Research Article
Volume 2 Issue 3 - 2018
Hematological and Serological Changes in Neonatal Diarrheic Calves Infected with Bovine Rotavirus
Department of Pathology and Parasitology, Chittagong Veterinary and Animal Sciences University, Khulshi, Chittagong 4225, Bangladesh
*Corresponding Author: Shama Ranjan Barua, Department of Pathology and Parasitology, Chittagong Veterinary and Animal Sciences University, Khulshi, Chittagong 4225, Bangladesh.
Received: May 30, 2018; Published: June 05, 2018
Abstract
Bovine rotavirus A is a leading cause of diarrhea in calves. Diarrhea causes excessive water and electrolyte loss through the gastrointestinal (GI) tract and subsequently produces dehydration, and in acute cases can lead to death. The aim of the study was to evaluate haemato-biochemical changes occurred in diarrheic calves as well as rotavirus affected diarrheic calves. The study was undertaken in calves between 1-45 days old at Chittagong metropolitan area in Chittagong, Bangladesh during the period from January 2017 to June 2017. Fifty blood samples from 40 diarrheic and 10 non-diarrheic cases were collected and subjected to analysis of hematological and serological parameters. Hematological values in both rotavirus positive and negative cases had no significant differences except increased number of lymphocytes (75.58%; SD: 7.34; 95% CI: 71.81-79.36) which clearly indicate viral causation. Serological profile revealed increase in chloride level (115.05%; SD: 19.18; 95% CI: 108.83-121.27) in rotavirus positive diarrheic calves as compared to rotavirus negative cases and suggested the presence of hyperchloremia in diarrheic calves.
Keywords: Hematology; Serology; Rotavirus Diarrhea; Calves
Introduction
Neonatal calf diarrhea is a major health problem in dairy hard management. It has a multifactorial etiology including viruses, bacteria, protozoa and also management factors [1,2]. In respect to the enter pathogens, Escherichia coli K99 (E. coli), Coronavirus, Cryptosporidium parvum (C. parvum) and Rotavirus causes 75–95% of intestinal infections in young calves and especially rotavirus accounts for 27–36% [3-5]. Rotavirus is responsible for impairment of the mature enterocytes of the villi (primarily in the middle and terminal segment) of the small intestine and enhances its exfoliation [6]. As a result disturbed water and electrolyte absorption is observed, which, in turn, causes its accumulation in the gut lumen resulting diarrhea [6]. It leads to morbidity and mortality, delayed growth rate, higher age at first calving that consequence to direct and indirect economic losses in dairy farming [1, 7-9].
Rapid diagnosis of etiology and estimation of physiological alteration are important factors for proper treatment and control of diseases. Hematological and biochemical variables are most frequently used as diagnostic tool for taking medical decision. Analyses of blood parameters are very helpful to have an insight in metabolic and health status of animal. It is also very useful to compare the values obtained from ill animal with normal values in healthy animals during diagnostic procedure [10]. The changes in biochemical and hematological constituents are important indicators of the physiological or pathological state of the animal. Blood examination is also performed for screening of general health [11]. Diarrheic animals loose fluid, becomes rapidly dehydrated and are suffered from electrolyte loss, acidosis. Infectious agents may cause initial damage to the intestine but death from scours usually results from dehydration, acidosis and loss of electrolytes [12].
Packed cell volume (PCV) may increase in diarrheic calves that indicates fluid loss from vascular compartment [13], and oral rehydration solution therapy along with other therapeutic measure were effective in bringing back the hydration status of diarrheic calves to normal [14]. Therefore, packed cell volume (PCV) could be an important indicator of change in extra cellular fluid (ECF) volume during diarrhea. Dehydration in calves that have diarrhea is accompanied by large decreases in the extracellular fluid volume along with small increases in intracellular fluid volume [15,16]. In general, significant increase in hematological parameters as Hemoglobin (Hb), packed cell volume (PCV) and total erythrocyte counts (TEC) due to haemoconcentration are related with dehydration [17]. Moreover, some biochemical alterations such as hypoglycemia [17], Hyperproteinaemia and hyperalbuminaemia [18], hyperkalemia, hyperchloremia and hypernatremia[19] were also found associated with diarrhea.
The study was designed to estimate the hematological and serological values in diarrheal/non-diarrheal, rotavirus positive/negative cases in calves that might support the diagnosis of the suspected etiology.
Methodology
Study area and sample size
The study was undertaken on cattle calves under the age of 45 days exhibiting symptoms of diarrhea in selected dairy farms in Chittagong. A total of 50 samples (blood and faeces), among which 40 from the calves showing diarrhea and 10 samples without diarrhea were collected.
The study was undertaken on cattle calves under the age of 45 days exhibiting symptoms of diarrhea in selected dairy farms in Chittagong. A total of 50 samples (blood and faeces), among which 40 from the calves showing diarrhea and 10 samples without diarrhea were collected.
Sample collection
The fecal samples were collected directly from rectum using latex gloves. In addition, five to ten ml of blood from each of the calves was collected from the jugular vein in aseptic conditions using labeled sterile disposable syringes. About half of the blood was transferred immediately after collection to the sterilized vial containing ethylene di-amine tetra acetic acid (EDTA) solution and half of the blood was transferred to sterilized labeled test tubes for serum. All the samples were transported using cool box to the Clinical Pathology laboratory, Department of Pathology and Parasitology, Chittagong Veterinary and Animal Sciences University, Khulshi, Chittagong.
The fecal samples were collected directly from rectum using latex gloves. In addition, five to ten ml of blood from each of the calves was collected from the jugular vein in aseptic conditions using labeled sterile disposable syringes. About half of the blood was transferred immediately after collection to the sterilized vial containing ethylene di-amine tetra acetic acid (EDTA) solution and half of the blood was transferred to sterilized labeled test tubes for serum. All the samples were transported using cool box to the Clinical Pathology laboratory, Department of Pathology and Parasitology, Chittagong Veterinary and Animal Sciences University, Khulshi, Chittagong.
Sample processing
A 10% fecal suspension of individual sample was prepared in phosphate buffered saline (PH 7.2) and clarified by centrifugation at 13,000 rpm at 4℃ for 10 minutes. The supernatant was separated and stored at -70℃ until RNA extraction for real time RT-PCR. After coagulation, coagulated blood was centrifuged in 3000rpm for 15 minutes. Serum was transferred to the eppendorf tube by using micropipette. The obtained serum samples were stored in -20℃ at laboratory for biochemical test.
A 10% fecal suspension of individual sample was prepared in phosphate buffered saline (PH 7.2) and clarified by centrifugation at 13,000 rpm at 4℃ for 10 minutes. The supernatant was separated and stored at -70℃ until RNA extraction for real time RT-PCR. After coagulation, coagulated blood was centrifuged in 3000rpm for 15 minutes. Serum was transferred to the eppendorf tube by using micropipette. The obtained serum samples were stored in -20℃ at laboratory for biochemical test.
Laboratory analysis of blood and serum samples
Hematological analysis of total erythrocyte count (TEC/RBC), total leukocyte count (TLC/WBC), differential leukocyte count (DLC), packed cell volume (PCV), hemoglobin concentration (Hb) were done as per standard procedures described by Nemi [20] and Sharma and Singh [21] . Consequently, serological investigation was conducted following proper aseptic measures at the time of serum analysis in the laboratory. Serum was placed in normal temperature approximately 30 minutes before the analysis. Serum samples were evaluated for electrolyte parameters by an automated biochemical analyzer (HumalizerR-3000).
Hematological analysis of total erythrocyte count (TEC/RBC), total leukocyte count (TLC/WBC), differential leukocyte count (DLC), packed cell volume (PCV), hemoglobin concentration (Hb) were done as per standard procedures described by Nemi [20] and Sharma and Singh [21] . Consequently, serological investigation was conducted following proper aseptic measures at the time of serum analysis in the laboratory. Serum was placed in normal temperature approximately 30 minutes before the analysis. Serum samples were evaluated for electrolyte parameters by an automated biochemical analyzer (HumalizerR-3000).
Detection of rotavirus by real time RT- PCR
All the fecal samples were used for viral RNA extraction. Rotavirus RNA was extracted from the fecal suspension using QIAamp Viral RNA mini kit (Qiagen/Westburg, Leusden, Netherlands) following the manufacturer’s instructions. The extracted RNA were stored at -80°C. Subsequently, the real time RT-PCR reaction was performed by using a QIAGEN One Step RT-PCR Kit (QIAGEN) for the confirmation of bovine rotavirus A. The real time RT-PCR assay was performed by using an ABI 7500 thermo cycler (Applied Biosystems). The TaqMan® assay primers and probe sequences and thermal cycles were selected for testing according to previously described by Jothikumar, Kang [22].
All the fecal samples were used for viral RNA extraction. Rotavirus RNA was extracted from the fecal suspension using QIAamp Viral RNA mini kit (Qiagen/Westburg, Leusden, Netherlands) following the manufacturer’s instructions. The extracted RNA were stored at -80°C. Subsequently, the real time RT-PCR reaction was performed by using a QIAGEN One Step RT-PCR Kit (QIAGEN) for the confirmation of bovine rotavirus A. The real time RT-PCR assay was performed by using an ABI 7500 thermo cycler (Applied Biosystems). The TaqMan® assay primers and probe sequences and thermal cycles were selected for testing according to previously described by Jothikumar, Kang [22].
Statistical analysis
The laboratory data was stored in Microsoft Excell-2007 and Statistical differences were calculated according to the Student t-test with significance level at P < 0.05 using the procedure of (SAS, 2004). Descriptive statistics analysis was done to measure the mean, SD, 95% confidence interval (CI) and P value of different parameters. The two sample paired t-test was done to compare the diarrhea/non-diarrhea, rotavirus positive/negative and diarrhea with rotavirus positive/negative variation.
The laboratory data was stored in Microsoft Excell-2007 and Statistical differences were calculated according to the Student t-test with significance level at P < 0.05 using the procedure of (SAS, 2004). Descriptive statistics analysis was done to measure the mean, SD, 95% confidence interval (CI) and P value of different parameters. The two sample paired t-test was done to compare the diarrhea/non-diarrhea, rotavirus positive/negative and diarrhea with rotavirus positive/negative variation.
Results
The blood samples were subjected to estimation of hematological parameters including Hemoglobin, packed cell volume, total erythrocyte count, total leukocyte count and differential leukocyte count and serological parameters including sodium, potassium and chloride. All the parameter estimates has been compiled in Table 3-8 and compared with the reference values of Table 1&2.
Comparison of hematological and serological parameters in non-diarrheic and diarrheic
The haemoglobin (Hb) values in both non-diarrheic and diarrheic calves were recorded below the average values of normal range (table 1) which was 8.01 and 10.17 g/dl in non-diarrheic and diarrheic calves accordingly. Similarly, other hematological parameters values were recorded below the normal ranges in both non-diarrheic and diarrheic calves except the values of lymphocyte and monocyte. The values of lymphocytes were 69.45% and 71.72% in non-diarrheic and diarrheic calves, respectively; the values of monocyte in the non-diarrheic and diarrheic calves were 5.54 and 4.23, respectively. On the other hand, biochemical parameters of non-diarrheic and diarrheic calves were ranged within the reference values (Table 4).
The haemoglobin (Hb) values in both non-diarrheic and diarrheic calves were recorded below the average values of normal range (table 1) which was 8.01 and 10.17 g/dl in non-diarrheic and diarrheic calves accordingly. Similarly, other hematological parameters values were recorded below the normal ranges in both non-diarrheic and diarrheic calves except the values of lymphocyte and monocyte. The values of lymphocytes were 69.45% and 71.72% in non-diarrheic and diarrheic calves, respectively; the values of monocyte in the non-diarrheic and diarrheic calves were 5.54 and 4.23, respectively. On the other hand, biochemical parameters of non-diarrheic and diarrheic calves were ranged within the reference values (Table 4).
| Parameters | Normal Range (Calves) | Diarrheic Calves |
| Haemoglobin (g/dl) | 11.3 (9.5-13.5) | 12.2 ± 0.221 |
| PCV (%) | 37.5 (36.0-49.0) | 44.2 ± 0.692 |
| TEC (million/cm3) | 8.1 | 8.54 ± 0.316 |
| TLC (thousands/cm3) | 9.7 | 12.4 ± 0.295 |
| Neutrophils (%) | 37 (12-38) | 46.8 ± 0.748 |
| Eosinophils (%) | 1.9 (2-30) | 1.7 ± 0.482 |
| Basophils (%) | 0.6 (0-2) | 0.5 ± 0.147 |
| Lymphocytes (%) | 68.6 (33-87) | 51.6 ± 0.514 |
| Monocytes (%) | 3.4 (1-5) | 2.72 ± 0.687 |
Courtesy by Mailk, Kumar (36)
Table 1: Hematological profiles of normal and diarrheic calves.
Table 1: Hematological profiles of normal and diarrheic calves.
| Parameters | Normal range (Calves)# | Diarrheic calves## |
| Na (mEq/litre) | 144 ± 2.9 | 126.16 ± 1.815 |
| K (mEq/litre) | 5.95 ± 0.48 | 5.6 ± 0.084 |
| Cl- (mEq/litre) | 102.3 ± 2.7 | 122.47 ± 1.943 |
# Klinkon and Ježek (10); ## Mailk, Kumar (36)
Table 2: Biochemical profile of normal and diarrheic calves.
Table 2: Biochemical profile of normal and diarrheic calves.
| Variable | Infection status | Mean | SD | 95% CI | P-value (Ttest) |
| Hemoglobin (g/dl) | Non-diarrhoea | 8.01 | 2.01 | 6.66-9.36 | |
| Diarrhoea | 10.17 | 2.30 | 9.43-10.92 | 3.05 | |
| PCV (%) | Non-diarrhoea | 31.45 | 9.71 | 24.93-37.98 | |
| Diarrhoea | 36.54 | 10.19 | 33.23-39.84 | 1.52 | |
| TLC (thousands/cm3) | Non-diarrhoea | 8.15 | 3.81 | 5.59-10.71 | 0.03 |
| Diarrhoea | 8.12 | 3.16 | 7.09-9.14 | ||
| TEC (million/cm3) | Non-diarrhoea | 4.95 | 2.87 | 3.03-6.88 | |
| Diarrhoea | 5.32 | 2.36 | 5.56-6.09 | 0.39 | |
| Lymphocytes (%) | Non-diarrhoea | 69.45 | 8.91 | 63.47-75.44 | |
| Diarrhoea | 71.72 | 10.05 | 68.46-74.97 | 0.72 | |
| Neutrophils (%) | Non-diarrhoea | 12.09 | 8.43 | 6.43-17.76 | |
| Diarrhoea | 19.79 | 11.52 | 16.06-23.53 | 2.45 | |
| Monocyte (%) | Non-diarrhoea | 5.54 | 3.67 | 3.08-8.01 | 1.12 |
| Diarrhoea | 4.23 | 2.47 | 3.43-5.03 | ||
| Basophil (%) | Non-diarrhoea | 0.09 | 0.30 | 0.11-0.29 | |
| Diarrhoea | 0.05 | 0.22 | 0.02-0.12 |
Table 3: Association of hematological parameters for non-diarrheic with diarrheic.
| Variable | Infection status | Mean | SD | 95% CI | P-value (Ttest) |
| Sodium (mEq/litre) | Non-diarrhoea | 152.52 | 27.06 | 134.34-170.70 | 2.47 |
| Diarrhoea | 128.59 | 18.87 | 122.47-134.71 | ||
| Potassium (mEq/litre) | Non-diarrhoea | 5.74 | 0.94 | 5.10-6.37 | |
| Diarrhoea | 6.23 | 1.44 | 5.77-6.70 | 1.35 | |
| Chloride (mEq/litre) | Non-diarrhoea | 99.55 | 9.21 | 93.36-105.74 | |
| Diarrhoea | 115.05 | 19.18 | 108.83-121.27 | 3.74 |
Table 4: Association of blood biochemistry parameters for non-diarrheic with diarrheic.
Comparison of hematological and serological parameters of Rota virus positive and negative calves
The hemoglobin values in both rotavirus positive and negative cases irrespective of diarrheic and non-diarrheic calves were lower than the average normal range. The mean value of hemoglobin was recorded as 9.75 and 9.59 g/dl as in rotavirus positive and negative cases, respectively (Table 5). However, the association was not found significant (P-value= 0.82).
The hemoglobin values in both rotavirus positive and negative cases irrespective of diarrheic and non-diarrheic calves were lower than the average normal range. The mean value of hemoglobin was recorded as 9.75 and 9.59 g/dl as in rotavirus positive and negative cases, respectively (Table 5). However, the association was not found significant (P-value= 0.82).
The PCV Values in both rotavirus positive and negative cases were lower than the average normal range. Average mean value of PCV was recorded as 36.33% and 33.64% in rotavirus positive and negative cases, respectively (Table 5). The P-value of the significant test was 0.38 (statistically non-significant).
The TEC Values in all the diarrheic calves were lower than the normal range. The mean values of the TEC were 4.44 and 5.65 million per cm3 in both rotavirus positive and negative cases, respectively (Table 5); the TLC values in all the diarrheic calves were lower than the normal range. The mean values of TLC were 7.67 and 8.35 thousands per cm3 in rotavirus positive and negative cases, respectively.
In the present study, the lymphocyte count was observed higher in diarrheic calves as compared with the normal values and average mean value of lymphocyte was estimated as 75.58% and 68.96% in rotavirus positive and negative cases, respectively. On the other hand, the neutrophil count was lower in diarrheic calves as compared with the normal values and the average mean value appeared as 15.41% and 19.48% in rotavirus positive and negative cases, respectively. The monocyte count in the diarrheic calves was slightly higher in comparison to that of normal values of healthy calves, whereas, the mean value of monocyte count was 4.11% and 4.72% in rotavirus positive and negative cases, respectively (Table 5).
| Variable | Infection status | Mean | SD | 95% CI | P-value (Ttest) |
| Hemoglobin(g/dl) | Negative | 9.75 | 2.40 | 8.89-10.60 | 0.82 |
| Positive | 9.59 | 2.44 | 8.33-10.85 | ||
| PCV (%) | Negative | 36.33 | 9.91 | 32.81-39.84 | 0.38 |
| Positive | 33.64 | 10.85 | 28.06-39.22 | ||
| TLC (thousands/cm3) | Negative | 8.35 | 3.42 | 7.14-9.56 | 0.49 |
| Positive | 7.67 | 3.01 | 6.12-9.22 | ||
| TEC (million/cm3) | Negative | 5.65 | 2.76 | 4.67-6.63 | 0.09 |
| Positive | 4.44 | 1.42 | 3.69-5.19 | ||
| Lymphocytes (%) | Negative | 68.96 | 10.18 | 65.35-72.58 | 0.02 |
| Positive | 75.58 | 7.34 | 71.81-79.36 | ||
| Neutrophils (%) | Negative | 19.48 | 12.66 | 14.99-23.97 | 0.23 |
| Positive | 15.41 | 7.69 | 11.46-19.35 | ||
| Monocyte (%) | Negative | 4.72 | 2.93 | 3.68-5.76 | 0.46 |
| Positive | 4.11 | 2.49 | 2.83-5.40 | ||
| Basophil (%) | Negative | 0.09 | 0.29 | 0.01-0.19 | 0.20 |
| Positive | 0 | 0 | 0 |
Table 5: Association of hematological parameters with rota virus positive and negative calves diagnosed by rtRT-PCR.
Three common electrolytes (sodium, potassium and chloride) as a measure of homeostasis and water balance were studied and the mean values of the results are shown in Table 6. It can be noted that the sodium value in diarrheic calves was lower than that of normal values. Sodium was ranged between 132.28 and 134.66 mEq per litre in rotavirus positive and negative cases, respectively (Table 6). In the present study, it was recorded that the potassium values in diarrheic calves were slightly higher than that of normal values. The mean values of potassium in rotavirus positive and negative cases were 5.81 and 6.28 mEq per litre, respectively. Similarly, estimated value of chloride was higher in the diarrheic calves as compared to that of normal values. The mean values of chloride in rotavirus positive and negative cases were 109.42 and 115.93 mEq per litre, respectively. However, none of the significance test showed any significant association between different biochemical parameter and diarrheic or rota virus positive calves.
| Variable | Infection status | Mean | SD | 95% CI | P-value (Ttest) |
| Sodium (mEq/litre) | Negative | 134.66 | 25.39 | 125.66-143.68 | 0.73 |
| Positive | 132.28 | 17.80 | 123.12-141.43 | ||
| Potassium (mEq/litre) | Negative | 6.28 | 1.40 | 5.78-6.77 | 0.25 |
| Positive | 5.81 | 1.25 | 5.17-6.46 | ||
| Chloride (mEq/litre) | Negative | 109.42 | 16.71 | 103.49-115.35 | 0.24 |
| Positive | 115.92 | 21.61 | 104.81-127.04 |
Table 6: Association of blood biochemistry parameters with rotavirus positive and negative calves diagnosed by rtRT-PCR.
Comparison of hematological and serological parameters in diarrheic rota virus positive and negative calves
The results of hematological and serological parameters for diarrheal calves with rotavirus positive and negative cases represented in the table 7 & 8. The average lymphocyte value of rotavirus positive diarrheal calves were 74.69% which were higher than the value of rotavirus negative diarrheal calves that represented 69.65% (Table 7).
The results of hematological and serological parameters for diarrheal calves with rotavirus positive and negative cases represented in the table 7 & 8. The average lymphocyte value of rotavirus positive diarrheal calves were 74.69% which were higher than the value of rotavirus negative diarrheal calves that represented 69.65% (Table 7).
| Variable | Infection status | Mean | SD | 95% CI | P-value (Ttest) |
| Hemoglobin (g/dl) | Negative | 10.4 | 2.35 | 9.38-11.41 | 0.72 |
| Positive | 9.86 | 2.27 | 8.65-11.07 | ||
| PCV (%) | Negative | 38 | 9.80 | 33.76-42.24 | 1.06 |
| Positive | 34.44 | 10.69 | 28.74-40.13 | ||
| TLC (thousands/cm3) | Negative | 8.71 | 3.45 | 7.22-10.20 | 1.51 |
| Positive | 7.26 | 2.57 | 5.89-8.63 | ||
| TEC (million/cm3) | Negative | 5.84 | 2.76 | 4.64-7.03 | 1.87 |
| Positive | 4.58 | 1.38 | 3.85-5.32 | ||
| Lymphocytes (%) | Negative | 69.65 | 11.59 | 64.64-74.66 | |
| Positive | 74.69 | 6.54 | 71.20-78.17 | 1.73 | |
| Neutrophils (%) | Negative | 22.39 | 13.21 | 16.68-28.10 | 1.91 |
| Positive | 16.06 | 7.42 | 12.11-20.02 | ||
| Monocyte (%) | Negative | 4.22 | 2.49 | 3.14-5.29 | |
| Positive | 4.25 | 2.52 | 2.91-5.59 | 0.04 | |
| Basophil (%) | Negative | 0.09 | 0.29 | 0.04-0.21 | 1.45 |
| Positive | 0 | 0 | 0 |
Table 7: Association of hematological parameters for diarrhea with rota virus positive and negative calves diagnosed by rtRT-PCR.
| Variable | Infection status | Mean | SD | 95% CI | P-value (Ttest) |
| Sodium (mEq/litre) | Negative | 127.41 | 20.74 | 118.44-136.38 | |
| Positive | 130.29 | 16.32 | 121.59-138.99 | 0.49 | |
| Potassium (mEq/litre) | Negative | 6.51 | 1.51 | 5.86-7.16 | 1.49 |
| Positive | 5.84 | 1.29 | 5.150-6.52 | ||
| Chloride (mEq/litre) | Negative | 114.23 | 17.18 | 106.81-121.67 | |
| Positive | 116.22 | 22.29 | 104.34-128.10 | 0.30 |
Table 8: Association of blood biochemistry parameters for diarrhoea with rota virus positive and negative calves diagnosed by rtRT-PCR.
Visual differences in the maximum, minimum and median values of hemoglobin, PCV, ESR, TLC and TEC, lymphocytes, neutrophils, eosinophil’s and monocytes according to sex of the study population can be noted in figure 1 & 2. Similarly, the maximum, minimum and median value of sodium, potassium and chloride according to sex of the study population showed visual differences in figure 3.
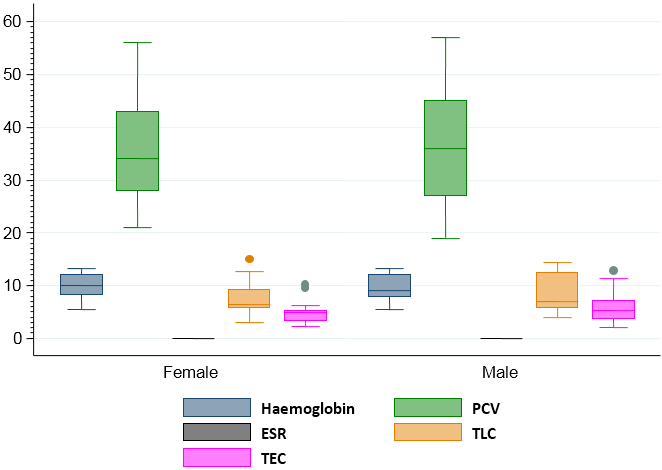
Figure 1: Boxplot showing the maximum, minimum and median value of haemoglobin, PCV, ESR, TLC and TEC according to sex of the study population.
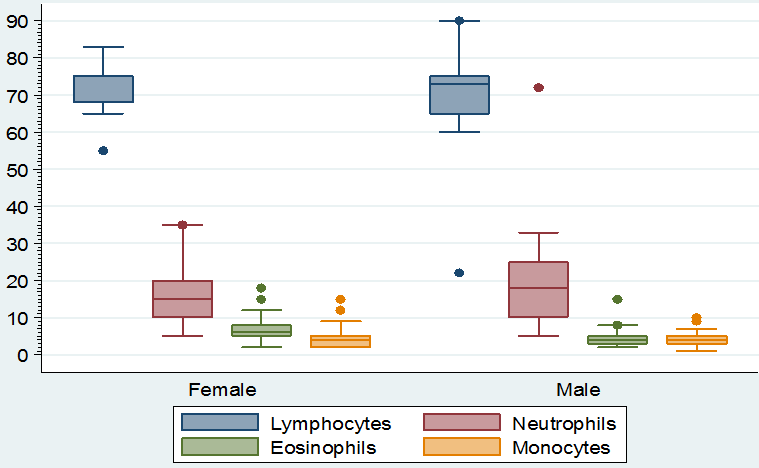
Figure 2: Boxplot showing the maximum, minimum and median value of lymphocytes, neutrophils, eosinophil’s and monocytes according to sex of the study population.
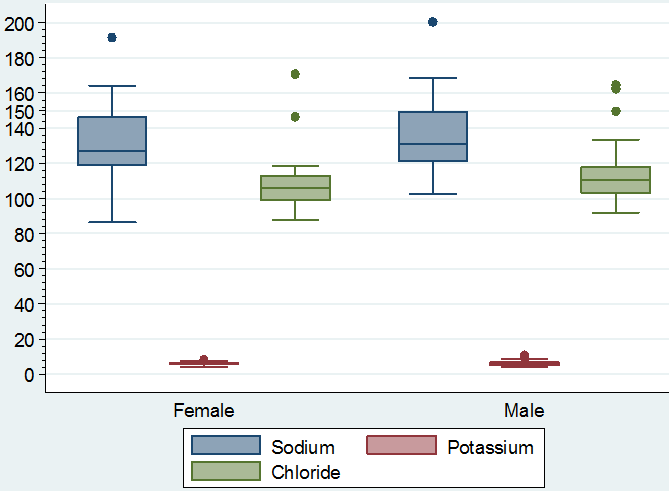
Figure 3: Boxplot showing the maximum, minimum and median value of sodium, potassium and chloride according to sex of the study population.
Discussion
In the present study, the hemoglobin value (Table 3, 5 &7) was lower in the study population compared to the reference value (Table 1) but it was higher in diarrheic than in non-diarrheic calves. The increase in hemoglobin in diarrheic calves might be due to haemoconcentration associated with dehydration. Our finding was in concordant with the findings of Schalm., et al. (1975) and [19, 23].
The elevation of PCV was observed in diarrheic calves compared to non-diarrheic. The increased PCV values in diarrheic calves indicate fluid loss from vascular compartment [13]. The elevation of PCV is an indicator of dehydration [24,25]. Thus, estimation of PCV is of utmost importance to monitor hydration status of animal and is a sensitive indicator for assessing the severity of dehydration. Increase in PCV in diarrheic calves was apparently due to hemo-concentration associated with dehydration and hypovolemia.
Elevated TEC was revealed in the diarrheic calves in the present study. The result is supported by the earlier findings by Schalm., et al. (1975) and [19] reported significant elevation of TEC in diarrheic calves. Haemoconcentration of blood due to diarrhea might be the underlying reason for this trend.
TLC value did not show any difference between diarrheic and non-diarrheic calves in the present study. Different studies revealed that the value of TLC increased in diarrheic calves and also reported significant leucocytosis which might have occurred due to normal reaction of body defense mechanism against infective cause of diarrhea and also due to haemoconcentration due to dehydration [19,26].
Differential leukocyte count (DLC) includes the estimation of relative percentage of neutrophils, eosinophil’s, basophils, lymphocytes and monocytes in per ml of blood. Increase in DLC is suggestive of infectious agents of different origin. The present study revealed increase in lymphocyte count in rotavirus positive cases than rotavirus negative indicates a viral etiology of the diarrhea (Table 5 & 7).
The sodium is the most important cation in extracellular fluid as it is responsible for maintenance of osmotic pressure[10]. Together with chlorine (Cl) it collaborates in metabolism of water and regulation of acid-base balance in the cell [10]. The present study revealed significant reduction of Na values in diarrheic calves. These findings were in agreement with observations of [19,27,28]. Similarly, [29] also reported hypernatremia in diarrheic calves due to an excessive secretion of sodium along with water into intestinal lumen. These findings are contrary to that of [30] who recorded hypernatraemia in diarrhoeic calves as compared to healthy calves. Most of the diarrhoea causing microorganisms disrupt the intestinal function and dehydrate the body either by increasing the chloride-secreting activity of the crypt cell or impairing the absorption of sodium by the villus cells or both [31].
Potassium is important for making electrical potential for transport of nerve impulses and for maintenance of muscle tonicity [10]. Potassium is also important for regulation of acid-base balance in the body [10]. Hypokalemia increase the membrane potential and cause hyper polarization block, that has influence on lower muscle tone and paralysis [32]. The present study revealed elevation of potassium levels in all the diarrheic calves. Hyperkalemia is caused by increased potassium retention by kidney and also due to cellular damage [33,34]. Hyperkalemia observed in the present study might be due to damage of enterocytes by microorganisms. Similar observations have been reported by other researchers [19,30,35]. In our study, serum chloride values of diarrheic calves showed elevation of chloride values in diarrheic calves.
Conclusions
It can be concluded that diarrheal condition of calves influences the hematological and serological variables that could be considered during diagnosis. In the study, though, no statistically significant alterations were observed between the diarrheic and non-diarrheic calves, the analysis of blood and serum samples of the diarrheic calves revealed the elevation of Hb, PCV, TLC and Chloride compared to non-diarrheal calves. Similarly, Rota viral positive diarrheal cases showed elevation in lymphocyte count along with some other parameters. Finally, the results of the study can be used to partially support the diagnosis of the rotavirus infection in calves.
Acknowledgement
This work is supported by University Grants Commission Higher Education and Quality Enhancement Project (CP-3220).
This work is supported by University Grants Commission Higher Education and Quality Enhancement Project (CP-3220).
Authors Contribution
SRB, TMR designed and conducted the research. SC, MM, MAH supervised the research. SD supported in manuscript preparation.
SRB, TMR designed and conducted the research. SC, MM, MAH supervised the research. SD supported in manuscript preparation.
Conflict of interest
Authors have no conflict of interest.
Authors have no conflict of interest.
References
- Bartels CJ., et al. “Prevalence, prediction and risk factors of enteropathogens in normal and non-normal faeces of young Dutch dairy calves”. Preventive Veterinary Medicine 93.2 (2010): 62-69.
- Lorenz I, editor Diarrhoea of the young calf: an update. Proceedings of the XXIV World Buiatrics Congress; (2006).
- Okur-Gumusova S., et al. “Seroprevalence of bovine viral respiratory diseases”. Acta veterinaria 57.1 (2007): 11-16.
- Uhde FL., et al. “Prevalence of four enteropathogens in the faeces of young diarrhoeic dairy calves in Switzerland”. The Veterinary Record 163.12 (2008): 362-366.
- Dhama K., et al. “Rotavirus diarrhea in bovines and other domestic animals”. Veterinary research communications 33.1 (2009): 1-23.
- Dratwa-Chałupnik A., et al. “Calves with diarrhea and a water-electrolyte balance”. Medycyna Weterynaryjna 68.1 (2012): 5-8.
- Bouzid M., et al. “Cryptosporidium pathogenicity and virulence”. Clinical microbiology reviews 26.1 (2013): 115-134.
- Stewart JR., et al. “The coastal environment and human health: microbial indicators, pathogens, sentinels and reservoirs”. Environmental Health 7 (2008).
- Chowdhury S., et al. “Survey of calf management and hygiene practices adopted in commercial dairy farms in Chittagong, Bangladesh”. Advances in Animal and Veterinary Sciences 5.1 (2017): 14-22.
- Klinkon M and Ježek J. “Values of blood variables in calves”. A bird’s-eye view of veterinary medicine’(Ed CC Perez-Marin) (2012): 301-320.
- Peinado VI., et al. “Basic hematological values in some wild ruminants in captivity”. Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 124.2 (1999): 199-203.
- Radostits O., et al. “Diseases of the newborn”. Veterinary medicine A textbook of the diseases of cattle, sheep, pigs, goats, and horses (2007):107-110.
- Naylor JM. “Severity and nature of acidosis in diarrheic calves over and under one week of age”. The Canadian Veterinary Journal 28.4 (1987): 168-173.
- Dhaliwal P. Studies of fluid and electrolyte imbalance in buffalo calves with special reference to its therapy: Ph. D. dissertation, Punjab Agriculture University, Ludhiana (1993).
- Constable P. “Fluid and electrolyte therapy in ruminants”. Veterinary Clinics: Food Animal Practice 19.3 (2003): 557-597.
- Smith GW. “Treatment of calf diarrhea: Oral fluid therapy”. Veterinary Clinics of North America: Food Animal Practice 25.1 (2009): 55-72.
- Schalm O., et al. Veterinary Haematology. 3rd (Ed.) Lea. And Febiger, Philadelphia, USA (1975).
- Gupta P. “Efficacy of psidium guajava (guava) leaves as an anti diarrhoeal in calves with special reference to E. Coli”. Nanaji Deshmukh Veterinary Science University Jabalpur (2013): 52.
- Brar A., et al. “Hematological changes in neonatal diarrheic calves of different age groups”. Indian Journal of Veterinary Pathology 39.1 (2015): 73-77.
- Nemi CJ. Schalm's veterinary hematology. Lea and Febiger Philadelphia Pa USA (1986).
- harma I and Singh H. Student's laboratory manual of veterinary physiology: Kalyani (2008).
- Jothikumar N., et al. “Broadly reactive TaqMan® assay for real-time RT-PCR detection of rotavirus in clinical and environmental samples”. Journal of virological methods 155.2 (2009): 126-131.
- Al-Robaiee I and Al-Farwachi M. “Prevalence of rotaviral infection in diarrheic neonatal calves in Mosul city, Iraq”. Veterinary World 6 (2013): 538-540.
- Rajora V and Pachauri S. “Laboratory assessment as an aid to rehydration therapy in neonatal diarrhoeic calves”. Indian journal of veterinary medicine 20.1 (2000): 18-20.
- Al-Robaiee I and Al-Farwachi M. “Changes in blood gases and electrolytes in local calves affected with diarrhea”. Iraqi Journal of Veterinary Sciences 26 (2012.
- Windeyer M., et al. “Factors associated with morbidity, mortality, and growth of dairy heifer calves up to 3 months of age”. Preventive veterinary medicine 113.2 (2014): 231-240.
- Singh M., et al. “A study on alteration in Haemato-biochemical parameters in Colibacillosis affected calves”. International Journal 2.7 (2014): 746-750.
- Shekhar S., et al. “Prevalence, Clinicohaemato-Biochemical Alterations in Colibacillosis in Neonatal Calves”. International Journal of Current Microbiology and Applied Sciences 6.9 (2017): 3192-3198.
- Singh K., et al. “Bacteriological investigation on buffalo calves suffering from gastrointestinal tract disorders and their In vitro drug sensitivity”. Indian Journal of Comparative Microbiology, Immunology and Infectious Diseases 27.1 (2006): 54-56.
- Kaur K., et al. “Haemato-biochemical profile of diarrhoeie dairy calves affected with colibacillosis”. Indian Journal of Veterinary Medicine (2006).
- Hirschhorn N and Greenough Wr. “Progress in oral rehydration therapy”. Scientific American 264.5 (1991): 50-56.
- Carlson GP. Fluid, electrolyte and acid-base balance. Clinical Biochemistry of Domestic Animals (Fifth Edition): Elsevier (1997): 485-516.
- Kirchner D., et al. “Dietary influences on the hydration and acid–base status of experimentally dehydrated dairy calves”. The Veterinary Journal 199.2 (2014): 251-257.
- Sobiech P., et al. “Changes in blood acid-base balance parameters and coagulation profile during diarrhea in calves”. Polish journal of veterinary sciences 16.3 (2013): 543-549.
- Ewaschuk JB., et al. “Lactobacillus GG Does Not Affect D‐Lactic Acidosis in Diarrheic Calves, in a Clinical Setting”. Journal of veterinary internal medicine 20.3 (2006): 614-619.
- Mailk S., et al. Journal of Animal Health and Production 1.2 (2013): 15-19.
Citation:
Shama Ranjan Barua., et al. “Hematological and Serological Changes in Neonatal Diarrheic Calves Infected with Bovine
Rotavirus”. Multidisciplinary Advances in Veterinary Science 2.3 (2018): 356-366.
Copyright: © 2018 Shama Ranjan Barua., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.





























 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.