Research Article
Volume 2 Issue 1 - 2018
Antimicrobial Resistance Profiles of Thermophilic Campylobacter Species in Rural Poultry in the North Western Nigeria.
1Department of Animal Health and Production Technology, School of Agricultural Technology, Federal Polytechnic Mubi. Adamawa State. Nigeria
2Department of Veterinary Medicine, Faculty of Veterinary Medicine, Usmanu Danfodiyo University Sokoto, Nigeria
3Department of Veterinary Medicine and Surgery, Faculty of Veterinary Medicine, Federal University of Agriculture, Abeokuta, Ogun State, Nigeria
4Department of Veterinary Microbiology, Faculty of Veterinary Medicine, Usmanu Danfodiyo University Sokoto, Sokoto State, Nigeria
2Department of Veterinary Medicine, Faculty of Veterinary Medicine, Usmanu Danfodiyo University Sokoto, Nigeria
3Department of Veterinary Medicine and Surgery, Faculty of Veterinary Medicine, Federal University of Agriculture, Abeokuta, Ogun State, Nigeria
4Department of Veterinary Microbiology, Faculty of Veterinary Medicine, Usmanu Danfodiyo University Sokoto, Sokoto State, Nigeria
*Corresponding Author: Abubakar SM Abba Maiha, Department of Animal Health and Production Technology, School of Agricultural Technology, Federal Polytechnic Mubi. Adamawa State. Nigeria.
Received: February 05, 2018; Published: February 23, 2018
Abstract
A Study was conducted to determine the antimicrobial sensitivity of thermophilic Campylobacters species in rural poultry in Kebbi State, the North Western part of Nigeria to ten (10) antimicrobial agents. A total of 400 cloacal swabs from rural poultry were screened and analyzed using standard culture isolation technique and biochemical characterization. A total of 177 (44%) were positive for Campylobacter species and were subjected to sensitivity testing using disc diffusion method. 89 (22.3%) of the isolates was identified as Campylobacter coli while 51(12.8%), and 37(9.3%) were identified as Campylobacter lari and Campylobacter jejuni respectively. Out of 177 Campylobacter species subjected to antimicrobial sensitivity test, 112(63%) were resistant at least to a minimum of one antimicrobial agents. High resistance was observed in ciprofloxacin (62.1%), followed by tetracycline (57.1%), erythromycin (54.8%), Azithromycin (50.3%) and Sulphamethoxazole/Trimetoprim (48%) for the three species. The low resistance profile was observed in streptomycin (1.7%), gentamycin (67.3%) and chloramphenicol (5.1%) in the three species. All the species were resistance to Cephalothin. It was also observed that amongst species that were multi-resistant, Ciprofloxacin, Trimetoprim/Sulphamethoxazole and tetracycline were present in most of the patterns. A minimum of three and maximum of seven multi-drugs resistance patterns were observed in the study. This study, show that the organism is increasingly resistant to antibiotics especially fluoroquinolones and macrolides, which are the most frequently used antimicrobials agent for the treatment of Campylobacteriosis when clinical therapy is warranted. As a zoonotic pathogen, sensitivity test is recommended for the choice of antibiotics for effective therapeutic purposes in animals and human when dealing with Campylobacters infections.
Keywords: Sensitivity; Campylobacter; Poultry
Introduction
Campylobacter is a leading food borne bacterial pathogen, which causes gastroenteritis in human and animals and colonize the gastrointestinal tract of birds causing diarrhea, less feed conversion ratio, decrease egg production and mortality in day old chicks (Ruiz-Palacios., et al. 1981; Butzler, 2004; Han., et al. 2007). Avian carriage of Campylobacter has been regarded as a potential hazard to animals and human health, either through consumption of raw or undercooked carcass or by contamination of water supplies (Varslot., et al. 1996). Normally, Campylobacter infections are self limiting in poultry and treatment with antibiotics in most of the time not required. However, when an antibiotic is recommended for treatment, macrolides e.g Erythromycin is considered as one of the drug of choice and fluoroguinolones e.g Ciprofloxacin are also recommended (Kassa., et al. 2006). In general, majority of thermotolerant Campylobacter species are resistant to a large number of beta-lactam antibiotics, particularly for ampicilin, amoxicilin, and cefotaxime (Tajada., et al. 1996). In addition, majority of C. jejuni and C. coli are resistant to trimethoprim and sulphonamides (Aarestrup and Engberg, 2001). Information on antimicrobial sensitivities of thermophilic Campylobacters differs from different countries (Van Loveren., et al. 2001; Ishihara., et al. 2004; Andersen., et al. 2006). This pathogenic organism have been reported to be increasingly resistant to antibiotics especially fluoroquinolones and macrolides which are the most frequently used antimicrobials for the treatment of Campylobacteriosis when clinical therapy is warranted (Rosef., et al. 2001; Asrestrup and Engberg, 2001). Development and transmission of antibiotic-resistant Campylobacter is complicated by the fact that Campylobacter is a zoonotic pathogen and is therefore exposed to antibiotics used in both animal production and human medicine. Campylobacters increasing resistant to clinically important antibiotics is of great concern for veterinary medicine and public health. Therefore an ecological approach is required to understand the emergence, transmission and persistence of antibiotic-resistant Campylobacter species by determining the sensitivity of Campylobacters species isolated in rural poultry (Alfredson and Korolik, 2007; Han., et al. 2007).
The aim of the study was to determine the antimicrobial sensitivity of thermophilic Campylobacter species in rural poultry in Kebbi State Northwestern Nigeria.
Materials and Methods
Study area
Kebbi State is geographically located to the North Western part of Nigeria at 11° 30’N 4° 00’E. Kebbi State falls within the Sudan Savanna with mean minimum temperature of 26°C (Kowal and Knabe, 1992). During the harmattan season (December to February), the temperature can go down to as low as 21°C and mean maximum temperature can go up to 40°C during the months of April to June (MANR, 1999). Annual rainfall is about 800 mm and relative humidity is low (40%) for most of the year except during the wet season when it reaches an average of 80%. The wet season lasts from June to September, the hot season April to June while cool dry season lasts from December to February (Odjugo, 2010). Kebbi State was ranked among the five states with the highest number of livestock in Nigeria. Agriculture is the main occupation of the people especially in rural areas (animal rearing and fishing).
Kebbi State is geographically located to the North Western part of Nigeria at 11° 30’N 4° 00’E. Kebbi State falls within the Sudan Savanna with mean minimum temperature of 26°C (Kowal and Knabe, 1992). During the harmattan season (December to February), the temperature can go down to as low as 21°C and mean maximum temperature can go up to 40°C during the months of April to June (MANR, 1999). Annual rainfall is about 800 mm and relative humidity is low (40%) for most of the year except during the wet season when it reaches an average of 80%. The wet season lasts from June to September, the hot season April to June while cool dry season lasts from December to February (Odjugo, 2010). Kebbi State was ranked among the five states with the highest number of livestock in Nigeria. Agriculture is the main occupation of the people especially in rural areas (animal rearing and fishing).
Research design
The study was a cross sectional study of Campylobacter infection in domestic birds. One hundred samples were collected from domestic birds at poultry markets from each of the selected four local government areas (Argungu, Birni Kebbi, Yauri and Zuru). Each was selected from one of the four local governments in the state. Random sampling techniques were used in sample collection.
The study was a cross sectional study of Campylobacter infection in domestic birds. One hundred samples were collected from domestic birds at poultry markets from each of the selected four local government areas (Argungu, Birni Kebbi, Yauri and Zuru). Each was selected from one of the four local governments in the state. Random sampling techniques were used in sample collection.
Sampling method
Domestic birds at live bird Markets were the target population while poultry at live bird Markets were the sampling frame. Purposive sampling as described by Paul (2006) was used for selection of local governments’ areas while simple random sampling as described by Valerie and John (1997) was used for sampling domestic birds in selected areas.
Domestic birds at live bird Markets were the target population while poultry at live bird Markets were the sampling frame. Purposive sampling as described by Paul (2006) was used for selection of local governments’ areas while simple random sampling as described by Valerie and John (1997) was used for sampling domestic birds in selected areas.
Sample size determination
The minimum sample size for this study was determined by the formula,
n = t2x pexp(1-pexp)/ d2 (Thrusfield, 2005)
Where n=sample size, t2 = the score for a giving interval which is 1.96 (S.E) at 95%, confidence interval, pexp = Known or estimated prevalence, and d2 = precision at 0.05.
The samples were calculated at 38.8% prevalence, (Salihu., et al. 2009) at 95% confidence interval, with desired precision of 5%.
n = (1.96)2 x 0.39 x (1-0.39)/(0.05)2,
n = 0.9139/0.0025 = 365.5
n = 366
For more precision of the study, 400 samples were collected.
Thus, n = 400
The minimum sample size for this study was determined by the formula,
n = t2x pexp(1-pexp)/ d2 (Thrusfield, 2005)
Where n=sample size, t2 = the score for a giving interval which is 1.96 (S.E) at 95%, confidence interval, pexp = Known or estimated prevalence, and d2 = precision at 0.05.
The samples were calculated at 38.8% prevalence, (Salihu., et al. 2009) at 95% confidence interval, with desired precision of 5%.
n = (1.96)2 x 0.39 x (1-0.39)/(0.05)2,
n = 0.9139/0.0025 = 365.5
n = 366
For more precision of the study, 400 samples were collected.
Thus, n = 400
Sample collection
Permission was obtained from the Ministry of Agriculture and Natural Resources and for each of the selected market 2 in every 5 bird (40%) counted were randomly sampled. A total of 400 domestic birds were sampled at poultry markets from four of the randomly selected local government, each from one of the four Emirate councils in the state. Cloacal swabs or freshly voided faeces were collected using sterile commercial swab sticks and were placed in Amies transport media, kept cold with the use of ice blocks (Butzler, 2004). Samples were transported within few hours after collection on the same day to the Veterinary Microbiology Laboratory, Faculty of Veterinary Medicine, Usmanu Danfodio University, Sokoto for processing.
Permission was obtained from the Ministry of Agriculture and Natural Resources and for each of the selected market 2 in every 5 bird (40%) counted were randomly sampled. A total of 400 domestic birds were sampled at poultry markets from four of the randomly selected local government, each from one of the four Emirate councils in the state. Cloacal swabs or freshly voided faeces were collected using sterile commercial swab sticks and were placed in Amies transport media, kept cold with the use of ice blocks (Butzler, 2004). Samples were transported within few hours after collection on the same day to the Veterinary Microbiology Laboratory, Faculty of Veterinary Medicine, Usmanu Danfodio University, Sokoto for processing.
Processing of samples
Samples were inoculated directly onto a selective medium, modified charcoal cefaperazone Deoxycholate Agar (mCCDA) and incubated at 42°C for 48 hrs (Butzler and Megraud, 2002). Suspected Campylobacter colonies on the selective mCCDA medium were identified based on their characteristics features as creamy or white, moist, flat or slightly raised, extending along the streak line, or regular circular discrete colony based on the description of Atabay and Corry (1998).
Samples were inoculated directly onto a selective medium, modified charcoal cefaperazone Deoxycholate Agar (mCCDA) and incubated at 42°C for 48 hrs (Butzler and Megraud, 2002). Suspected Campylobacter colonies on the selective mCCDA medium were identified based on their characteristics features as creamy or white, moist, flat or slightly raised, extending along the streak line, or regular circular discrete colony based on the description of Atabay and Corry (1998).
Suspected Campylobacter isolates were confirmed based on their biochemical reactions as follows: Oxidase test, Hippurate hydrolysis test, Catalase test, Hydrogen sulphide production test (Atabay and Corry, 1998) and sensitivity to Cephalothin, Nalidixic acid using agar disc diffusion method (CLSI, 2014).
Antimicrobial sensitivity testing of Campylobacter
Antimicrobial sensitivity test to determine the resistance profiles of the Campylobacter isolates to Ciprofloxacin (CIP 5 μg), Tetracycline (TET 30 μg), Erythromycin (ERY 15 μg) Streptomycin (STREP 10 μg) Azithromycin (AZM 10 μg), Gentamicin (GEN 10μg), Chloramphenicol (C 30μg) Cephalothin (CEP 30g), Nalidixic acid (NAL 30g) and Trimetoprim/Sulphamethoxazole (SXT 30 μg) were carried out using disc diffusion method. The tests were performed on Mueller-Hinton agar. The presence of the zones of inhibition were regarded as sensitive while absence of zones of inhibition were regarded as resistance as described by Kirby-Bauer (Bauer., et al. 1996; NCCLS 2002) method. This method allowed for the rapiddetermination of the efficacy of a drug by measuring the diameter of the zone of inhibition that resulted from diffusion of the agent into the medium surrounding the disc.
Antimicrobial sensitivity test to determine the resistance profiles of the Campylobacter isolates to Ciprofloxacin (CIP 5 μg), Tetracycline (TET 30 μg), Erythromycin (ERY 15 μg) Streptomycin (STREP 10 μg) Azithromycin (AZM 10 μg), Gentamicin (GEN 10μg), Chloramphenicol (C 30μg) Cephalothin (CEP 30g), Nalidixic acid (NAL 30g) and Trimetoprim/Sulphamethoxazole (SXT 30 μg) were carried out using disc diffusion method. The tests were performed on Mueller-Hinton agar. The presence of the zones of inhibition were regarded as sensitive while absence of zones of inhibition were regarded as resistance as described by Kirby-Bauer (Bauer., et al. 1996; NCCLS 2002) method. This method allowed for the rapiddetermination of the efficacy of a drug by measuring the diameter of the zone of inhibition that resulted from diffusion of the agent into the medium surrounding the disc.
A test organism in a nutrient agar from a slant bottle was prepared by overnight culture for 24 hours at 42°C. Selected colonies were streaked using sterile swab stick homogenously on the medium. Antibiotic disc were applied aseptically on tothe surface of the inoculated plates at an appropriate special arrangement with the help of a sterile pair offorceps on Mueller-Hinton agar plates. The plates were then inverted and incubated at 42°C for 24 hours.
Results
Out of the 400 samples analyzed, a total of 177 samples were positive for Campylobacter species. 89, 51 and 37% prevalence were recorded for Campylobacter coli, Campylobacter lari and C. jejuni, respectively (Table 1).
| LGA | Total sampled |
Total Positive (%) |
C. jejuni | Species C. Coli |
C. lari |
| Argungu | 100 | 53 | 10 (18.9) | 26 (49.1) | 17 (17.1) |
| Birni Kebbi | 100 | 58 | 13 (22.4) | 29 (50) | 16 (27.6) |
| Yauri | 100 | 36 | 8 (22.2) | 17 (47.2) | 11 (30.6) |
| Zuru | 100 | 30 | 6 (20) | 17 (23.3) | 7 (23.3) |
| 400 | 177 | 37 (9.3) | 89 (22.3) | 51 (12.8) |
Table 1: Prevalence of Campylobacter infection in rural poultry in kebbi state.
Out of 177 Campylobacter species subjected to antimicrobial sensitivity test, 112(63%) were resistant at least to a minimum of one antimicrobial agents. High resistance was observed in ciprofloxacin (62.1%), followed by tetracycline (57.1%), erythromycin (54.8%), Azithromycin (50.3%) and Sulphamethoxazole/Trimetoprim (48%) for the three species (Table 2). The low resistance was observed in streptomycin (1.7%), gentamycin (67.3%) and chloramphenicol (5.6%) in the three species (Table 2). All the species were resistance to Cephalothin (Table 2). Figure 1 represents percentage resistance profile of all the three species to ten (10) antimicrobial agents used in the study. It was also observed that amongst species that were multi-resistant, Ciprofloxacin, Trimetoprim/Sulphamethoxazole and tetracycline were present in most of the patterns (Table 3). A minimum of three and maximum of seven multidrug resistant patterns were exhibited in the study (Table 3).
| Antimicrobial agent | Species/Number (%) Resistance | Total (n = 177) | ||
| C. Jejuni (n = 37) | C. coli (n = 89) | C. lari (n = 51) | ||
| Ciprofloxacin | 23 (62.2) | 59 (66.3) | 28 (54.9) | 110 (62.1) |
| Azithromycin | 17 (46.0) | 55 (61.8) | 17 (33.3) | 89 (50.3 |
| Erythromycin | 11 (30.0) | 70 (78.7) | 16 (31.3) | 97 (54.8) |
| Tetracycline | 14 (37.8) | 61 (68.5) | 25 (49.0) | 101 (57.1) |
| Nalidixic acid | 11 (30.0) | 0 (0) | 0 (0) | 11 (6.2) |
| Chloramphenicol | 2 (5.4) | 6 (6.7) | 1 (2.0) | 9 (5.1) |
| Gentamycin | 3 (8.1) | 7 (7.9) | 3 (5.9) | 14 (7.9) |
| Cephalothin | 37 (100) | 89 (100) | 51 (100) | 177 (100) |
| Streptomycin | 3 (8.1) | 0 (0) | 0 (0) | 3 (1.7) |
| Trimetoprim/ Sulphamethoxazole | 27 (73.0) | 49 (55.1) | 9 (17.6) | 85 (8.0) |
Table 2: Antimicrobial Resistance Profile of Thermophilic Campylobacter Species from rural poultry.
| Multi - drugs Resistant Patterns | No (% Resistance Profile) S | Total (n = 79) | ||
| C Jejuni (n = 21) | C. Coli (n = 32) | C. Lari (n = 26) | ||
| CIP, TET, ERY | 2 (9.5) | 1 (3.1) | 1 (3.8) | 4 (5.1) |
| CIP, ERY, AZM | 1 (4.8) | 1 (3.1) | (3.8) | 3 (3.8) |
| TET, ERY, AZM | 1 (4.8) | 2 (6.3) | 1 (3.8) | 4 (5.1) |
| ERY, AZM, GEN | 1 (4.8) | 1 (3.1) | 0 (0) | 2 (2.5) |
| CIP, TET, ERY, NAL | 1 (4.8) | 0 (0) | 0 (0) | 1 (1.3) |
| CIP, TET, ERY, SXT | 1 (4.8) | 2 (6.3) | 7 (7.7) | 6 (7.6) |
| CIP, ERY, AZM, SXT | 1 (4.8) | 1 (3.1) | 1 (3.8) | 3 (3.8) |
| TET, ERY, AZM, NAL | 1 (4.8) | 0 (0) | 0 (0) | 1 (1.3) |
| TET, ERY, C, SXT | 1 (4.8) | 3 (9.4) | 2 (7.7) | 5 (6.3) |
| CIP, TET, ERY, AZM, SXT | 1 (4.8) | 7 (21.9) | 9 (34.6) | 17 (21.5) |
| CIP, TET, C, STREP, SXT | 1 (4.8) | 1 (3.1) | 1 (3.8) | 3 (3.8) |
| CIP, TET, ERY, GEN, SXT | 1 (4.8) | 3 (9.4) | 2 (7.7) | 6 (7.6) |
| CIP, TET, ERY, NAL, SXT | 1 (4.8) | 0 (0) | 0 (0) | 1 (1.3) |
| CIP, TET, ERY, AZM, STREP, SXT | 1 (4.8) | 2 (6.3) | 3 (11.5) | 6 (7.6) |
| CIP, ERY, AZM, GEN, STREP, SXT | 1 (4.8) | 3 (9.4) | 1 (3.8) | 5 (6.3) |
| CIP, TET, ERY, AZM, GEN, C, SXT | 0 (0) | 5 (15.6) | 2 (7.7) | 7 (8.9) |
| CIP, TET, ERY, AZM, GEN, NAL, SXT | 5 (23.8) | 0 (0) | 0 (0) | 5 (10.1) |
Table 3: Multi-Drug resistance patterns for isolates of Campylobacter species from rural poultry
CIP = Ciprofloxacin, TET = Tetracycline, ERY = Erythromycin, STREP = Streptomycin, AZM = Azithromycin,
NAL = Nalidixic acid, GEN = Gentamycin, C = Chloramphenicol, SXT = Trimetoprim/Sulphamethoxazole.
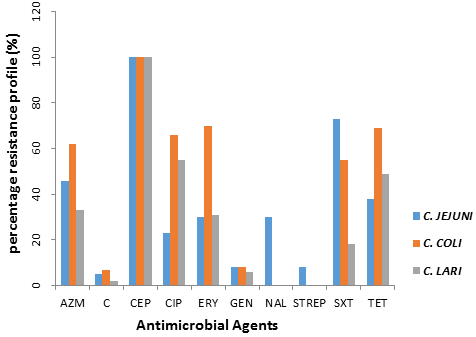
Figure 1: Percentage resistance profile of thermophilic Campylobacter
species in rural poultry to ten (10) antimicrobial agents.
Discussion
In this study, high resistance rates were observed for ciprofloxacin (61%). The level of resistance is higher than 50% reported in Plateau State by Ngulukun. (2010), However, the resistance profile was lower than 71.7% in Czech Republic reported by Bardon., et al. (2009) in poultry. Enrofloxacin is closely related to ciprofloxacin and widely used in Nigeria to treat infectious diseases in poultry and could be the responsible for high resistance rate to ciprofloxacin seen in this study.
Resistance to tetracycline (57.1%) recorded in this study is comparable to 48.8% reported by Taremi., et al. (2006) in Iran. However, it is lower than 67.9% reported by Ngulukun, (2010) in Plateau State. The higher resistance in this study may be due to extensive use and misuse of oxytetracycline in poultry for prophylaxis and treatment of bacterial diseases. The next antimicrobial agents isolated with higher rate of resistance in rural poultry were macrolides which includes erythromycin (54.8%) and Azithromycin (50%). This drug is used intermittently in the poultry industry in feed formulations. Also high resistance to macrolides (Erythromycin and Azithromycin) may be attributed to extensive use and misuse of Tylosin for the control and treatments of respiratory diseases in poultry. The detection of isolates resistant to erythromycin may impose therapeutic problems in poultry-borne gastroenteritis caused by Campylobacter in dogs and humans. C. coli (79%) being more resistant than C. jejuni (30%). The resistance profile was 48% for Trimetoprim/Sulphamethoxazole (SXT) with 73% C. jejuni and 55% C. coli being more resistant to this agent. Sulphanomides are used in Nigeria in poultry industry, being easily available, soluble and readily applied in water and feeds (Adesiyun and Oni, 1989). This could be responsible for the high resistance in this study.
The low resistance profile to gentamycin (7.9%), streptomycin (1.7%) and chloramphenicol (5.1%) in this study is consistent with finding by Ngulukun (2010), which recorded low resistance profile to gentamycin (10.8%), streptomycin (4.6%) and chloramphenicol (7.7%). This may be attributed, in part to the fact that they are rarely used in the poultry industry either for prophylactic or therapeutically due to their intramuscular route of administration.
In this study, remarkable differences between C. jejuni and C. coli were observed with regard to the antimicrobial agents used. The occurrence of resistance to most of the antimicrobial agents tested was generally higher for C. coli than for C. jejuni. This finding is similar to other studies previously reported (Ngulukun. 2010). However, results from some other studies have demonstrated that C. jejuni are generally more resistant than C. coli (Cokal., et al. 2008).
Forty five percent (45%) of the isolates from domestic birds in this study exhibited multiple resistances to 3 or more antimicrobial agents tested. This is higher than 33% reported by Saenz., et al. (2000) in Spain. However, the rate reported in the current study is lower than 52% as revealed by Ngulukun. (2010) in Plateau State and 64% reported by Rodrigo., et al. (2007) in Trinidad.
In this study, it was observed that amongst isolates that were multi-resistant, Ciprofloxacin, Trimetoprim/Sulphamethoxazole and tetracycline were present in most of the patterns. The occurrence of multi-resistant isolates is of major concern since Campylobacter is a gram- negative organism and there is a phenomenon of transfer of resistant genes to other gram-negative pathogens in the environment (Barlow., et al. 2004). Consequently, this could have therapeutic implications in the treatment of animals and human bacterial diseases originating from the consumption of Campylobacter contaminated chickens. The public health significance of the findings in this study cannot be over emphasized more so as ciprofloxacin and erythromycin commonly used in the therapy of Campylobacteriosis featured prominently in the resistance patterns. Further investigation and surveillance is necessary to determine the extent of the resistant situation in the country to suggest control measures. The use of antimicrobial agents in food animal production has become a source of great concern in recent years. Concerns over the emergence of bacterial pathogens resistant to antimicrobial agents commonly used to treat infections in animals and humans, and the potential transfer of resistant pathogenic strains from food animals products to humans has led to changes in antimicrobial usage in livestock and poultry production worldwide (Andersen., et al. 2006; Ruiz-Palacios, 2007; Cokal., et al. 2008).
Conclusion
Remarkable differences in term of drug resistance between C. jejuni and C. coli were observed with regard to the antimicrobial agents used. The occurrence of resistance to most of the antimicrobial agents tested was generally higher for C. coli than for C. jejuni.
Recommendation
Indiscriminate use of antibiotics should be avoided. As a zoonotic pathogen, sensitivity test is recommended for the choice of antibiotics for effective therapeutic purposes in animals and human when dealing with Campylobacters infections.
Indiscriminate use of antibiotics should be avoided. As a zoonotic pathogen, sensitivity test is recommended for the choice of antibiotics for effective therapeutic purposes in animals and human when dealing with Campylobacters infections.
Acknowledgement
We are grateful to Dr. Yusuf Yakubu of the Department of Public Health and Preventive Medicine for his help in data analysis and the staff of Central Research Laboratory Faculty of Veterinary Medicine, Usmanu DanFodiyo University Sokoto, Abdulmalik Shuaibu Bello and Nafiu, for their technical assistance during sampling processing.
We are grateful to Dr. Yusuf Yakubu of the Department of Public Health and Preventive Medicine for his help in data analysis and the staff of Central Research Laboratory Faculty of Veterinary Medicine, Usmanu DanFodiyo University Sokoto, Abdulmalik Shuaibu Bello and Nafiu, for their technical assistance during sampling processing.
References
- Aarestrup FM and Engberg J. “Antimcrobial resistance of themophilic Campylobacter”. VeterinaryResearch 32.3 (2001): 311-321.
- Adesiyun AA and Oni OO. “Prevalence and antibiograms of salmonellae in slaughter cattle, slaughter areas and effluents in Zaria abattoir, Nigeria”. Journal of Food Protection 52.4 (1989): 232-234.
- Alfredson DA and Korolik V. “Antibiotic resistance and resistance mechanisms in Campylobacter jejuni and Campylobacter coli”. FEMS Microbiology Letter 277.2 (2007): 123–132.
- Andersen SR., et al. “Antimicrobial resistance among Campylobacter jejuni isolated from raw poultry meat at retail level in Denmark”. International Journal of Food Microbiology 107.3 (2006): 250-255.
- Atabay H and Corry J EL. “Isolation and prevalence of Campylobacters from the dairy using variety of methods”. Journal of Applied Microbiology84.4 (1998): 733-740.
- Bardon J., et al. “Prevalence of Campylobacter jejuni and its resistance to antibiotics in poultry in the Czech Republic”. Zoonoses and Public Health 56.3 (2009): 111-116.
- Barlow RS., et al. “Isolation and characterization of integron-containing bacteria without antibiotic selection”. Antimicrobial Agents Chemotherapy48.3 (2004): 838-842.
- Bauer AW., et al. “Antibiotic susceptibility testing by a standard single disc method. American Journal of Clinical Pathology 45.4 (1996): 493-496.
- Butzler JP. “Campylobacter, from obscurity to celebrity”. Clinical Microbiology and Infection10.10 (2004): 868-876.
- Butzler JP., et al. “Expanding indication for the new macrolides, azalides and streptogramins”. New York (2002): 237-249.
- Clinical and Laboratory Standard Institute (CLSI). “Performance standards for antimicrobial disc and dilution susceptibility tests for bacteria isolated from animals”.Approved Standards 28.3 (2014).
- Cokal Y., et al. “Campylobacter species and their antimicrobial resistance patterns in poultry: An epidemiological survey study in Turkey”. Zoonoses and Public Health 56.3 (2008): 105-110.
- Han K., et al. “Prevalence, genetic diversity, and antibiotic resistance patterns of Campylobacter jejuni from retail raw chickens in Korea”. Journal of Food Microbiology 114 (2007): 50-59.
- Ishihara K., et al. “Antimicrobial susceptibilities of Campylobacter isolated from food-producing animals on farms (1999 - 2001): Results from Japanese Veterinary Microbial Resistance Monitoring Programme”. International Journal of Antimicrobial Agents 24 (2004): 63-69.
- Kassa T., et al. “Antimicrobial susceptibility patterns of thermotolerant Campylobacter strains Isolated from food animals in Ethiopia”. Veterinary microbiology 119.1 (2007): 82-87.
- Kowal JM and Knabe DT. “An agrodimatological atlas of the northern States of Nigeria with explanatory notes. Ahmadu Bello University Press, Zaria, Nigeria (1992).
- Ministry of Agriculture and Natural Resources (MANR). The Report, Nigeria Livestock Resources1999.
- National Committee for Clinical Laboratory Standards. “Performance standards for antimicrobial disc and dilution susceptibility tests for bacteria isolated from animals”.Approved Standard s22.6 (2002)
- Ngulukun and Sati Samuel (2010). Molecular epidemiology of thermophilic Campylobacter infection in poultry, cattle and humans in plateau state, Nigeria. Doctoral thesis. University of Nigeria Nsukka Pp 133-175.
- Nwankwo IO., et al. “Epidemiology of Campylobacter species in poultry and humans in the four agricultural zones of Sokoto State, Nigeria”. Journal of Public Health and Epidemiology (2016): 185-190.
- Odjugo PAO. “Regional evidence of climatic change in Nigeria”. Journal of Geography and Regional Planning 3.6 (2010): 142-150.
- Paul O., SAGE Research Method.The SAGE Dictionary of Social Research Methods (2006): 121-156.
- Rodrigo S., et al. “Antimicrobial resistance of Campylobacter species. Isolated from broilers in small poultry processing operations in Trinidad”. Food Control18.4 (2007): 321-325.
- Rosef O., et al. “Thermophilic Campylobacter in surface water: a potential risk of Campylobacteriosis”. International Journal of Environment Health Research 11.4 (2001): 321327.
- Ruiz-Palacios GM., et al. “Experimental Campylobacter diarrhea in chickens”. Infectious and Immunology 34.4 (1981): 250-255.
- Ruiz-Palacios GM. The health burden of Campylobacter infection and the impact of antimicrobial resistance: playing chicken. Clinical Infectious Disease 44.5 (2007): 701-703.
- Sáenz, Y., et al. “Antibiotic resistance in Campylobacter strains isolated from animals, foods, and humans in Spain in 1997–1998”. Antimicrobial Agents and Chemotherapy 44.2 (2000): 267-271.
- Tajada P., et al. “Antimicrobial susceptibilities of C. jejuni and C. coli to 12 B-lactam agents and combinations with B-lactamase inhibitors”. Antimicrobial Agents and Chemotheraphy 40.8 (1996): 1924 -1925.
- Taremi M., et al. “Prevalence and antimicrobial resistance of Campylobacter isolated from retail raw chicken and beef meat, Tehran, Iran”. International Journal of Food Microbiology 108.3 (2006): 401-403.
- Thrusfield M. “Veterinary Epidemiology”. Oxford OX4 IJF (2005) 180-198.
- Valerie JE and John HM (1997). Statistics Glossary v1.1
- Van Loveren M., et al. “Antimicrobial susceptibilities of Campylobacter strains isolated from food animals in Belgium”. Journal of Antimicrobial Chemotheraphy48.2 (2001): 235-240.
- Varslot M., et al. “Water-borne outbreaks of Campylobacter gastroenteritis due to pink-footed geese in Norway in 1994 and 1995”. Tidsskrift for Den Norske Laegeforening 116.28 (1996): 3366-3369.
Citation:
Abubakar SM Abba Maiha., et al. “Antimicrobial Resistance Profiles of Thermophilic Campylobacter Species in Rural Poultry
in the North Western Nigeria.” Multidisciplinary Advances in Veterinary Science 2.1 (2018): 268-275.
Copyright: © 2018 Abubakar SM Abba Maiha., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.





























 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.