Research Article
Volume 2 Issue 1 - 2018
Evidence on the Presence of the Critically Endangered Philippine Crocodile, Crocodylus mindorensis (Schmidt 1935) in the Highlands of Daguma Mountain Range, Lake Sebu, The Philippines
1Crocodile Research and Conservation Program, Crocodylus Porosus Philippines Incorporated, Kapalong, Davao Del Norte, Philippines
2DNA Barcoding Laboratory, Institute of Biology, College of Science, University of the Philippines, Quezon City, Philippines
3College of Agriculture, University of Southern Mindanao, North Cotabato, Philippines
2DNA Barcoding Laboratory, Institute of Biology, College of Science, University of the Philippines, Quezon City, Philippines
3College of Agriculture, University of Southern Mindanao, North Cotabato, Philippines
*Corresponding Author: Rainier I Manalo, Crocodylus Porosus Philippines Incorporated, Pag-asa Farm, Kapalong, Davao Del Norte, Philippines.
Received: February 15, 2018; Published: February 23, 2018
Abstract
The presence of crocodiles in the highland region of Daguma Mountain Range, Lake Sebu is new to science. This study aimed to determine the species level of crocodile artifacts retrieved from the local resident. A skull fragment and dorsal skins of relatively described small crocodiles was morphologically examined in 2012. In 2017, the cytochrome oxidase I (COI) barcoding gene was initially sequenced to identify the species level of dried tissue specimens recovered from the postorbital and squamosal region of the skull. Results showed that the analysis of cranial measurements do not differ from the cranial indices of Crocodylus mindorensis. The resultant estimated total length and dorsal skin scalation pattern of the specimens have resembled to be C. mindorensis. The BLAST search and phylogenetic analysis of the COI fragment were 100% C. mindorensis. DNA sequencing has concluded the long standing uncertainty of relatedness based on cranial morphology and dorsal skin scalation patterns. C. mindorensis have existed in high altitude (750–800 mASL) of Lake Sebu and concerns about the altitudinal limitations for C. mindorensis were decisively diminished.
Keywords: Philippine crocodile; Crocodylus mindorensis; highlands; DNA barcoding
Introduction
In 1992, the Philippine crocodile, Crocodylus mindorensis is considered the most endangered crocodilian in the world. Ross and Alcala (1983) indicated that crocodiles were once a conspicuous element of lowland fauna of the Philippines. The presence of C. mindorensis according to Messel., et al. (1992) remains in minor pockets of habitat and none appear protected. It was listed by the International Union for the Conservation of Nature (IUCN) in 1996 as Critically Endangered due to decreasing population caused by a decline in the quality of habitat and severe fragmentation of population. Ross (1998) has estimated the population to be no more than 100 non-hatchlings in the wild. Recent population that there are 92–137 matured C. mindorensis in the wild with continued decline (van Weerd., et al. 2016), but causes are understood and potentially reversible.
Two important populations are recorded in 1999, the western slopes of Sierra Madre Mountain Range and Rio Grande de Mindanao, Bukidnon Central Cordillera (Pontillas 1999). The discovery of these populations has generated perceptions that C. mindorensis can thrive in high elevation. Highland areas were investigated and recorded their presence for the first time in the tributaries of Cordillera Central, Abra Province. The occurrence of C. mindorensis was documented in high elevation (850 m ASL) thereby challenging the altitudinal limitations of the supposed lowland species (Manalo 2008).
The continued exploration for new populations across the landscapes had engaged the review of previously discounted localities. Reports on local consumption on crocodiles in the highland of southern Mindanao prompted an opportunity for survey in 2012. Remains of a reported small crocodiles could provide an important contribution on the presence of C. mindorensis in highland of the Philippines. It will shed light as to reduce the preconceived perceptions on the altitudinal limitation on the C. mindorensis. Moreover, this study aimed to determine the species level of crocodile artifacts retrieved from the local resident. The unearthing of new locality and altitudinal record for this species is new to science.
Materials and Methods
Study site
In the vicinity of Daguma mountain range, Seven Lake, Barangay Ned, Municipality of Lake Sebu, South Cotabato, Philippines, a reported presence of crocodile biological specimens was received and visited on 22-26 November 2012. A skull fragment and dorsal skin of relatively described adult small crocodiles slaughtered by local residents in 2009 from Pugwan Lake (6°17’10.2“N, 124°26’19.6”E; 798 m ASL) and a displayed dorsal skin from a juvenile crocodile from Pangalman Lake (6°18’03.3“N, 124°25’14.9”E; 753 m ASL) in 2007 was obtained. Several geologic depressions with reports of crocodile sightings from 2007-2010 was surveyed and examined.
In the vicinity of Daguma mountain range, Seven Lake, Barangay Ned, Municipality of Lake Sebu, South Cotabato, Philippines, a reported presence of crocodile biological specimens was received and visited on 22-26 November 2012. A skull fragment and dorsal skin of relatively described adult small crocodiles slaughtered by local residents in 2009 from Pugwan Lake (6°17’10.2“N, 124°26’19.6”E; 798 m ASL) and a displayed dorsal skin from a juvenile crocodile from Pangalman Lake (6°18’03.3“N, 124°25’14.9”E; 753 m ASL) in 2007 was obtained. Several geologic depressions with reports of crocodile sightings from 2007-2010 was surveyed and examined.
An unidentified crocodile cranium and sun-dried dorsal skins were photographed, examined, and digitally drawn using CorelDRAW ver.11 2002. Morphometric measurements and descriptions of the cranium were compared with cranial indices by Hall (1989) adapted from Iordansky (1973) using Chi-square. Two sun-dried dorsal crocodile skins were analyzed and scalation counts were compared on the descriptions of Schmidt (1935), Ross (1982), and Ross & Alcala (1983). Leftover dried tissue slab attached in the margins of postorbital and squamosal region of the skull was retrieved and stored in a sealed plastic container in 2012 (Figure 1).
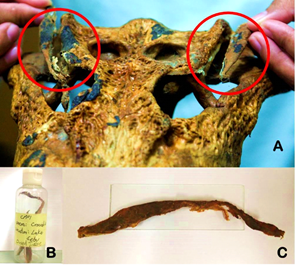
Figure 1: (A) Unidentified crocodile skull with leftover tissue.
(B) Sample CPP1 in the original container. (C) Dried tissue
removed from the container to be excised for DNA extraction.
In 2017, dried tissue specimens were transferred in 95% ethanol after tissue excision. A slice of tissue was dissected and used for DNA extraction using the Purelink® Genomic DNA Extraction Kit (Invitrogen) following the manufacturer’s protocol. The cytochrome oxidase I (COI) barcoding gene was initially sequenced to identify the samples to the species level. Amplification of the gene fragment was done using the primers VF1 (5′-TTCTCAACCAACCACAARGAYATYGG-3′) and VR1 (5′-TAGACTTCTGGGTGGCCRAARAAYCA-3) (Folmer., et al. 1994). A 50 µL PCR mastermix was made from 10 µL of 5x PCR Buffer with dNTPs (Bioline, UK), 1.5 µL of each primers (10 mM), 0.25 µL Taq Polymerase (5 units/uL) (Bioline, UK), 2.5 µL molecular grade DMSO (Sigma), 1 µL of 50 mM MgCl2 (Bioline, UK), 10-30 ng of genomic DNA, and diluted with DNAse free water. Thermal cycling was done in a LabNet Thermal Cycler using the following protocol: a 5 min denaturation step at 94°C followed by: 43 cycles of 30 sec at 94°C, 30 sec at 45°C, and 60 sec at 65°C, and a final extension of 5 min at 72°C. The PCR products were visualized in a 1% agarose gel stained with ethidium bromide in a UV transilluminator. Positive products were excised and purified using the QIAquick® DNA Extraction Kit (Qiagen, USA). Purified samples were then sent to 1st Base Malaysia for single pass capillary sequencing.
Results and Discussion
Morphological description of specimens - The crocodile cranium showed a massive structure, distinctively broader and short snout of prominent maxillary angulation, prominent lachrymal groove, antorbital or maxillary ridge high and abrupt laterally, pronounced festooning of maxillary teeth, more rounded premaxillary with 5 teeth sockets, palatine-pterygoids suture nearly transverse and the length of maxillary symphysis is shorter than the length of premaxillary symphysis (MXS < PXS) (Manalo., et al. 2013a; Manalo., et al. 2013b). This conformed to the findings of Schmidt (1935), Ross (1982), Ross and Alcala (1983), and Hall (1989) that generally described Crocodylus mindorensis as robust with neither extremely broad nor elongated skull with palatine-pterygoids suture nearly transverse but never bisecting.
The analysis of cranial measurements did not significantly differ (X2 = 4.758, 8df, P > 0.05) from the relative growth cranial indices obtained by Hall [7] for C. mindorensis (Table 1). Estimation of Total Length (TL) following the widely assumed constant DCL: TL ratio of 1:7–7.5 generally for C. porosus. C. niloticus and C. acutus (Bellairs 1969, Platt., et al. 2011, and Fukuda., et al. 2013) reflects that the specimen had a total length ranging from 216–235 cm. This resultant estimate is within the average total length of 150–250 cm. for C. mindorensis described by Ross (1998). The estimated size was almost the same with the recorded 217 cm. adult female C. mindorensis in Caucauayan Creek, Dalupiri in 2005 (Oliveros., et al. 2005). According to Fukuda., et al. (2013), a TL/HL ratio of 1:8.8 was obtained from the largest C. porosus caught in Philippines. In this ratio, a total length of 271.92 cm. can be obtained, and is higher than average total length of C. mindorensis. However, Ross and Alcala (1983) and Ross (1984) recorded a size range estimate of 288-326 cm. with a maximum length of 350 cm. examined in captivity.
| Character | Crocodylus mindorensis relative growth cranial indices* by Hall (1989) (mm.) |
Lake Pugwan Skull Specimen |
||
| Mean ± SE | Range | Class Mark | ||
| DCL | 227.7 ± 20.6 | 140-387 | 263.5 | 309 |
| RWST | 57.5 ± 1.0 | 51.9-66.3 | 59.1 | 67.53 |
| RLST | 63.3 ± 0.6 | 59.2-66.4 | 62.8 | 62.78 |
| RCW | 46.4 ± 1.4 | 43.9-49.8 | 46.85 | 59.55 |
| RWI | 53.1 ± 3.5 | 34.5-70.5 | 52.5 | 65.38 |
| RLR | 76.8 ± 1.1 | 71.2-84.2 | 77.7 | 72.57 |
| ROL | 15.8 ± 0.4 | 13.4-18.4 | 15.9 | 16.83 |
| ROW | 76.1 ± 2.0 | 65.9-91.3 | 78.6 | 69.23 |
| RWN | 19.2 ± 0.7 | 16.7-23.1 | 19.9 | 21.74 |
Table 1: Comparative relative growth skull indices by Hall (1989) and Lake Pugwan skull specimen.
The examination of dorsal scalation pattern revealed that the two sun-dried skin specimens had 17-18 transverse dorsal rows, 10 dorsal midbody scale rows within post-caudal (PC) 10-15 for adult and PC 9-14 in juvenile crocodile, and an ossified dorsal armor (Figure 3). These scale characteristics were within the descriptions of Schmidt (1935), and Ross and Alcala (1983) for C. mindorensis. But the absence of nuchal shield cluster (PC 19-23) and post-occipital or occipitals (PC 24-26) for both specimens reflects difficulties in direct association with C. mindorensis. However, the presence of a relatively large ventral scales in adult specimen and 23 large ventral scale rows from the cloaca to the thoracic collar in juvenile specimen somehow related with C. mindorensis description of Ross and Alcala (1983) ranging from 22 to 25. In addition, incomplete caudal scale rows and ventral scale row for adult specimen do not provide better comparison with juvenile skin specimen.
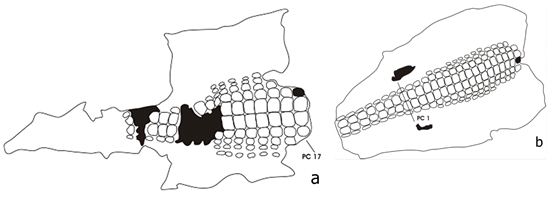
Figure 3: Sketch of a sun-dried skins from an adult (a) and a juvenile (b) crocodile
taken from Lakes Pugwan and Pangalman respectively. Lake Sebu 2012.
DNA sequencing - A 658 bp fragment of the COI gene was sequenced from each sample using the standard barcoding primers VF1 and VR1. These sequences were then subject to a BLAST search on the NCBI GenBank database (Table 2). The results of the BLAST search and phylogenetic analysis of the COI fragment concluded that the specimens in this case were C. mindorensis with 100% identity to the GenBank accession of the same species and 100 bootstrap supports in the phylogenetic tree with members of the subfamily Crocodilinae. A neighbor-joining tree was constructed in MEGA 7 using a trimmed 461 bp fragment of the COI gene (Figure 4). Osteolaemus tetraspis (Crocodilidae: Crocodilinae) was used as an outgroup.
| Sample ID | BLAST Result | %Identity | Accession |
| CPP1 | Crocodylus mindorensis | 100% | GU144287 |
Table 4: BLAST results table for the 658 bp fragment of the COI gene confirming the identity of CPP1 as C. mindorensis with 100% identity to the whole mt genome sequence in GenBank (GUI144287).
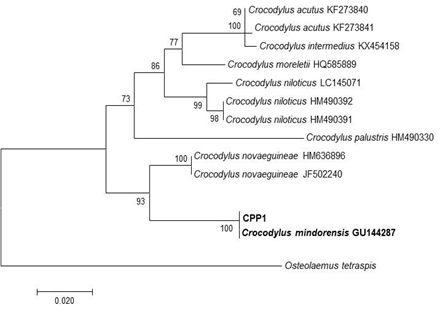
Figure 3: Sketch of a sun-dried skins from an adult (a) and a juvenile (b) crocodile
taken from Lakes Pugwan and Pangalman respectively. Lake Sebu 2012.
Habitat Assessment – Thirty – eight percent of the 21 highland crests and inundated basins or small lakes have reports of crocodile sightings from 2007-2010. Three of this wetland has high ecological value and viability with partly forested and intact natural catchment condition. Probable viable habitat was observed in Lake Ubodan (6°18’10.8“N, 124°25’34.8”E; 792m ASL) with layers of shrub and marsh associated vegetation in its clear and greenish surface water habitat. A lower montane marginal forest surrounded the lake shore peripheral vegetation.
Conclusion
The presentation of the long standing findings on cranial morphology and dorsal skin scalation patterns that resembled with C. mindorensis has been cemented by DNA sequencing. This suggests that crocodile artifacts and specimens retrieved were from Philippine crocodile, Crocodylus mindorensis. The presence C. mindorensis in this highland region of Daguma Mountain Range was the second record of population existed in high altitude (750–800 m ASL) next to Abra, Cordillera Central Mountain (850 m ASL). The preconceived impressions on altitudinal limitations for C. mindorensis were decisively diminished. A new viable populations and potential habitats can be unearthed and considered for restocking program.
Acknowledgements
The authors would like to extend gratitude to the DENR Provincial Environment and Natural Resources Office – Cotabato and the Biodiversity Management Bureau for their support in the retrieval of specimens. We also wished to acknowledge the field efforts rendered by Julius Gil and Armel Delante, students of the University of Southern Mindanao, the coordination support of the Philippine Crocodile Rescue and Breeding Center (PCRBC) and the Environment and Livelihood Organization for Advancing Development, and to our local informants/guides, Trivetth Tupas, Enrique Besa and Romy Baay, who assist in on-field logistics is remarkable.
The authors would like to extend gratitude to the DENR Provincial Environment and Natural Resources Office – Cotabato and the Biodiversity Management Bureau for their support in the retrieval of specimens. We also wished to acknowledge the field efforts rendered by Julius Gil and Armel Delante, students of the University of Southern Mindanao, the coordination support of the Philippine Crocodile Rescue and Breeding Center (PCRBC) and the Environment and Livelihood Organization for Advancing Development, and to our local informants/guides, Trivetth Tupas, Enrique Besa and Romy Baay, who assist in on-field logistics is remarkable.
Conflict of interest
The authors declared no financial interest or any potential conflict of interest with respect to authorship and publication of this article.
The authors declared no financial interest or any potential conflict of interest with respect to authorship and publication of this article.
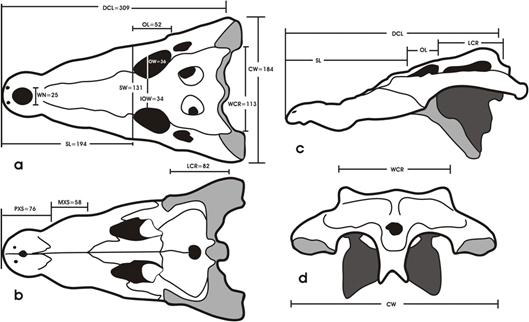
View of skull specimen from Lake Pugwan, Bgy. Ned, Lake Sebu, Philippines showing measurements taken. DCL = dorsal cranial length, CW = cranial width, SW = basal snout width, SL = snout length, IOW = minimal interorbital width, OW = maximal orbital width, OL = maximal orbital length, LCR = length of postorbital cranial roof, WCR = posterior width of the cranial roof, WN = maximal width of external nares, PXS = length of premaxillary symphysis, and MXS = length of maxillary symphysis. 1mm.
*DCL = dorsal cranial length; RWST (relative width of snout) = (basal width of snout x 100)/snout length; RLST (relative length of snout) = (snout length x 100)/DCL; RCW (relative cranial width) = (cranial width x 100)/DCL; RWI (relative interorbital width) = (minimal interorbital width x 100)/maximal orbital length; RLR = (relative length of postorbital cranial roof) = (length of postorbital cranial roof x 100)/posterior width of cranial roof; ROL = (relative orbital length) = (maximal orbital length x 100)/DCL; ROW = (relative orbital width) = (maximal orbital width x 100)/maximal orbital length; RWN = (relative width of external nares) = (maximal width of external nares x 100)/DCL – snout length. (adapted from Hall, 1989).
References
- Bellairs A. “The life of reptiles. Weidenfeld & Nicolson” (1969).
- Fukuda Y., et al. “Estimation of Total Length from Head Length of Saltwater Crocodiles (Crocodylus porosus) in the Northern Territory, Australia”. Journal of Herpetology 47.1 (2013): 34–40.
- Hall PM. “Variation in geographic isolates of the New Guinea crocodile (Crocodylus novaeguinae Schmidt) compared with the similar, allopatric Philippine crocodile”. Copeia 1 (1989):71-80.
- Iordansky NN. ‘Biology of the Reptilia”. Academic Press (1973): 201-262.
- Manalo RI., et al. “A New Distribution Record for the Philippine Crocodile (Crocodylus mindorensis, Schmidt 1935)”. International Union for Conservation of Nature Gland 49.1 (2015): 208-213.
- Manalo RI., et al. “Philippine crocodile (Crocodylus mindorensis, Schmidt 1935) population recorded in highlands of Lake Sebu, southern Mindanao, Philippines”. Crocodile Specialist Group Newsletter 31.1 (2013): 15-17.
- Manalo RI. Occurrence of Crocodylus mindorensis in the Cordillera Central. Abra Province, Luzon Island. In: Alba, E. & Lagartija, M. (Eds) National Museum Papers. Proceedings Forum on Crocodiles in the Philippines. National Museum of the Philippines, Ermita, Manila 14 (2008): 109–115.
- Messel HF., et al. The Present Status of the Two Species in the Philippines, the Analysis on the Activities of Crocodile Farming Institute (CFI), and the Future Strategy of Species Conservation. Summary report on the workshop on the prospect and future strategy of crocodile conservation of the two species (Crocodylus mindorensis and Crocodylus porosus) occurring in the Philippines. In: Crocodile Conservation Action. A Special Publication of the Crocodile Specialist Group of the Species Survival Commission of the IUCN-The World Conservation Union. (1992): 98-101.
- Oliveros C., et al. “The Philippine crocodile recorded on Dalupiri Island”. Crocodile Specialist Group Newsletter 24.3 (2005): 14-15.
- Platt SG, et al. “Size estimation, morphometrics, sex ratio, sexual size dimorphism, and biomass of Crocodylus acutus in the coastal zone of Belize”. Salamandra 47.4 (2011): 179-192.
- Pontillas UFA. The distribution, abundance, and population genetics and conservation Philippine crocodile Wildlife Conservation Society, Bronx, New York, USA (1999): 6.
- Ross CA and Alcala AC. “Distribution and status of the Philippine Crocodile (Crocodylus mindorensis). Kalikasan” Philippine Journal of Biology 12.1(1983): 169-173.
- Ross CA. “Crocodiles in the Republic of the Philippines”. In: Crocodiles. Proceeding of the 6th Working Meeting of the Crocodile Specialist Group, IUCN – The World Conservation Union, Gland, Switzerland and Cambridge UK (1984):84-90.
- Ross CA. Final Report. Smithsonian Institution/World Wildlife Fund Philippine Crocodile Project. WWF Report 1489 (1982): 34.
- Ross JP. “Crocodiles, status, survey, and conservation action plan”. IUCN Library system (1998): 136.
- Schmidt KP. “A new crocodile from the Philippine islands”. Field Mus. Nat. Hist. Zool. Ser 20 (1935): 67-70.
- Van Weerd, et al. Crocodylus mindorensis. The IUCN Red List of Threatened Species (2016): e.T5672A3048281 Downloaded on 13 February 2018.
Citation:
Rainier I Manalo., et al. “Evidence on the Presence of the Critically Endangered Philippine Crocodile, Crocodylus
mindorensis (Schmidt 1935) in the Highlands of Daguma Mountain Range, Lake Sebu, The Philippines”. Multidisciplinary Advances in
Veterinary Science 2.1 (2018): 276-282.
Copyright: © 2018 Rainier I Manalo., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.





























 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.