Case Report
Volume 1 Issue 6 - 2018
Non-invasive Assessment of Lameness in Horses with Dorsal Spinous Process Impingement “Kissing spine”: A Case Study.
1IVH - Physiology, Faculty of Health & Medical Sciences, Copenhagen University,
Grønnegaardsvej 7, 1870 Frederiksberg C, Denmark
2Hillerød Hestedyrlæger, Baunevej 17, Bendstrup, 3400 Hillerød, Denmark
2Hillerød Hestedyrlæger, Baunevej 17, Bendstrup, 3400 Hillerød, Denmark
*Corresponding Author: Adrian P Harrison, IVH - Physiology, Faculty of Health & Medical Sciences, Copenhagen University, Grønnegaardsvej 7, 1870 Frederiksberg C, Denmark.
Received: January 24, 2018; Published: January 29, 2018
Abstract
The assessment of muscle function in connection with an injury or during recovery is of paramount importance in the veterinary field, where animals often attempt to conceal their pain and impaired mobility. In recent years, such techniques as accelerometry. bioimpedance analysis and mechanomyography more precisely referred to as acoustic myography have been used for the assessment of human muscular problems. However, these techniques have yet to be applied routinely in the veterinary world, and have not been used in connection with injury in horses.
The combined use of these novel and non-invasive techniques was applied to the case of a 6-year-old Danish Warmblood mare that presented with recurring and shifting lameness. Acoustic myography, assessing both the amplitude and frequency of active muscles, was employed to locate the specific area of muscle injury. Thereafter this specific region was assessed in terms of its bioimpedance properties, assessing muscle resistance (R) and reactance (Xc) and confirming a regional loss of muscle mass and cellular integrity compared with the contralateral site. Finally, accelerometry was used to reveal the effects of the injury on the gait, as measured using such parameters as the stride interval, strike force and stance interval. It is concluded that acoustic myography, bioimpedance and accelerometry when used in combination, provide a useful set of diagnostic tools for the rapid and non-invasive determination of muscular injury in the horse.
Keywords: Bioimpedance analysis; Accelerometry; Acoustic myography; lameness
Introduction
Back problems are often one of the most common causes of poor performance and disobedience in horses, yet all too often they go undiagnosed and untreated, becoming first apparent when lameness occurs (Valberg, 1996; Allen., et al. 2010).“Kissing spine” also referred to as dorsal spinous process impingement (DSPI) or Basstrup’s disease, occurs when the dorsal spinous processes, and/or to a lesser extent, the transverse spinous processes of the horse’s vertebrae start to rub together, causing pain and swelling, especially when horses are active or being ridden (Turner, 2011).
Typically this problem is most commonly associated with the thoracic vertebral region, where the largest dorsal spinous processes are to be found, but this condition is also seen in the lumbar region (Turner, 2011). “Kissing spine” is a major cause of poor performance and gait abnormality but is often not detected by the rider until it has significantly begun to affect the horse’s performance. In addition to poor performance, other symptoms associated with this condition include resentment towards certain schooling exercises, difficulty in maintaining a three-beat canter gait, irritability when the saddle is placed on the back and or the girth is tightened and resentment of pressure or grooming over the back (Valberg, 1996; Turner, 2011).
In recent years great advances have been made in imaging procedures such as radiography, ultrasonography and nuclear scintigraphy, allowing visualization of structures and lesions that were not accessible before (Fonseca., et al. 2006). In addition to imaging techniques, it is also possible to inject local anaesthetics between the spinal processes where a kissing spine problem is suspected and then to work the horse again to see if there is any improvement in performance (Allen., et al. 2010).
Different grades of severity can be identified from radiographs of the abnormal vertebral processes (Allen., et al. 2010). Grade 1 lesions involve only a narrowing of the space between adjacent processes. Grade 2 lesions show an increase in bone density at the margins of the processes. In contrast, Grade 3 lesions show loss of bone adjacent to the margins, and Grade 4 lesions involve severe remodelling of the processes, due to chronic and profound irritation of the structures (Patel., et al. 2009). It should be noted though, that kissing spine can be found in performance and sport horses without back pain, and without apparently restricting back movement in any way (Jeffcott, 1979). Therefore, in each case, the clinical significance of suspected lesions must be carefully assessed and the degree of damage to soft muscle tissue around a region of injury determined (Dermas., et al. 2017). Bioimpedance analysis (BIA) uses the components of impedance (Z), resistance (R) that is to say the opposition to the flow of an alternating current through intra- and extracellular ionic solutions, and reactance (Xc), which is the delay in passage of a current by the cell membranes and tissue interfaces (Van Der Aa Kuhle., et al. 2006; Harrison., et al. 2015).
Resistance is inversely related to fluid content and Xc indicates cell membrane mass, function and interface. In this way, BIA enables characterization or classification of relative changes in hydration and cell health/damage in a non-invasive fashion (Lindinger., et al. 2004). This case report presents the application of three non-invasive techniques, namely acoustic myography, bioimpedance and accelerometry used in conjunction to assess a case of lameness in a horse known to be suffering from “kissing spine”.
Materials & Methods
Considerable care was taken to make sure that the horse was not stressed in any way by the non-invasive measurements. Furthermore, the owners were informed of the measurements to be performed and gave their full consent.
Subject: A 6-year-old Danish Warmblood mare was bought by the current owner after a pre-purchase examination despite the fact that it was identified as having a tendency towards “kissing spine” (see Figure 1).
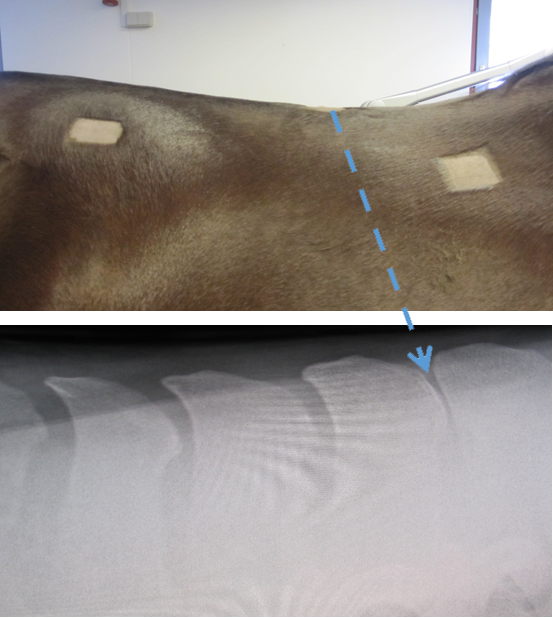
Figure 1: UPPER PANEL: A photo of the right-hand side of the spine of the
horse, showing the regions used for the placement of the mfBIA electrodes,
as well as the region of the spine where the anaesthetic was administered.
LOWER PANEL: An X-ray of the region of the spine at the point of T14-T15 showing the close relation of the spinous processes indicative of “kissing spine”.
LOWER PANEL: An X-ray of the region of the spine at the point of T14-T15 showing the close relation of the spinous processes indicative of “kissing spine”.
Approximately 6 months after being purchased, the horse presented with stiffness in the back, an asymmetric head position whilst being ridden, lameness and a reluctance to take a saddle or be ridden. In total, a period of lameness lasting 7 months passed by from the date of purchase until the present muscle measurements, bioimpedance and gait analyses were undertaken.
Acoustic MyoGraphy: A custom built acoustic myography (AMG) unit (CURO, MyoDynamik ApS; www.myodynamik.com) (Fenger & Harrison, 2017), capable of recording remotely at a distance of 100 m, was placed on the horse at the point of the withers and mounted as part of a customized training girth. The unit was set to record muscle contractions transdermally from the muscles adjacent to the back at four sites along the spine at the regions of T10/11, T14/15, T18/L1 and L3/4. The recorded data were analyzed for their frequency (Hz) and amplitude (mV) parameters (Harrison, 2017).
The central nervous system (CNS) coordinates muscle function by increasing or decreasing the recruitment and synchronization of motor units (equating to signal amplitude – Spatial summation), as well as altering the frequency with which active motor units fire (equating to signal frequency – Temporal summation) (Fenger & Harrison, 2017; Harrison, 2017).
Bioimpedance: The horse was restrained in a standing position and kept free of all metal surfaces. The back muscles (m. Latissimus dorsi; m. Longissimus dorsi) were then prepared using a conductive paste applied by rubbing it gently into the skin (Ten20, Aurora, CO 80011, US). Four pure platinum electrodes (1 x 3 cm) were subsequently placed onto the prepared sites.
The region associated with the back muscles (m. Latissimus dorsi; m. Longissimus dorsi) was assessed in this way. A bioimpedance unit (BIA; ImpediVET BIS 1, Pinkenba, QLD 4008 Australia), providing 800 μA of current was attached to the platinum electrodes and recordings of the resistance (R) and reactance (Xc), as well as the impedance (Z) at 256 frequencies ranging 3 kHz to 850 kHz were made. The BIA unit was set to record six consecutive measurements and the mean of these values was used. In this way any slight movement artefacts or changes in the resistance and reactance values due to cable movement etc. was kept to a minimum.
Accelerometry: An accelerometer (Waveland, MS 39576, US), was attached firmly to both hind legs of the horse at the level of the cannon bones, using a standard dressage leg bandage. The units were programmed to record at 100 Hz (100 samples / second) and they were sensitive to directional acceleration above 0.1g and up to a maximum of 8.5g. The recorded data were analyzed for their acceleration with particular emphasis on the lateral “g” forces produced during trotting. These data revealed information as to the coordination of the hind limbs, the acceleration of the left and right-hand sides of the horse during movement and gait analysis for each hind leg in terms of stride length (sec), strike force (g) and stance time (sec).
Results
The CURO sensors was placed along the spine at the regions of T10/11, T14/15, T18/L1 and L3/4 and the frequency and amplitude values for the left and right hand sides of the back of the horse are given in Figure 2.
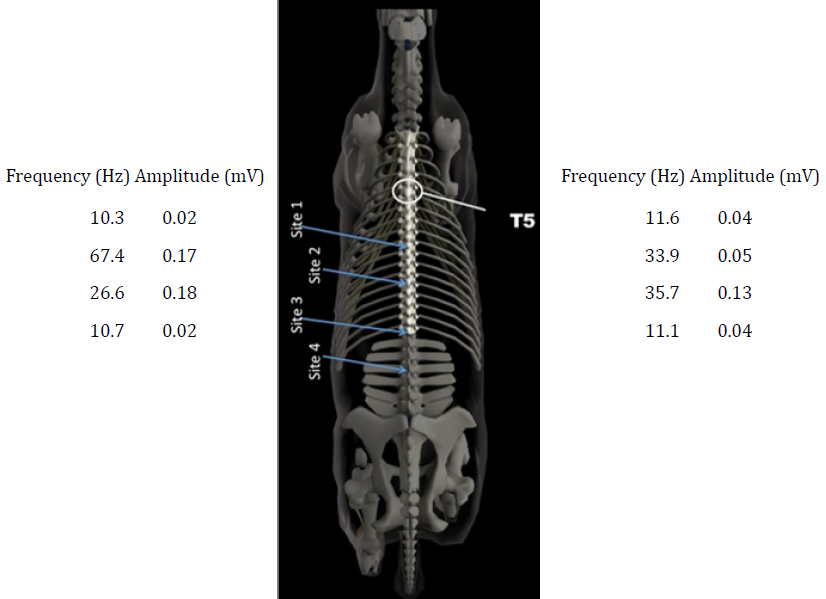
Figure 2: A schematic of the spine of a horse, showing the vertebra in relation to the
neck and the hips. Values for the acoustic myography signal are presented in terms of
their spatial summation (amplitude; mV) and temporal summation (frequency; Hz)
for the four regional sites, T10/11, T14/15, T18/L1 and L3/4. Acoustic myography
recordings were taken during a free trot and without either a saddle or anaesthetic.
The acoustic myography system revealed a normal signal for both regions T10/11 and L3-4, whilst for region T14/15 there were signs of elevated muscle contraction with a frequency of 67 Hz and an amplitude of 0.17 mV on the left side and a slightly lower, albeit still elevated, level of contraction on the right side (33.9 Hz). The region T18/L1 also showed indications of elevated muscle contraction, although here the elevated signal was more symmetrical for both sides.
The data recorded by the BIA unit revealed typical signal values (see Table 1). The signals exhibited a clear Cole-Cole plot of reactance against resistance for the 256 frequencies used, enabling the collection of normal resistance and reactance data for the regions measured. Using this technique, a clear difference in the Z, R and Xc values between the left and right sides of the horse at the region of the back muscles (m. Latissimus dorsi; m. Longissimus dorsi) was revealed.
| Measurements | Left-hand side | Right-hand side | |
| m.L.dorsi | Impedance (Z) Ω | 49.3 | 58.8 |
| Resistance (R) Ω | 47.3 | 56.8 | |
| Reactance (Xc) Ω | 14.0 | 15.4 |
Table 1: Measured BIA values for the left-hand and right-hand sides of the horse, measured for the back muscle (m. Latissimus dorsi; m. Longissimus dorsi) region. Values are shown for the 50 kHz frequency. The horse, was measured as having a height (S1-S2) of 169.5 cm and a calculated weight of 560 kg.
The impedance, resistance and reactance were lower on the left- cf the right-hand sides of the back (Z=16%; R=17%; Xc=9%), indicative of a reduced muscle mass, inflammation and swelling as well as impaired cellular energy storage, respectively. Data obtained from the two accelerometers likewise revealed typical signal values (see Table 2). A very constant time (approx. 0.8 sec) between steps was measured as the stride interval. Moreover, a general strike force of approx. 8.0 g was measured with an average stance interval of 0.1 sec.
2a – Without Saddle
| Hind Limb | Stride Interval (sec) Right Left |
Strike Force (g) Right Left |
Stance Interval (sec) Right Left |
|||
| Free Trot | 0.79 | 0.83 | 7.8 | 7.9 | 0.11 (14%) | 0.16 (19%) |
2b – With Saddle
| Hind Limb | Stride Interval (sec) Right Left |
Strike Force (g) Right Left |
Stance Interval (sec) Right Left |
|||
| Free Trot | 1.21 | 1.21 | 8.0 | 7.5 | 0.37 (30%) | 0.16 (31%) |
2c – With Anaesthesia
| Hind Limb | Stride Interval (sec) Right Left |
Strike Force (g) Right Left |
Stance Interval (sec) Right Left |
|||
| Free Trot | 0.82 | 0.78 | 8.0 | 7.9 | 0.15 (18%) | 0.11 (14%) |
2d – With Anaesthesia & Saddle
| Hind Limb | Stride Interval (sec) Right Left |
Strike Force (g) Right Left |
Stance Interval (sec) Right Left |
|||
| Free Trot | 0.82 | 0.79 | 7.8 | 7.9 | 0.12 (14%) | 0.10 (12%) |
Table 2: Measured accelerometer values for the hind limbs during periods of free trot, without a saddle (2a), with a saddle (2b), following localized anaesthesia (2c), and following anaesthesia with the saddle in place once again (2d). The accelerometer units were placed as outlined in the materials and methods, and recordings were taken at a sampling rate of 100 Hz. Values in brackets represent the percentage of the stride interval spent at stance.
With a saddle in place there was a considerable increase (149%) to a new stride interval of 1.21 sec and an increase in the stance time by 30%. Once a saddle, but no rider, was placed on the horse, there was a dramatic increase in the stride interval (0.8 to 1.2 sec; 149% increase) with an equally dramatic increase in the stance interval (0.1 to 0.3 sec). It was also visually clear that the horse was in discomfort and not only slowed down its free trot movement but also soon came to a stop and was reluctant to resume a trot.
Following a local anaesthetic (carbocaine) at the point of the “kissing spine” lesion (T14/15) a repeat of the previous exercise, but without a saddle, revealed a return to the previous stride-(0.8 sec) and stance- interval (0.1 sec). Moreover, the addition of a saddle after administration of a local anaesthetic did not result in an adverse change in the accelerometer parameters as seen earlier, quite the reverse, the stance interval continued to improve very slightly.
Discussion & Conclusion
The combination of muscle parameters presented in this case study alongside the accelerometer parameters not only confirmed the x-ray diagnosis but also identified the most affected side of the horses back.
The acoustic myography data support published findings that have reported temporal summation in human subjects with fibromyalgia, a physiological parameter that is not only elevated with referred pain, but one that can be reduced with administration of an NMDA-antagonist (ketamine) (Graven-Nielsen., et al. 2000). The highest frequency recording (temporal summation) was found to be localized to the left-hand side of the horses back at the region of T14/15 where the worst kissing spine image was found using x-ray. In addition, the highest acoustic myography signal amplitude was noted for the left-hand side of the back, extending to T18/L1 and clearly involving a greater number of muscle fibres than the right-hand side of the horses back. Often muscles are used in this way to stabilize joints that are painful and thereby reduce pain and discomfort. Muscles carry out two functions when they contract in such situations, they act firstly as a nonlinear spring to slow down impact and secondly they function as a viscous damper to absorb painful impact (Sarvazyan., et al. 2014).
The BIA data further supports the findings from the acoustic myography recordings and indicates that the back region of this horse had suffered a regional loss of muscle mass (Z) and a loss of cellular integrity (Xc) predominantly on the left-hand compared with the contralateral side. The extracellular resistance was also lower on the left- compared to the right-hand side of this horse.
Bioimpedance has been applied in the medical clinic and in the sports field for assessment of human muscles (Nescolarde., et al. 2013), where injury to a muscle with associated swelling has been shown to result in a drop in the extracellular resistance (R). Nescolarde and her colleagues were able to show in the leg muscle of a football player that had sustained a grade 2 injury that the extracellular resistance dropped immediately after the injury occurred and rose again back to resting values once localized inflammation had subsided (Nescolarde., et al. 2013).
If we assume the right-hand side value to be a healthy reference then the drop in R from right to left in this horse constitutes a 17% change which is close to the value of 19% found by Nescolarde and colleagues for grade 2g injuries that is to say a myotendinous or myofascial muscle injury with feather and gap as assessed by MRI (Nescolarde., et al. 2017).
It is likewise interesting that the accelerometer data confirm that with a change in muscle function, suggestive of a grade 2 injury in terms of the BIA data for the back muscles that there is a change in gait when a saddle is placed on the horses back, covering the region under investigation. It is equally interesting that this change in gait disappears after a local anaesthetic is applied to the region identified by x-ray as having “kissing spine”.
Furthermore, once the anaesthetic had been applied, not only was a normal gait observed, it could also be maintained despite the addition of a saddle that had previously had such an adverse effect on the stride and stance intervals. These accelerometer findings are in keeping with the realization that present-day gait analysis systems can identify changes in the movement of the back and that knowledge of the relation between such changes and the site of injury can help veterinarians towards a better localization and diagnosis of disorders of the equine back (Wennerstrand., et al. 2009).
It should further be noted that a recent study of 49 interspinous spaces in equine cadavers, yielding 274 measurements, concluded that x-ray beam angle significantly affected the measured width of interspinous spaces (Djernaes., et al. 2017). Djernaes and colleagues concluded that width did not follow a consistent pattern, such that interspinous space widths in focus position were highly significantly smaller on radiographs compared with matched reconstructed CT images for backs diagnosed as having “kissing spine” syndrome. (Djernaes., et al. 2017)
Such a measureable imbalance in muscle parameters and function in the back region as the one in this case is most likely the result of a localized painful region. Muscular compensation, intended to stabilize a spinous impingement region during periods of physical activity, induces muscle injury with associated swelling and inflammation.
Whilst some consider cases of “kissing spine” to be the result of overuse of the back, giving rise to inflammation and pain at specific sites on the spine and ultimately spinous impingement, others believe it to occur more often in competitive jumping or dressage horses, while still others find it to be related to conformation and development (Turner, 2011).
Whatever the cause, a protocol of shockwave therapy, mesotherapy and exercise is usually recommended for cases of “kissing spine” (Turner, 2011; Coomer., et al. 2012). For some horses, however, treatment requires surgery. Typically an incision is made over the top of the spinous processes and the top half of one or more processes is removed. The bone doesn’t re-grow and the space left by the removal of the spinous process is gradually filled with fibrous tissue. The overall success of such a surgical approach generally depends on the adoption of a comprehensive rehabilitation and recovery programme after surgery (Coomer., et al. 2012).
It is concluded that such non-invasive measurements of muscle function as acoustic myography, bioimpedance and accelerometry in combination can provide a useful set of diagnostic data that may assist in not only rapidly determining the site and extent of muscular injury but quite possibly in directing treatment and rehabilitation in afflicted horses. Moreover, for cases involving horses already diagnosed as having changes similar to those of “kissing spine” on an x-ray, the aforementioned tools could be used to assess the function of the muscles in the area affected and thereby help in the evaluation of the soundness of a horse with regard to planned sports competitions or in connection with a presales check.
Acknowledgement
The authors are indebted to the horse owner for allowing us to perform these non-invasive measurements.
The authors are indebted to the horse owner for allowing us to perform these non-invasive measurements.
Conflicts of interests
APH is currently trying to commercialize the AMG recording system and is establishing a company to cover the costs of future development.
APH is currently trying to commercialize the AMG recording system and is establishing a company to cover the costs of future development.
References
- Allen A.K., et al. “How to Diagnose and Treat Back Pain in the Horse”. AAEP Proceedings 56 (2010): 384-388.
- Alves N and Chau T. “Mechanomyography as an access pathway: corporeal contraindications”. Assistive Technology 6.6 (2011): 552-563.
- Bajaj P., et al. “Mechanomyography and electromyography force relationships during concentric, isometric and eccentric contractions”. Journal of Electromyography and Kinesiology 11.2 (2001): 126-136.
- Coomer RP., et al. “A controlled study evaluating a novel surgical treatment for kissing spines in standing sedated horses”. Veterinary Surgery41.7 (2012): 890-897.
- Djernaes JD., et al. “Effects of X-ray beam angle and geometric distortion on width of equine thoracolumbar interspinous spaces using radiography and computed tomography – a cadaveric study”. Veterinary Radiology & Ultrasound 58.2 (2017): 169-175.
- Elbrønd VS., et al. ”Multi-frequency bioimpedance and myofascial release therapy: An equine “AtlasOrange1” validation study”. Medical Research Archives 3 (2015): 2375-1924.
- Fenger C and Harrison AP. “The Application of Acoustic Myography in Canine Muscle Function and Performance Testing”. SOJ | Veterinary Sciences 3.1 (2017): 1-6.
- Fonseca BPA., et al. “Thermography and Ultrasonography in Back Pain Diagnosis of Equine Athletes”. Journal of Equine Veterinary Science 26.11 (2006): 507-516.
- Graven-Nielsen T., et al. ”Ketamine reduces muscle pain, temporal summation, and referred pain in fibromyalgia patients”. Pain 85.3 (2000): 483-491.
- Harrison AP. “A more precise, repeatable and diagnostic alternative to surface electromyography – an appraisal of the clinical utility of acoustic myography”. Clinical Physiology and Functional Imaging2.3 (2017).
- Harrison AP., et al. “Multi-frequency bioimpedance in equine muscle assessment”. Physiological Measurement36.3 (2015): 453-464.
- Herda TJ., et al. “A Noninvasive log-transform Method for Fiber Type Discrimination using Mechanomyography,” Journal of Electromyography and Kinesiology 20.5 (2010): 787-794.
- Jeffcott LB. “Radiographic examination of the equine vertebral column”. Veterinary Radiology & Ultrasound20.3.6 (1979):135-139.
- Järvinen TA., et al. “Muscle injuries: biology and treatment”. The American Journal of Sports Medicine33.5 (2005): 745-764.
- Kearns CF., et al. “Overview of horse body composition and muscle architecture: implications for performance”. Veterinary Journal164.3 (2002): 168-70.
- Lindinger MI., et al. “Time course and magnitude of changes in total body water, extracellular fluid volume, intracellular fluid volume and plasma volume during submaximal exercise and recovery in horses”. Equine and Comparative Exercise Physiology 1.2 (2004): 131-139.
- Nescolarde L., et al. “Localized bioimpedance to assess muscle injury”. Physiological Measurement34.2 (2013): 237-45.
- Nescolarde L., et al. “Detection of muscle gap by L-BIA in muscle injuries: clinical prognosis”. Physiological Measurement38.7 (2017): 1-9.
- Patel AA., et al. “Thoracolumbar spine trauma classification: the Thoracolumbar Injury Classification and Severity Score system and case examples”. Journal of Neurosurgery Spine 10.3 (2009): 201-206.
- Qi L., et al. “Spectral properties of electromyographic and mechanomyographic signals during isometric ramp and step contractions in biceps brachii,” Journal of Electromyography and Kinesiology 21.1 (2011): 128-135.
- Salomons KK., et al. “Surface electromyography during both standing and walking in m. Ulnaris lateralis of diversely trained horses”. M Schwartz (ed.), in EMG methods for evaluating muscle and nerve function. InTech (2012): 209-224.
- Sarvazyan A., et al. “Muscle as a molecular machine for protecting joints and bones by absorbing mechanical impacts”. Medical Hypotheses83.1 (2014): 6-10.
- Stokes M and Blythe M. “Muscle Sounds in physiology, sports science and clinical investigation”. Medintel Medical Intelligence Oxford (2001).
- Turner T. “Overriding spinous processes (“kissing spines”) in horses: diagnosis, treatment and outcome in 212 cases”. AAEP Proceedings57 (2011): 424-430.
- Valberg SJ. “Muscular causes of exercise intolerance in horses”. Veterinary Clinics of North America: EquinePractice 12.3 (1996): 495-515.
- Van Der Aa Kuhle KS., et al. “Application of bioimpedance for the determination of equine body composition”. In: Australian Equine Science Symposium. Australian Equine Science Symposium Gold Coast (2006): 59-59.
- Walker VA., et al. “The effect of collection and extension on tarsal flexion and fetlock extension at trot”. Equine Veterinary Journal 45.2 (2012): 1-4.
- Walmsley EA., et al. “Muscle strain injuries of the hindlimb in eight horses: diagnostic imaging, management and outcomes”. Australian Veterinary Journal 88.8 (2010): 313-321.
- Wennerstrand J., et al. “Spinal kinematics in horses with induced back pain”. Veterinary and Comparative Orthopaedics and Traumatology 22.6 (2009): 448-454.
Citation:
Adrian P Harrison., et al. “Non-invasive Assessment of Lameness in Horses with Dorsal Spinous Process Impingement
“Kissing spine”: A Case Study.” Multidisciplinary Advances in Veterinary Science 1.6 (2018): 257-265.
Copyright: © 2018 Adrian P Harrison., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.





























 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.