L) and tet (M) genes. In the B1.2 were grouped isolates that showed the bopABCD, ace and tet (L) genes. The four strains of B1.2 were isolated from vegetables such as potatoes and sweet potatoes in 2006.
The majority of collected isolates (150 isolates, 82.41%) were grouped in group B2. In this group are 67 of 70 isolates from broiler cloacal swabs. Isolates in this group had three or four genes of the operon bop, the gene tet (L), tet (M), besides the ace or agg genes, according to the classification of subgroups. The B2 group was divided into two subgroups with 75% similarity each the B2.1 and B2.2.
The B2.1 was divided again in “a” and “b”, and included 35 isolates. The subtype a (bopABCD, agg and, ace genes) contains 14 isolates (10 clinical isolates from urine, two food isolates from chicken carcass in 2007 and two broilers isolated in 2008). The subtype b comprises isolates (6 clinical, 11 from chicken carcass and dairy isolates in 2006 and 2007 and 4 broilers cloacal) positive to bopABCD and ace genes.
In the B2.2 subgroup was pooled 115 isolates and divided into subtypes “c”, “d”, “e” and “f” with similarity coefficients 0.8. In subtype "c" contains four isolates of cheese and one isolate from cloacal swabs chickens. These strains contain virulence genes bopBCD, ace, agg, tet (M). The subtype "e" grouped four isolates of clinical origin, two isolates from food and three cloacal swabs and your present genes are bopABCD, agg, tet(M). The subtype “d” is the biggest group of the phylogenetic tree and contains 92 isolates, where 25 are isolated from food samples, 20 clinical and 47 are most isolated of chicken’s cloacal swabs and has all the genes studied. The subtype “e” contain the genes bopABCD, agg, tet(M) with four clinical isolates, two isolates from food and three of poultry (cloacal swabs), while the subtype “f” grouped one clinical isolate, one isolate from food and seven poultry isolates from swabs cloacal which contains the genes bopACD, agg, ace, tet(M). Group C contains two isolates of clinical origin (urine) and group D, showed seven isolates, three swabs of chickens, two food (cheese) and two clinical origin (urine) and were more distant from the other isolates (similarity coefficients 0.53).
Discussion
Frequency of genes of virulence factors between samples
Frequencies of virulence genes between the samples are consistent to those observed in other studies. McBride., et al. (2007) demonstrated that the virulence factors can be found in various strains of E. faecalis.
Frequencies of virulence genes between the samples are consistent to those observed in other studies. McBride., et al. (2007) demonstrated that the virulence factors can be found in various strains of E. faecalis.
The prevalence of the agg gene among isolates from food and clinical in the present study agree with those observed by Dupont., et al. (2008), Bittencourt and Suzart (2004) and Eaton and Gasson (2001). The agg gene was detected at a high frequency between samples of E. faecalis isolated from broilers cloacae when compared with Poeta., et al. (2006) that detected a frequency of 39.5% in agg in fecal samples of broilers. However, the frequency reported by the authors was still considered high, since the presence of this gene was only detected in isolates of E. faecalis enterococci among all surveyed.
The ace gene encoding collagen adhesin of E. faecalis (Ace) also showed high levels among isolates from broilers cloacal swabs (94.2%). This is a cellular protein, specific surface area E. faecalis, which allows the binding of bacteria to the extracellular matrix proteins, collagens type I and IV and laminin may play a role in the pathogenesis of endocarditis (Nallaparedy., et al. 2000; Koch., et al. 2004).
Diarra., et al. (2010) demonstrated that 100% of E. faecalis isolated from feces or cecum of broilers contained the ace gene. Once again, Poeta., et al. (2006) agrees with the results obtained in this study, with a prevalence of 62.8% of E. faecalis isolated from fecal samples of broilers, with ace being detected only in isolates of this species. Like those observed for the ace among clinical samples was found by Nallapareddy., et al. (2000) who detected a frequency 60% ace gene. The prevalence of 86.6% of the ace gene in food isolates also corroborate those determined by Cariolato., et al. (2008) in food samples. Olsen., et al. (2011) investigated the gene in human isolates with bacteremia and cloacal swabs of broilers and achieved 100% attendance and 99% gene similarity ace among different isolates studied in both origins. Silva., et al. (2013) demonstrated the presence of different levels of ace gene ranging between 75 and 94.2% for E. faecalis isolated from poultry and clinical specimens, respectively.
The genes of the operon bopABCD were observed with high frequency among all isolates. The gene encodes a glycosyltransferase, bopA, located immediately downstream bopB responsible for the polymerization of phosphoglucomutase enzyme, followed by bopC encoding an aldolase-1-epimerase. The last gene in the operon, the bopD, the transcriptional regulator is a sugar binder and is involved in biofilm formation by E. faecalis (Hufnagel., et al. 2004). To date, no studies on the prevalence of genes in the operon bopABCD isolates of E. faecalis isolated from clinical and food samples.
The results observed for the tet (M) and tet (L) genes are according to studies by several authors (Aarestrup., et al. 2000; De Leener., et al. 2004; Cauwerts., et al. 2007; Frazzon., et al. 2009). The tet (L) gene has been found in fewer screened isolates (19.2%). This gene encodes a membrane protein, called efflux, which carries molecules out of the cell such as tetracycline and doxycycline (Chopra and Roberts, 2001). The gene tet (M) is commonly located on the bacterial chromosome and can be adduced by the conjugative transposons Tn916/Tn1545 family (Clewell., et al. 1995; Poeta., et al. 2006).
A similar situation was found during a survey conducted in hospitals in France, where 229 enterococci were isolated for 10 collections. In this study, the tet (M) and tet (L) genes have high prevalence among samples and were determinants for tetracycline resistance (Charpentier., et al. 1994). The lowest frequency of tet (L) gene can be explained because their transfer to other cells since they are not able to transfer themselves independently, depending on the presence of conjugative plasmids, so its spread in the population happens slowly. Moreover, the association of tet (M) gene of conjugative elements such as transposons have been an important factor in the spread of tetracycline resistance in enterococci (Chopra and Roberts, 2001).
Phylogenetic E. faecalis divergence of virulence and resitant genes content
Genetic characteristics variables provide a niche specialization and virulence of E. faecalis. Thus, by colonizing ability in a wide range of hosts and environments most suitable for a particular niche variant can proliferate and fill the niche, where the phylogenetic analysis evaluating the presence of virulence factors revealed clusters isolated from all origins and different periods of years. However, the selective forces that lead to convergence of these characteristics and adaptability continue to be studied. Our study assessed the diversity of E. faecalis from different sources and noted that the isolated show a similarity coefficients value ranging from 0.65 to 1.0. Castillo-Rojas., et al. (2013) reported that E. faecalis isolated from samples of urine, pleural fluid and blood and water content similarity coefficients 0.6, suggesting that the strains were related.
Genetic characteristics variables provide a niche specialization and virulence of E. faecalis. Thus, by colonizing ability in a wide range of hosts and environments most suitable for a particular niche variant can proliferate and fill the niche, where the phylogenetic analysis evaluating the presence of virulence factors revealed clusters isolated from all origins and different periods of years. However, the selective forces that lead to convergence of these characteristics and adaptability continue to be studied. Our study assessed the diversity of E. faecalis from different sources and noted that the isolated show a similarity coefficients value ranging from 0.65 to 1.0. Castillo-Rojas., et al. (2013) reported that E. faecalis isolated from samples of urine, pleural fluid and blood and water content similarity coefficients 0.6, suggesting that the strains were related.
Enterococcus faecalis originating from clinical samples isolated from blood, urine, pus and vaginal fluid from a hospital in Malaysia, demonstrated a high level of diversity in typing by Pulse Field Gel Eletroforesis (PFGE) and Multilocus Sequence Type (MLST), agreeing with studies observed in this study and studies by Lloyd., et al. (1998) and from the same or other hospitals has also been reported (Silva., et al. 2013).
The technique of random amplified polymorphic DNA (RAPD) is also commonly used in species-specific identification and typing as use of M13 in a primer sequence developed by Rossetti and Giraffa (2007) used on analysis of genetic diversity of Enterococcus by Riboldi., et al. (2008). The PCR phylotyping technique was described by Clermont., et al. (2000) and has been used to evaluate the different phylogenetic groups of E. coli strains (Derakhshandeh., et al. 2013). Is a technique based on a PCR using a combination of several virulence genes for E. faecalis, although can only indicate the presence or absence of genes, showed results strongly correlate with those obtained by RAPD method (Costa., et al. 2009). It is an excellent technique for rapid and inexpensive assigning of E. faecalisstrains in different phylogenetic groups.
These studies support the possibility of E. faecalis possess great ability to adapt to different environments, with their establishment and possible isolation of different sample origins. Another study about resistance phenotype between populations of E. faecalis isolates from chicken carcasses after cooling processing in five different places, showed that all plants have a high degree of diversity, despite their resistance phenotype were largely grouped into a single cluster, reporting that the likely standardization of procedures exert selective pressures operating uniforms, characterizing the environment as phenotypic determinant (Olsen., et al. 2011).
Cobo Molinos., et al. (2008) suggests that the presence of the operon ebp (encoding pilli) in E. faecalis, there are a role in the ubiquity of the species isolated from clinical and food origin, where the presence of certain genetic traits increases their adaptation to specific environments. This fact exalts the importance of dissemination of enterococci containing determinants of virulence and antimicrobial resistance genes across different environments, increasing their potential and ability to interact with human hosts.
In conclusion, the differences in frequencies of virulence genes in E. faecalis isolates from different sources shows that genetic gains and losses are important and crucial role of adaptation to a new habitat and the emergence of new strains contribution. However, the phylogenetic tree shows that despite the different origins of the isolates and the various genotypic profiles, the E. faecalis species exhibits a great adaptive capacity in different environments, even where selective pressure and high frequency of gene exchange occurs. Such characteristics may allow their survival in these environments and gives its characteristic of ubiquity.
Also, important to note that the different period of isolation of the samples between 2005 and 2009 did not interfere during the cluster, not being considered a determinant of genetic diversity. In addition, PCR phylotyping technique proved to be effective, rapid and inexpensive for the study of diversity of E. faecalis strains in different phylogenetic groups.
Acknowledgments
Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the financial assistance provided.
Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the financial assistance provided.
References
- Aarestrup FM., et al. “Comparison of antimicrobial resistance phenotypes and resistance genes in Enterococcus faecalis and Enterococcus faecium from humans in the community, broilers, and pigs in Denmark”. Diagnostic Microbiology and Infectious Disease 37.2 (2000): 127-137.
- Bittencourt E and Suzart S. “Occurrence of virulence-associated genes in clinical Enterococcus faecalis strains isolated in Londrina, Brazil”. Journal of Medical Microbiology 53.11 (2004):1069-1073.
- Cariolato D., et al. “Occurrence of virulence factors and antibiotic resistances in Enterococcus faecalis and Enterococcus faecium collected from dairy and human samples in North Italy”. Food Control 19 (2008): 886-892.
- Cassenego APV., et al. “Species distribution and antimicrobial susceptibility of enterococci isolated from broilers infected experimentally with Eimeria spp. and fed with diets containing different supplements. Brazilian Journal of Microbiology 42.2 (2011): 480-488.
- Cassenego APV., et al. “Virulência e formação de biofilme microbiano por Enterococcus faecalis isolados de swabscloacais de frangos de corte infectados com Eimeria spp”. Pesquisa Veterinária Brasileira 33.12 (2013): 1433-1440.
- Castillo Rojas G., et al. “Comparison of Enterococcus faecium and Enterococcus faecalis Strains Isolated from Water and Clinical Samples: Antimicrobial Susceptibility and Genetic Relationships”. Plos one 8.4 (2013).
- Cauwerts K., et al. “High prevalence of tetracycline resistance in Enterococcusisolates from broilers carrying the erm(B) gene”. Avian Pathology 36.5 (2007): 395-399.
- Charpentier E., et al. “Presence of Listeria tetracycline resistance genes tet(S) in Enterococcus faecalis”. Antimicrobial Agents and Chemotherapy 38.10 (1994): 2330-2335.
- Chopra I and Roberts M “Tetracycline antibiotics: mode of action, applications, molecular biology, and epidemiology of bacterial resistance”. Microbiology and Molecular Biology Reviews 65.2 (2001): 232-260.
- Clermont O., et al. “Rapid and simple determination of the Escherichia coli phylogenetic group”. Applied and Environmental Microbiology 66.10 (2000): 4555-4558.
- Clewell DB., et al. “Unconstrained bacterial promiscuity: the Tn916-Tn1545 family of conjugative transposons”. Trends in Microbiology 3.6 (1995): 229-236.
- Cobo Molinos A., et al. “Detection of ebp (endocarditis- and biofilm-associated pilus) genes in enterococcal isolates from clinical and non-clinical origin”. International Journal of Food Microbiology 126.1.2 (2008): 123-126.
- Costa AG Frazzon APG., et al. “Perfil genotípico de Enterococcus faecalis resistentes a antimicrobianos isolados de carne de frango e de infecção urinária pela técnica molecular RAPD-PCR”. Biociências 17.1 (2009): 74-71.
- d'Azevedo PA., et al. “Genetic diversity and antimicrobial resistance of enterococcal isolates from southern region of Brazil”. Revista do Instituto de Medicina Tropical de São Paulo 48.1 (2006): 11-16.
- Diarra MS., et al. “Distribution of antimicrobial resistance and virulence genes in Enterococcusspp. and characterization of isolates from broiler chickens”. Applied and Environmental Microbiology 76.24 (2010): 8033-8043.
- De Leener E., et al. “Distribution of the erm(B) gene, tetracycline resistance genes, and Tn1545-like transposons in macrolide- and lincosamide-resistant enterococci from pigs and humans”. Microbial Drug Resistance 10.4 (2004): 341-345.
- Depardieu F., et al. “Detection of the van Alphabet and Identification of Enterococci and Staphylococci at the Species Level by Multiplex PCR”. Journal of Clinical Microbiology 42.12 (2004): 5857-5860.
- Derakhshandeh A., et al. “Phylogenetic analysis of Escherichia coli strains isolated from human samples”. Molecular Cell Biology Research Communications 2.4 (2013):143-149.
- Donlan RM and Costerton JW. “Biofilms: survival mechanisms of clinically relevant microrganisms”. Clinical Microbiology Reviews 15.2 (2002): 167-193.
- Dupont H., et al. “Prospective evaluation of virulence factors of enterococci isolated from patients with peritonitis: impact on outcome”. Diagnostic Microbiology and Infectious Disease 60.3 (2008): 247-253.
- Duprè S., et al. “Incidence of virulence determinants in clinical Enterococcus faecium and Enterococcusfaecalis isolates collected in Sardinia (Italy)”. Journal of Medical Microbiology 52.6 (2003): 491-498.
- Eaton TJ and Gasson MJ. “Molecular screening of Enterococcus virulence determinants and potential for genetic exchange between food and medical isolates”. Applied and Environmental Microbiology 67.4 (2001):1628-1635.
- Frazzon APG., et al. “Prevalence of antimicrobial resistance and molecular characterization of tetracycline resistance mediated by tet(M) and tet(L) genes in Enterococcus spp. isolated from food in Southern Brazil”. World Journal of Microbiology and Biotechnology 26.2 (2010): 365-370.
- Giraffa G. “Enterococci from food”. FEMS Microbiology Reviews 26.2 (2002): 163-171.
- Hufnagel M., et al. ”A Putative Sugar-Binding Transcriptional Regulator in a Novel Gene Locus in Enterococcus faecalis Contributes to Production of Biofilm and Prolonged Bacteremia in Mice”. The Journal of Infectious Diseases 189.3 (204): 420-430.
- Hummel A., et al. “Characterization and transfer of antibiotic resistance genes from enterococci isolated from food”. Systematic and Applied Microbiology 30.1 (2007): 1-7.
- Huys G., et al. “Prevalence and molecular characterization of tetracycline resistance in Enterococcus isolates from food”. Applied and Environmental Microbiology 70.3 (2004): 1555-1562.
- Jackson CR., et al. “A Comparison of BOX-PCR and Pulsed-Field Gel Electrophoresis to Determine Genetic Relatedness of Enterococci from Different Environments”. Microbial Ecology 64.2 (2012): 378-387.
- Koch S., et al. “Enterococcal infections: host response, therapeutic and prophylatic possibilities”. Vaccine 22.7 (2004): 822-830.
- Lebreton Y., et al. Maltose utilization in Enterococcus faecalis. Journal of Applied Microbiology 98.4 (2005): 806-813.
- Lloyd S., et al. “Risk factors for enterococcal urinary tract infection and colonization in a rehabilitation facility”. American Journal of Infection Control 26.1 (1998): 35-39.
- Mannu L., et al. “Comparison of the incidence of virulence determinants and antibiotic resistance between Enterococcusfaeciumstrains of dairy, animal and clinical origin”. International Journal of Microbiology 88.2.3 (2003): 291-304.
- McBride SM., et al. “Genetic Diversity among Enterococcus faecalis”. Plos one 2.7 (2007): 582.
- Nallaparedy SR., et al. “Enterococcus faecalisadhesin, ace, mediates attachment to extracellular matrix proteins collagen type IV and laminin as well as collagen type I”. Infection and Immunity 68.9 (2000): 5218-5224.
- Olsen RH., et al. “Enterococcus faecalisof human and poultry origin share virulence genes supporting the zoonotic potential of E. faecalis”. Zoonoses and public health 59.4 (2012): 256-263.
- Poeta P., et al. “Detection of genes encoding virulence factors and bacteriocins in fecal Enterococci of poultry in Portugal”. Avian Diseases 50.1 (2006): 64-68.
- Qin X., et al. “Effects of Enterococcus faecalis fsrgenes on production of gelatinase and serine protease and virulence”. Infection and Immunity 68.5 (2000): 2579-2786.
- Riboldi G., et al. “Phenotypic and genotypic heterogeneity of Enterococcusspecies isolated from foods in Southern Brazil”. Journal of Basic Microbiology 48.1 (2008): 31-37.
- Rice LB and Carias LL. “Transfer of Tn5385, a Composite, Multiresistance Chromosomal Element from Enterococcus faecalis”. Journal of Bacteriology 180.3 (1998): 714-721.
- Rossetti L and Giraffa G. “Rapid identification of dairy lactic acid bacteria by M13-generated, RAPD-PCR fingerprint databases”. Journal of Microbiological Methods 63.2 (2007): 135-44.
- Semedo T., et al. “Virulence factors in food, clinical and reference enterococci: a common trait in the genus?” Systematic and Applied Microbiology 26.1 (2003): 13-22.
- Silva J., et al. “Detección de genes de virulência em cepas de Enterococcus faecalis susceptibles y resistentes a aminoglucósidos”. Revista chilena de infectología 30.1 (2013): 17-22.
Citation:
Camila O. Arent., et al. “Comparison of Virulence Factors and Genetic Relationships of Enterococcus Faecalis Strains Isolated
from Clinical, Food and Poultry Samples ”. Multidisciplinary Advances in Veterinary Science 1.3 (2017): 106-115.
Copyright: © 2017 Camila O. Arent., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.





























 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.