Editorial
Volume 2 Issue 4 - 2018
Heat stress and Immune Function in Livestock
1Animal Nutrition Division, ICAR-National institute of animal Nutrition and Physiology, Bangalore, India
2Animal Physiology Division, ICAR-National institute of animal Nutrition and Physiology, Bangalore, India
2Animal Physiology Division, ICAR-National institute of animal Nutrition and Physiology, Bangalore, India
*Corresponding Author: M Bagath, Scientist, Animal Nutrition Division, ICAR-National institute of animal Nutrition and Physiology,
Bangalore, India.
Received: July 07, 2018; Published: July 26, 2018
Keywords: Heat stress; TLR; Cytokines; HSPs; Innate and Adaptive immunity
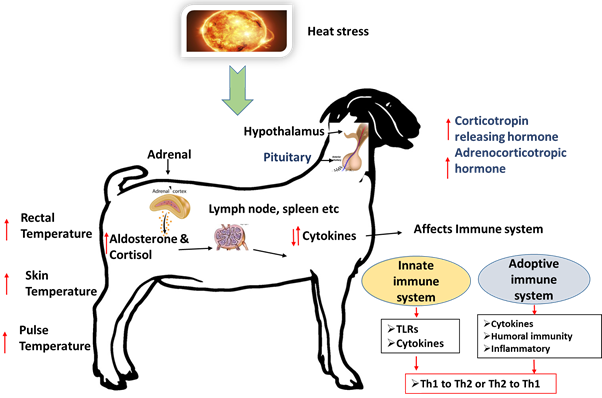
Agriculture sector provides the highest number of livelihood than any other sector [1]. In this sector, livestock provides milk, meat, draught, fuel and manure to the farmers, thus playing a huge role in the life of landless, small and marginal farmers [2]. Stress due to the erratic weather pattern has become a factual burden to the livestock especially due to the Heat Stress (HS). HS is one of the important stresses that affect the livestock in the present climate change scenario. In recent years, climate change has gained its importance owing to its impact on both humans as well as livestock. Impact of HS has not only affected the animal’s growth and production but also their immune system. The brain perceives the HS situation and stimulates the hypothalamic–pituitary–adrenal axis (HPA axis) and the sympathetic-adrenal–medullary (SAM) axis. The activated hypothalamus releases corticotropin-releasing hormone (CRH) which acts on the pituitary to produce adrenocorticotropin hormone (ACTH). ACTH acts on the adrenal gland to produce glucocorticoids and nor-adrenaline and adrenaline (catecholamines). The primary glucocorticoid molecule is cortisol. The glucocorticoids efficiently act to abate the HS response and has an anti-inflammatory effect on the immune response while the catecholamines have an inflammatory effect.
The immune system is an essential component of the defense system that has evolved and changed through evolution, to safeguard the livestock against the invading pathogens. The invading pathogens are neutralized by the innate and adaptive immune system. The balance between the innate and the adaptive immune systems are controlled by the combinatorial action of both HPA axis and SAM axis. The genes coding for the stressor proteins like heat shock proteins (HSPs), Toll-like receptors (TLRs), NOD-like receptors (NLRs), C-type lectin receptors (CLRs), RIG-I like receptors (RLRs), and the AIM2-like receptors (ALRs) and cytokines play a vital role in immune regulation [3].
HS an important challenge in the forthcoming years due to escalating environmental temperature at a global scale. This rise in temperature along with decreasing agriculture land and increasing human population to feed, is really a concern for the farmers and to the livestock industry [4]. Extreme temperature can cause distress to animals leading to changes in pulse rate, respiration rate, temperature, and metabolism, thus affecting their growth, production and immune system. Hence to overcome the HS, the animal moves into/near water bodies or exhibit shade-seeking behavior. Animals maintain their body temperature in a balanced state by the process of thermoregulation. Metabolism helps the animal to produce heat for various body functions. Increased metabolic heat and elevating weather temperature simultaneously, leads to affect the dissipation of excess heat gained. HS also plays an important role in affecting animal’s immune system thus making them more susceptible and increasing the morbidity and mortality rate. Better adaptability of the animals together with the strong immune system is the key for the livestock survival.
Immunity is of two type’s innate and adaptive immunity. Innate immunity is a non-specific immunity, forming the first line of defense which is also present in the lower strata of the phylum, including the plants. The adaptive immune system gets activated after the entry of the pathogen. The adaptive immune system is divided into 1, Cell Mediated Immunity (CMI) and 2, Humoral Mediated Immunity (HMI). Under the CMI, T-lymphocyte plays a vital role while the B-lymphocyte play a vital role in HMI. Cells involved in the adaptive immune system include natural killer cells, mast cells, eosinophils, basophils; and the phagocytic cells (macrophages, neutrophils, and dendritic cells). Though CMI and HMI are classified into two subtypes, they are interdependent and maintain a balance between the two for effective functioning [5]. HS affects both humoral and cellular immunity, by decreasing the production of both primary and secondary immunoglobulin as well as by regulating the production of various inflammatory and anti-inflammatory cytokines leading to impairment of the normal immune function.
Plasma cortisol decreased the production of L-selectin expression on the surface of neutrophil, which is responsible for recruitment of neutrophil. Thus, inhibiting the movement of the neutrophils into the tissue and their phagocytic activity. During HS condition, the HSPs increases and encourages the innate immune system cells to act against the invading pathogen. The migration of the circulating White Blood Cells into the mammary glands decreased during HS. In pigs, during the summer season, the level of IgG and IgM decreased during summer while the level of IgA did not change on comparison with spring autumn and winter [6]. In utero HS condition affects the IgG absorption due to the closure of the gut which aid in absorption [7] and serum level of IgG in calves decreased during HS condition [8]. Similarly, IgG decreased during HS in the broiler, this could be due to the decrease in the size of the lymphoid organs [9]. HS decreased the production of specific immunoglobulin thus affecting the humoral immunity.
HSPs play a crucial role in antigen processing and presentation in addition to their known chaperonin activities. Antigen transport associated with HSP70 requires ATP for transport. HSP70 and 60 associated with TLR4 is needed for dendritic cell stimulation. HSPs activate both adaptive and innate immune response [10]. Exposure to HS induced mRNA HSP70 expression in PBMC [11], testis [12] liver [13], adrenal gland [13] might play a role in the reduction of membrane permeability, cells resilience capacity and immunity. They also help in promoting anti-inflammatory cytokines and inhibit the pro-inflammatory cytokines thus boosting the immunity.
Cytokines that play a major role in the innate immune system include, TNF-α, IL-1, IL-10, IL-12, type I interferons (IFN-α and IFN-β), IFN-γ, likewise the IL-2, IL-4, IL-5, TGF-β, and IFN-γ play a vital part in the adaptive immune system. There was upregulation of the mRNA expression of TNF-α and IL-8 cytokine gene expression in HSed cows during the transitional period [14]. Lactating cows subjected to HS showed an increase in the serum TNF-alpha and IL-10 levels [15].
During HS the expression of TLR2 and TLR4 was increased in PBMC in response to lipoteichoic acid or LPS [16]. Malabari goats subjected to HS for 45 days, also showed increased expression of TLR2 in their lymph node [17]. The TLR2 and TLR4 mRNA expression were upregulated during in vivo and in vitro conditions in PBMCs [18]. Likewise, Osmanabadi goats showed increased splenic mRNA expression level of TLR 1, 2, 3, 5, 6, 8 and 10, where TLR3 could act as a suitable marker during the environmental HS [19]. The liver mRNA expression of TLR8 and TLR10 showed significantly higher expression level and might also act as an immunological marker during HS in the Osmanabadi goats [20]. The expression of TLR2 was not elevated in the HSed pigs [21] and in HSed chicken also showed decreased mRNA expression of TLR2 in spleen and cecal tonsils infected with Salmonella enteritidis [22]. The increase in the TLR expression is to facilitate the exclusion of the pathogen in a non-specific way and in short time on comparison with the doptive immunity which takes days to weeks to eliminate the pathogen. Thus increase in the TLR expression could be an adaptation sign to counter the invading pathogens.
HS immunomodulated the immune function response of the chickens by shifting the B-lymphocyte to a T-cytotoxic and T-helper lymphocyte profile in both HSed and HSed vaccinated group while, there was increase the igG in the HSed group and there was an increase in IgM in HSed non vaccinated group [23]. Under normal condition, the animal maintains equilibrium between Th1 which favors the CMI and Th2 which favors the HMI. During HS condition the balance between the two is disturbed due to secretion of the glucocorticoids also cause a shift from Th-1 to Th2 responses. During chronic HS, the immune system favors a shift in the response towards the HMI. Thus chronic HS could negatively impact the immune system by altering the innate and adaptive immune system during HS conditions, however, the mild or acute HS could be beneficial.
HS affects both the innate and the adaptive immunity. However, various pathways related to innate immune-based receptors have not been studied in the livestock. Similarly, in-depth studies on livestock immunity in relation to HS are lacking. Hence, importance should be given to study the immune system in detail. Studying the immune mechanism along with ameliorative measures like nutritional interventions and managemental measures to abate HS could be the key to improve immunity in the livestock without affecting the productivity.
Impact of heat stress on immunity
References
- Upton M. "The role of livestock in economic development and poverty reduction”. Pro-poor Livestock Policy Initiative (2004).
- Smith J., et al. "Beyond milk, meat, and eggs: Role of livestock in food and nutrition security”. Animal Frontiers 3.1 (2013): 6-13.
- Vidya MK., et al. "Toll-like receptors: Significance, ligands, signaling pathways, and functions in mammals”. International reviews of immunology 37.1 (2018): 20-36.
- Renaudeau D., et al. "Adaptation to hot climate and strategies to alleviate HS in livestock production”. Animal 6.5 (2012): 707-728.
- Medzhitov R. "Recognition of microorganisms and activation of the immune response”. Nature 449.7164 (2007): 819.
- Chu GM and Song YM. "Growth performance, blood characteristics and immune responses of fattening pigs in different seasons”. Asian Journal of Animal and Veterinary Advances 8.5 (2013): 691-702.
- Ahmed BMS., et al. "Maternal HS affects calf passive immunity: Effects on intestinal cell apoptosis”. Journal of Dairy Science 98.Suppl. 2 (2016): 713.
- Genc M. and Coban O. "Effect of Some Environmental Factors on Colostrum Quality and Passive Immunity in Brown Swiss and Holstein Cattle”. Israel Journal of Veterinary Medicine 72.3 (2017): 28-34.
- Park SO., et al. "Effects of Extreme HS on Growth Performance, Lymphoid Organ, IgG and Cecum Microflora of Broiler Chickens”. International Journal of Agriculture & Biology 15.6 (2013).
- Torigoe T., et al. "Heat shock proteins and immunity: application of hyperthermia for immunomodulation”. International Journal of Hyperthermia 25.8 (2009): 610-616.
- Shilja S., et al. "Adaptive capability as indicated by behavioral and physiological responses, plasma HSP70 level, and PBMC HSP70 mRNA expression in Osmanabadi goats subjected to combined (heat and nutritional) stressors”. International journal of biometeorology 60.9 (2016): 1311-1323.
- Niyas PA., et al. "Effect of heat and nutritional stress on growth and testicular HSP70 expression in goats”. Journal of Agro meteorology 19.3 (2017): 189-194.
- Shaji S., et al. "Summer season related heat and nutritional stresses on the adaptive capability of goats based on blood biochemical response and hepatic HSP70 gene expression”. Biological Rhythm Research 48.1 (2017): 65-83.
- Tao S., et al. "Effect of HS during the dry period on gene expression in mammary tissue and peripheral blood mononuclear cells”. Journal of dairy science 96.1 (2013): 378-383.
- Zhang FJ., et al. "Effects of temperature–humidity index and chromium supplementation on antioxidant capacity, heat shock protein 72, and cytokine responses of lactating cows”. Journal of Animal Science 92.7 (2014): 3026-3034.
- Zhou S., et al. "Discovery of a novel TLR2 signaling inhibitor with anti-viral activity”. Antiviral research 87.3 (2010): 295-306.
- Vandana GD., et al. "Summer season induced HS impact on the expression patterns of different toll-like receptor genes in Malabari goats”. Biological Rhythm Research (2018): 1-17.
- Ju XH., et al. "HS upregulation of Toll-like receptors 2/4 and acute inflammatory cytokines in peripheral blood mononuclear cell (PBMC) of Bama miniature pigs: an in vivo and in vitro study”. Animal 8.9 (2014): 1462-1468.
- Sophia I., et al. "Influence of different environmental stresses on various spleen toll like receptor genes expression in Osmanabadi goats”. Asian Journal of Biological Sciences 10 (2017): 9-16.
- Sophia I., et al. "Quantitative expression of hepatic toll-like receptors 1–10 mRNA in Osmanabadi goats during different climatic stresses”. Small Ruminant Research 141 (2016): 11-16.
- Eicher S., et al. "Toll-like receptors 2 (TLR2) and 4 (TLR4) of porcine blood leukocytes during heat-stress”. Veterinary Immunology International Symposium 2004.
- Quinteiro-Filho WM., et al. "HS decreases expression of the cytokines, avian β-defensins 4 and 6 and Toll-like receptor 2 in broiler chickens infected with Salmonella Enteritidis”. Veterinary immunology and immunopathology 186 (2017): 19-28.
- Honda BT., et al. "Effects of HS on peripheral T and B lymphocyte profiles and IgG and IgM serum levels in broiler chickens vaccinated for Newcastle disease virus”. Poultry science 94.10 (2015): 2375-2381.
Citation:
M Bagath and V Sejian. “Heat stress and Immune Function in Livestock”. Multidisciplinary Advances in Veterinary Science 2.4
(2018): 395-398.
Copyright: © 2018 M Bagath and V Sejian. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.





























 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.