Research Article
Volume 2 Issue 5 - 2018
Mercury Traced to the Tissue and Cells of the Testes of the Marine Teleostean Fish Leiostomus xanthurus, and Mercury-induced Testicular Histopathology.
National Oceanic and Atmospheric Administration, National Ocean Service, National Centers for Coastal Ocean Science, Marine Spatial Ecology Division, 101 Pivers Island Road, Beaufort, North Carolina, 28516 USA
*Corresponding Author: John J Govoni, North Carolina State University, School of Life Sciences, Department of Applied Ecology, CMAST, 303 College Circle, Morehead City, NC 28557 USA.
Received: October 21, 2018; Published: October 26, 2018
Abstract
Fishes take up Hg from the environment directly in its inorganic (HgII) form, and from their food primarily as methylated organic (MeHg). The effects of HgII and MeHg on fish reproduction are well documented for ovaries, but less so for testes. Auto metallography visualized Hg-sulfide granules in testes. Whether assimilated from their environment, or amplified by feeding on Hg-contaminated food, identifiable Hg granules were evident in spermatogenic germ cells, epithelial cells, and connective tissue surrounding sperm ducts, Sertoli cells of the walls of spermatocytes, and spermatozoa. Tissue and cellular locations of granules coincided with areas where Histopathological effects are evident in other fishes. These effects compromise testicular function.
Introduction
Mercury (Hg), deposited from the atmosphere into marine and freshwater environments in its inorganic (HgII) form, biomagnifies through food webs in its methylated organic (MeHg) form (Driscoll., et al. 2013). Both HgII and MeHg are toxic to aquatic and marine invertebrates and fish (Kasper., et al. 2009; Liu., et al. 2012), terrestrial vertebrates, and humans (Sweet and Zelikoff, 2001).
Both HgII and MeHg, absorbed from ingested food in the alimentary canal, have particular pathological effects on the reproduction of fishes (Crump and Trudeau, 2009). Effects are well documented for ovaries (Govoni., et al. 2017); less comprehensively for testes. In ovaries, dietary exposure to HgII and MeHg results in Histopathological effects, including apoptosis of follicles and increases in atretic oocytes, along with compromised ovarian production, registered as decreased egg production, decreased egg size, and decreased hatch rate (Govoni., et al. 2017). In testes, Hg exposure results in Histopathological effects that culminate in compromised spermatogenesis; reduced sperm production and abnormal or necrotic spermatozoa (Table 1). Batchelar., et al. (2013) found an association between the proportion of primary spermatocytes and total Hg concentration in muscle in fish caught in a heavily contaminated lake.
| Histopathological effect | Reference |
| Tissue level | |
| Suppressed testicular development or suppressed spermatogenesis | Ram and Sathyanesan (1983); Wester (1991); Kirubagaran and Joy (1992); Friedmann., et al. (1996); Vergílio., et al. (2014); Aziz., et al. (2017) |
| Testicular degeneration, hypertrophy, or atrophy | Wester and Canton (1992); Friedmann., et al. (1996): Miles-Richardson., et al.(1999); Weis (2009); Ebrahimi and Taherianfard (2009); Rajan and Kuzhivelil (2013); Vergílio., et al. (2014) |
| Disorganization of testicular tissue or disorganization of cyst structure | Wester and Canton (1992); Hammerschmidt., et al. (2002); Liao., et al. (2006); Ebrahimi and Taherianfard (2009); Vergílio., et al. (2014); Aziz., et al. (2017) |
| Congestion in testicular circulatory vessels | Vergílio., et al. (2014) |
| Proliferation of interstitial tissue | Rajan and Kuzhivelil (2013); Vergílio., et al. (2014) |
| Increased prevalence of ovotestes | Wester (1991); Stentiford., et al. (2003) |
| Testicular tumors (Sertoli cell adenoma, fibroplasia and fibrosis) | Granado-Lorencio., et al. (1987); Řehulka (2013) |
| Cellular level | |
| Germ cell atrophy | Vergílio., et al. (2014) |
| Leydig cell hypertrophy and changes in pycnosis | Kirubagaran and Joy (1992) |
| Sertoli cell atrophy | Wester and Canton (1992) |
| Spermatozoa | |
| Aggregation | Vergílio., et al. (2014) |
| Altered morphology | Vergílio., et al. (2014) |
| Arrested sperm development or reduced abundance | Wester 1991; Wester and Canton (1992); Miles-Richardson., et al. (1999); Rajan and Kuzhivelil (2013); Vergílio., et al. (2014) |
| Necrosis (pycnosis and karyorhexus) or apoptosis | Wester and Canton (1992); Miles-Richardson., et al.(1999); Blazer (2002); Drevnick., et al. (2006) |
Table 1: Histopathological effects associated with mercury in the testes of fishes.
The tracing of Hg to muscle and organs of fishes has used various methods. Intraperitoneal injection of the radionuclide 203Hg (NO3)2 resulted in accumulation the nuclide in the gall bladder, spleen, eye, kidney, intestines, and gonads (Weisbart, 1973). 203HgCl2 and CH3 203HgCl2 in seawater was traced to bulk samples of blood plasma, red blood cells, bone, muscles, and various visceral organs including ovaries and testes (Pentreath, 1976a, b). Histochemically, Hg was traced with auto metallography (AMG) to the kidney, hepatopancreas, spleen, and intestine of fishes (Kaewamatawong., et al. 2013). Zarnescu (2009) used AMG to trace Hg to ovarian tissues and oocytes of a fish, and confirmed localization of Hg with immunochemistry.
Hg was traced through the liver to the ovaries with AMG by Govoni., et al. (2017). In the liver, Hg-sulphide granules were observed throughout the cytoplasm of hepatocytes, primarily near the nucleus. In the ovaries, granules were observed in tissue and cells of the ovarian stroma, in developing oocytes, and in spawned eggs of the marine fish Leiostomus xanthurus. Here, we report Hg traced to the testes of the same fish experimentally exposed to dietary Hg as described in Govoni., et al. (2017).
Materials and Methods
Experimental design
Methods for the treatment of adult fish were those of Govoni., et al. (2017). Groups of adult L. xanthurus, were caught in the wild, held in the laboratory for 90d, and fed: (1) a control diet of Finfish Hi-Performance feed pellets; and (2) a treatment diet of ground axial muscle of Makaira nigricans with naturally high concentrations of Hg (Barber and Whaling, 1983), mixed with ground pellets. Muscleof M. nigricans provided a natural dietary vector for tracing Hg into testes. Hg concentrations in muscle of M. nigricans and in ovaries of L. xanthurus were estimated in solids and in solution by thermal decomposition, amalgamation, and atomic absorption spectrophotometryusing a Milestone DMA-80 Hg analyzer. The concentration of total Hg in muscle of M. nigricans was 3.37 μg/g wet weight (WW); the concentration of extracted MeHg was 0.48 μg/g (WW); and by difference, the concentration of HgII was 2.89 μg/g WW. Fish were fed ad libitum for 90 d. Gametogenesis, including spermatogenesis was induced by shortening photoperiod and increasing temperature.
Methods for the treatment of adult fish were those of Govoni., et al. (2017). Groups of adult L. xanthurus, were caught in the wild, held in the laboratory for 90d, and fed: (1) a control diet of Finfish Hi-Performance feed pellets; and (2) a treatment diet of ground axial muscle of Makaira nigricans with naturally high concentrations of Hg (Barber and Whaling, 1983), mixed with ground pellets. Muscleof M. nigricans provided a natural dietary vector for tracing Hg into testes. Hg concentrations in muscle of M. nigricans and in ovaries of L. xanthurus were estimated in solids and in solution by thermal decomposition, amalgamation, and atomic absorption spectrophotometryusing a Milestone DMA-80 Hg analyzer. The concentration of total Hg in muscle of M. nigricans was 3.37 μg/g wet weight (WW); the concentration of extracted MeHg was 0.48 μg/g (WW); and by difference, the concentration of HgII was 2.89 μg/g WW. Fish were fed ad libitum for 90 d. Gametogenesis, including spermatogenesis was induced by shortening photoperiod and increasing temperature.
Histology and Histochemistry
Sections (5 µm) were cut from treatment and control livers, ovaries, and batches of spawned eggs (Govoni., et al. 2017), and from the testes of treatment fish. Sections were treated with AMG following Danscher and Møller-Madsen (1985), with developing solutions and protocols of Danscher., et al. (2000), and counter stained with Mayer’s ̶ Harris’s hematoxylin and eosin-y-phloxine. A preliminary study to assess the efficacy of AMG in demonstrating Hg in liver and ovaries with densitometry indicated that Hg-sulfide granules, the reaction products of AMG, were significantly related to total Hg concentration in ovaries (Govoni., et al. 2017, Supplement 1). Total Hg in treated ovaries ranged from 0.018 to 1.0 µg/g wet weight. In Govoni., et al. (2017), Hg-sulfide granules were demonstrated in hepatic and ovarian tissues and cells of treatment fish, with some granules were evident in fish from control fish, not fed muscle of M. nigricans, but the prevalence of granules was greater in treatment fish; the presence of some granules in control fish was attributed to environmental exposure before capture (Govoni., et al. 2017).
Sections (5 µm) were cut from treatment and control livers, ovaries, and batches of spawned eggs (Govoni., et al. 2017), and from the testes of treatment fish. Sections were treated with AMG following Danscher and Møller-Madsen (1985), with developing solutions and protocols of Danscher., et al. (2000), and counter stained with Mayer’s ̶ Harris’s hematoxylin and eosin-y-phloxine. A preliminary study to assess the efficacy of AMG in demonstrating Hg in liver and ovaries with densitometry indicated that Hg-sulfide granules, the reaction products of AMG, were significantly related to total Hg concentration in ovaries (Govoni., et al. 2017, Supplement 1). Total Hg in treated ovaries ranged from 0.018 to 1.0 µg/g wet weight. In Govoni., et al. (2017), Hg-sulfide granules were demonstrated in hepatic and ovarian tissues and cells of treatment fish, with some granules were evident in fish from control fish, not fed muscle of M. nigricans, but the prevalence of granules was greater in treatment fish; the presence of some granules in control fish was attributed to environmental exposure before capture (Govoni., et al. 2017).
Nomenclature
Spermatogenesis is complex in fishes and consists of multiple phases and stages with alternate nomenclature (Schultz., et al. 2010; Uribe., et al. 2014). Here, we apply the simple, standardized nomenclature for phases of Brown-Peterson., et al. (2011), with the tissue and cellular definitions of Shaw., et al. (2012).
Spermatogenesis is complex in fishes and consists of multiple phases and stages with alternate nomenclature (Schultz., et al. 2010; Uribe., et al. 2014). Here, we apply the simple, standardized nomenclature for phases of Brown-Peterson., et al. (2011), with the tissue and cellular definitions of Shaw., et al. (2012).
Results
Prevalence of Hg-sulphide granules
All 20 slides prepared from testes (from separate fish) exhibited Hg-sulphide granules, although the extent and distribution in tissues and cells varied among slides, and therefore among specimens. Ten slides exhibited slight distribution with granules scattered in developing germ cells and support tissue around internal lobular sperm ducts, not the main sperm duct. Testicular tissue was in various phases of spermatogenesis, with developing and spawning-capable phases prevalent; one slide was from a testis in immature stage.
All 20 slides prepared from testes (from separate fish) exhibited Hg-sulphide granules, although the extent and distribution in tissues and cells varied among slides, and therefore among specimens. Ten slides exhibited slight distribution with granules scattered in developing germ cells and support tissue around internal lobular sperm ducts, not the main sperm duct. Testicular tissue was in various phases of spermatogenesis, with developing and spawning-capable phases prevalent; one slide was from a testis in immature stage.
Tissue and cellular localization of Hg-sulphide granules
Hg-sulphide granules were evident in spermatogenic germ cells, epithelial cells and connective tissue surrounding sperm ducts, Sertoli cells surrounding spermatocytes, and in spermatozoa. Granules were widespread in spermatogenic cells and in the epithelial cells that surround sperm ducts (Figure 1). In testes that were in developing phase, Sertoli cells in the germinal epithelium of spermatocytes exhibited granules (Figure 2). Sertoli cells associated with either collapsed spermatocytes or collapsed, small, sperm ducts had a high prevalence of granules. Granules were evident in melano-macrophages in interstitial tissue and in association with Sertoli cells (Figure 3). In testes that were in spawning capable phase, spermatozoa within the main sperm duct (Figure 4) and in smaller, radiating sperm ducts within lobular tissue were heavily invested with granules. These spermatozoa appeared necrotic and cellular debris was present within the ducts (Figure 5).
Hg-sulphide granules were evident in spermatogenic germ cells, epithelial cells and connective tissue surrounding sperm ducts, Sertoli cells surrounding spermatocytes, and in spermatozoa. Granules were widespread in spermatogenic cells and in the epithelial cells that surround sperm ducts (Figure 1). In testes that were in developing phase, Sertoli cells in the germinal epithelium of spermatocytes exhibited granules (Figure 2). Sertoli cells associated with either collapsed spermatocytes or collapsed, small, sperm ducts had a high prevalence of granules. Granules were evident in melano-macrophages in interstitial tissue and in association with Sertoli cells (Figure 3). In testes that were in spawning capable phase, spermatozoa within the main sperm duct (Figure 4) and in smaller, radiating sperm ducts within lobular tissue were heavily invested with granules. These spermatozoa appeared necrotic and cellular debris was present within the ducts (Figure 5).
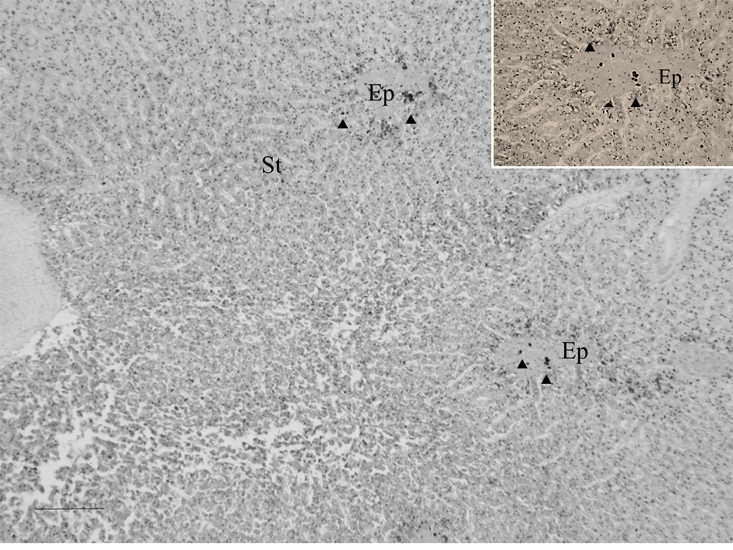
Figure 1: Widespread distribution of Hg-sulphide granules revealed by auto metallography in spermatogenic tissue and in epithelial tissue in the walls of ducts of the marine fish Leiostomus xanthurus; Hg-sulphide granules in epithelium surrounding sperm ducts (inset). Arrowheads are example Hg-sulphide granules or aggregations of granules; St is spermatogenic tissue and Ep is epithelium; scale bar is 100 µm.
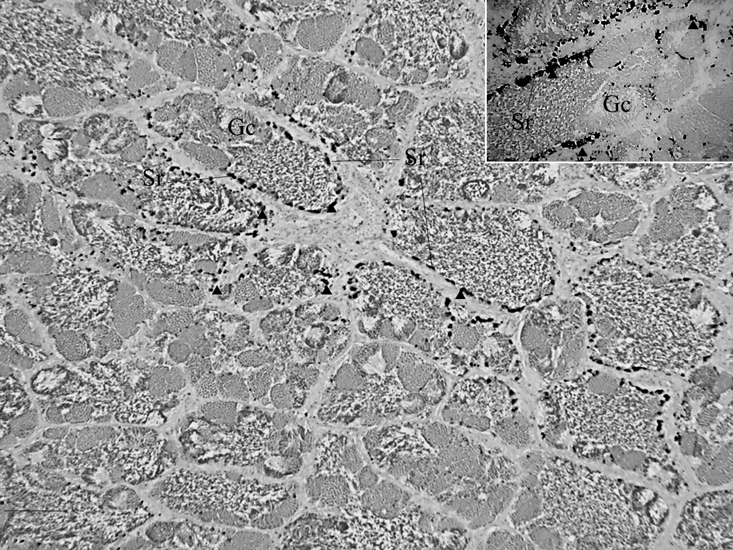
Figure 2: Hg-sulphide granules revealed by auto metallography in Sertoli cells surrounding spermatogenic tissue with spermatocytes of the marine fish Leiostomus xanthurus; Hg-sulphide granules in a spermatocyst (and inset). Arrowheads are example Hg-sulphide granules or aggregations of granules; Sr are Sertoli cells and Gc are various germ cells; scale bar is 100 µm.

Figure 3: Hg-sulphide granules revealed by auto metallography in melano-macrophages in interstitial tissue and in association with Sertoli cells of the marine fish Leiostomus xanthurus (and inset). Arrowheads are example Hg-sulphide granules or aggregations of granules; Mm are melano-macrophages; Sr are Sertoli cells; scale bar is 500 µm.
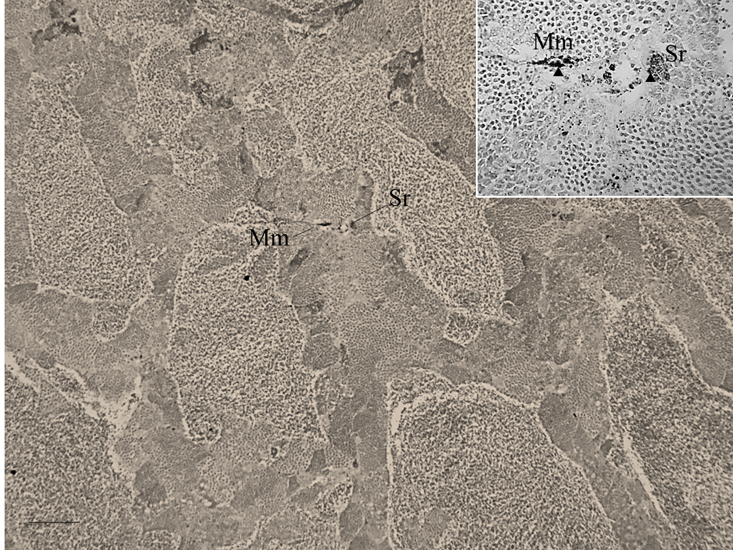
Figure 4: Hg-sulphide granules revealed by auto metallography in spermatozoa within sperm ducts embedded in the interior of lobules of the marine fish Leiostomus xanthurus and apparent necrotic spermatozoa and cellular debris. Arrowheads are example Hg-sulphide granules or aggregations of granules; Sz are necrotic spermatozoa; scale bar 4500 µm.
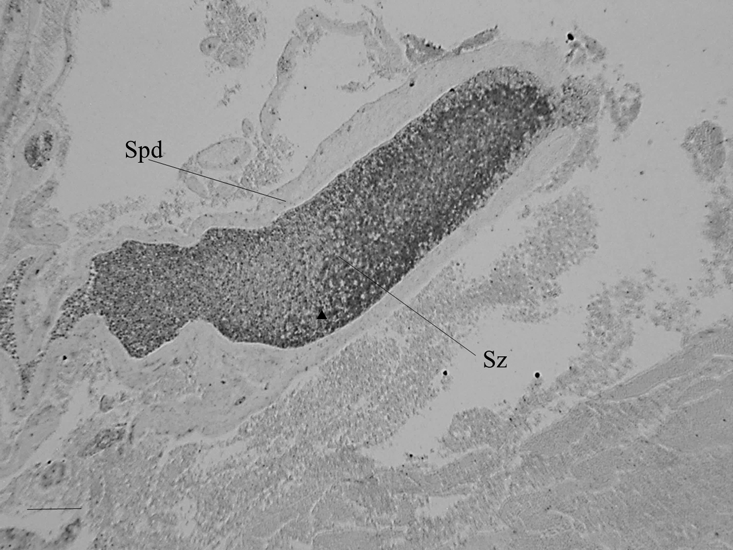
Figure 5: Hg-sulphide granules revealed by auto metallography in spermatozoa within sperm ducts embedded in the interior of lobules of the marine fish Leiostomus xanthurus and apparent necrotic spermatozoa and cellular debris (inset). Arrowheads are example Hg-sulphide granules or aggregations of granules; Sz are necrotic spermatozoa; scale bar 4500 µm.
Discussion
Variable feeding by fish on Hg-contaminated food accounts for variation in the extent and distribution of Hg-sulphide granules among slides (and specimens). Observations during experiments indicated variable feeding among fish, although gut content load was not measured when fish were collected for histological preparation.
Auto metallography is a proven methodology that demonstrates Hg in tissues and cells, including testicular tissue, of other vertebrates, along with fishes. With AMG, Danscher and Møller-Madsen (1995) demonstrated Hg-sulphide granules in the testes of rats, where granules are found in Sertoli cells, Leydig cells, and in melano-macrophages in the interstitial connective tissue. Hg has been traced with AMG to melano-macrophages of the liver of frogs (Loumbourdis and Danscher, 2004). Melano-macrophages are common in hepatic (Stentiford., et al. 2003), splenic (Kaewamaatawong., et al. 2013), and ovarian and testicular tissue of fishes (García-López., et al. 2005; Blazer 2002). In livers of fish, proliferation of melano-macrophages is associated with Hg intoxication (Adams and Sonne, 2013). In testes, aggregations of melano-macrophages are associated with the proliferation of Sertoli cells and their phagocytic activity, possibly accelerated by Hg (Blazer, 2002).
The testicular-tissue and cellular deposits of Hg-sulphide granules in L. xanthurus coincide with areas where Histopathological effects are evident in the testes of other fishes (Table 1). In L. xanthurus, Hg-sulphide granules were evident in the germ cells, epithelium surrounding sperm ducts, in Sertoli cells in the lining of spermatocytes, in spermatozoa, and possibly in melano-macrophages. Testicular tissues, where granules were visible, often lacked cyst Arian structure, a histopatholical effect recognized in other fishes (Table 1). Sertoli cells surround cysts through their cytoplasmic extensions and thereby define spermatocytes structurally, support the germ cells throughout spermatogenesis, and phagocytize residual bodies left from the transition of spermatids to spermatozoa, as well as residual spermatozoa after spawning (Schulz., et al. 2010; Uribe., et al. 2014). Disruption of the function of Sertoli cells might account for the lack of cyst Arian structure observed in some of the testes examined for L. xanthurus. Apoptosis is observed in the ovaries (Drevnick., et al. 2006) and testes (Řehulka, 2013) of fishes. Some apoptosis is normal in fish spermatogenesis (Vilela., et al. 2003; Nóbrega., et al. 2009), yet necrosis and apoptosis of is also stressor-induced (Yabu., et al. 2001). Hg-induced proliferation of Sertoli cells is also evident (Miles-Richardson., et al. 1999). Necrosis and apoptosis might account for the collection of necrotic spermatozoa and cellular debris found in spermatic ducts (Řehulka, 2013).
Fishes take up Hg from the environment directly as HgII, and through their food, primarily as MeHg. Among all tissues and organs of fishes, Hg is redistributed minimally to the organs of reproduction (Johnston., et al. 2001; Kasper., et al. 2009), and the least to testes (Pelletier and Audet, 1995). Yet, AMG demonstrated Hg-sulphide granules in the testicular tissues and cells of a marine fish, L. xanthurus. Concomitant, Histopathological effects that coincide with Hg-induced Histopathological effects in other fishes and that compromise reproduction were observed.
Acknowledgements
This paper is written in memory of D. W. Evans. Auto metallography was prepared by the North Carolina State University, College of Veterinary Medicine, Raleigh, NC; S. Horton and M. Mattmuller. P. H. Crumley assisted in experiments. We thank C. A. Harms (N.C. College of Veterinary Medicine) and M. Vandersea (National Oceanic and Atmospheric Administration, National Ocean Service, National Centers for Coastal Ocean Science, Marine Spatial Ecology Division) for their review of this manuscript. Protocols of fish experimentation were in accord with the standards recommended by the Guide for the Care and Use of Laboratory Animals and Directive 63/2010. Mention of trade names does not imply endorsement by NOAA. The U.S. Government has the right to retain a non-exclusive, royalty-free license in and to any copyright covering this paper.
This paper is written in memory of D. W. Evans. Auto metallography was prepared by the North Carolina State University, College of Veterinary Medicine, Raleigh, NC; S. Horton and M. Mattmuller. P. H. Crumley assisted in experiments. We thank C. A. Harms (N.C. College of Veterinary Medicine) and M. Vandersea (National Oceanic and Atmospheric Administration, National Ocean Service, National Centers for Coastal Ocean Science, Marine Spatial Ecology Division) for their review of this manuscript. Protocols of fish experimentation were in accord with the standards recommended by the Guide for the Care and Use of Laboratory Animals and Directive 63/2010. Mention of trade names does not imply endorsement by NOAA. The U.S. Government has the right to retain a non-exclusive, royalty-free license in and to any copyright covering this paper.
References
- Adams DH and Sonne C. “Mercury and histopathology of the vulnerable goliath groper, Epinephelus itajara, in U.S. waters: a multi-tissue approach”. Environmental Research 126 (2013): 254-263.
- Aziz FZA., et al. “A histological study on mercury-induced gonadal impairment in Javanese medaka (Oryziasjavanicus)”. Turkish Journal of Fisheries and Aquatic Science 17 (2017): 621-627.
- Barber RT and Whaling PJ. “Mercury in marlin and sailfish”. Marine Pollution Bulletin 14.10 (1983): 395-396.
- Batchelar KL., et al. “Reproductive health of yellow perch (Perca flavescens) from a biological mercury hotspot in Nova Scotia, Canada”. Science of the Total Environment 454.455 (2013): 319 -327.
- Blazer VS. “Histopathological assessment of gonadal tissue in wild fishes”. Fish Physiology and Biochemistry 26.1 (2002): 85-101.
- Brown-Peterson NJ., et al. “A standardized terminology for describing reproductive development in fishes”. Marine and Coastal Fisheries 3.1 (2011): 52-70.
- Crump KL and Trudeau VL. “Mercury-induced reproductive impairment in fish”. Environmental Toxicology and Chemistry 28.5 (2009): 895-907.
- Danscher GM and Møller-Madsen B. “Silver amplification of mercury sulfide and elenide: a histochemical method for light and electron microscopic localization of mercury in tissue”. Journal of Histochemistry and Cytochemistry 33.3 (1985): 219-228.
- Danscher GM., et al. “Bismuth autometallography: protocol, specificity, and differentiation”. Journal of Histochemistry and Cytochemistry 48.11 (2000): 1503-1510.
- Drevnick PE., et al. “Increased ovarian follicular apoptosis in fathead minnows (Pimephales promelas) exposed to dietary methylmercury”. Aquatic Toxicology 79.1 (2006): 49-54.
- Driscoll CT., et al. “Mercury as a global pollutant: sources, pathways, and effects”. Environmental Science and Technology 47.10 (2013): 4967-4983.
- Ebrahimi M and Taherianfard M. “The effects of heavy metals exposure on reproductive systems of cyprinid fish from Kor River”. Iranian Journal of Fisheries Science 10.1 (2011): 13-24.
- Friedmann AS., et al. “Low levels of dietary methylmercury inhabit growth and gonadal development in juvenile walleye (Stizostedion vitreum)”. Aquatic Toxicology 35 (1996): 265-278.
- García-López Á., et al. “Male reproductive system in Senegalese sole Solea s enegalensis (Kaup): Anatomy, histology and histochemistry”. Histolology and Histopathology 20.4 (2005): 1179-1189.
- Govoni JJ., et al. “Tracing dietary mercury histochemically, with auto metallography, through the liver to the ovaries and spawned eggs of the spot, a temperate coastal fish”. Journal of Aquatic Animal Health29.3 (2017): 173-180.
- Granado-Lorencio C., et al. “Testicular tumors in carp-funa hybrid: annual cycle and effect on a wild population”. Journal of Wildlife Diseases 23.3 (1987): 422-427.
- Hammerschmidt CR., et al. “Effects of dietary methyl mercury on reproduction of fathead minnows”. Environmental Science and Technology 36.5 (2002): 977-883.
- Johnston TA., et al. “Intra- and interpopulation variability in maternal transfer of mercury to eggs of walleye (Stizostedion vitreum)”. Aquatic Toxicology 52.1 (2001): 73-85.
- Kaewamatawong T., et al. “Short-term exposure of Nile tilapia (Oreochromis niloticus) to mercury histopathological changes, mercury bioaccumulation, and protective role of metallothioneins in different exposure routes”. Toxicological Pathology 41.3 (2013): 470-479.
- Kasper D., et al. “Mercury distribution in different tissues and trophic levels of fish from a tropical reservoir, Brazil”. Neotropical Ichthyology 7.4 (2009): 751-758.
- Kirubagaran R and Joy KP. “Toxic effects of mercury on testicular activity in the freshwater teleost, Clarias batrachus (L.)”. Journal of Fish Biology41.2 (1992): 305-315.
- Liao CY., et al.“Methylmercury accumulation, histopathology effects, and cholinesterase activity alterations in medaka (Oryzias latipes) following sublethal exposure to methylmercury chloride”. Environmental Toxicology and Pharmacology22.2 (2006): 225-233.
- Liu G., et al. “Overview of mercury in the environment. In: Environmental chemistry and toxicology of mercury”. John Wiley & Sons (2012): 1-12.
- Loumbourdis NS and Danscher G. “Autometallographic tracing of mercury in frog liver”. Environmental Pollution 129.2 (2004): 299-304.
- Miles-Richardson SR., et al. “Effects of waterbourne exposure of 17 β-estradiol on secondary sex characteristics and gonads of fathead minnows (Pimephales promelas)”. Aquatic Toxicology 47.2 (1999): 129-145.
- Nóbrega RH., et al. “An overview of functional and stereological evaluation of spermatogenesis and germ cell transplantation in fish”. Fish Physiology and Biochemistry 35.1 (2009): 197-206.
- Pelletier E and Audet C. “Tissue distribution and histopatholigical effects of dietary Methylmercury in benthic grubby Myoxocephalus aeneu”. Bulletin of Environmental Contamination Toxicology 54.5 (1995): 724-730.
- Pentreath RJ. “The accumulation of inorganic mercury from sea water by the plaice, Pleuronectes platessaL”. Journal of Experimental Marine Biology and Ecology 24.2 (1976a): 103-119.
- Pentreath RJ. “The accumulation of organic mercury from sea water by the plaice, Pleuronectes platessa L”. Journal of Experimental Marine Biology and Ecology 24.2 (1976b): 121-132.
- Ram RN and Sathyanesan G. “Effects of mercuric chloride on the reproductive cycle of the teleostean fish Channa puctatus”. Bulletin of Environmental Contamination and Toxicology30.1 (1983): 24-27.
- Rajan P and Kuzhivelil B. “Mercury induced histopathological changes in the testis of the freshwater fish, Rasbora dandia”. International Journal of Science and Research 4 (2013): 252-254.
- Řehulka J. “Testicular tumour in northern pike, Esox luciasL.”. Journal of Fish Diseases 36.7(2013): 669-673.
- Schulz R., et al.“Spermatogenesis in fish”. General and Comparative Endocrinology 165.3 (2010): 390- 411.
- Shaw FR., et al. “An atlas of reproductive development in rockfishes, genus Sebastes”. National Oceanic and Atmospheric Administration Professional Paper, National Marine Fisheries Service 14, Seattle, WA. (2012).
- Stentiford GD., et al. “Histopathological biomarkers in estuarine fish species for assessment of biological effects of contaminants”. Marine Environmental Research 55.2 (2003): 137-159.
- Sweet LI and Zelikoff JT. “Toxicological and immunotoxicology of mercury: a comparative review in fishes and humans”. Journal of Toxicology and Environmental Health 4.2 (2001): 161-205.
- Uribe MC., et al.“Comparative testicular structure and spermatogenesis in bony fishes”. Spermatogenesis4.3 (2014): 3e983400.
- Vergílio CS., et al. “Effects of in vitro exposure to mercury on male gonads and sperm structure of the tropical fish tuvira (Gymnotus carapo (L.)”. Journal of Fish Diseases37.6 (2014): 543-561.
- Vilela DAR., et al. “Spermatogenesis in teleost: insights from the Nile tilapia (Oreochromis niloticus) model”. Fish Physiology and Biochemistry 28.1.4 (2003): 187-190.
- Yabu T., et al. “Stress-induced apoptosis by heat shock, UVand γ-ray irradiation in zebrafish embryos detected by caspase activity and whole-mount TUNEL staining”. Fisheries Science67.2 (2001): 333-340.
- Weis J S. “Reproductive, developmental, and neurobehavioural effects of methylmercury in fishes”. Journal of Environmental Science and Health 27.4 (2009): 212-225.
- Weisbart M. “The distribution and tissue retention of mercury-203 in goldfish (Carassius auratus)”. Canadian Journal of Zoology 51.2 (1973): 143-150.
- Wester PW. “Histopathogical effects of environmental pollutants β-HCH and methyl mercury on reproductive organs in freshwater fish”. Comparative Biochemistry and Physiology100.1.2 (1991): 237- 239.
- Wester PW and Canton JH. “Histopathological effects in Poecilia reticulate (guppy) exposed to methyl mercury chloride”. Toxicologic Pathology 20.1 (1992): 81-92.
- Zarnescu O. “Tracing the accumulation and effects of mercury uptake in the previtellogenic ovary of crucian carp, Carassius auratus gibelio by autometallography and caspase-3 immunohistochemistry” Histology and Histopathology 24.2 (2009): 141-148.
Citation:
John J Govoni., et al. “Mercury Traced to the Tissue and Cells of the Testes of the Marine Teleostean Fish Leiostomus xanthurus,
and Mercury-induced Testicular Histopathology.” Multidisciplinary Advances in Veterinary Science 2.5 (2018): 413-421.
Copyright: © 2018 John J Govoni., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.





























 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.