Review Article
Volume 1 Issue 2 - 2016
Healing of Diabetic Foot Wounds-The Role of Basic Science and Underlying Pathology in Management of Diabetic Foot Wounds
1,2,3,4Department of Orthopaedic Surgery, National University Health System, Singapore
*Corresponding Author: Aziz Nather, Senior Consultant Division of Foot and Ankle, University Orthopaedics, Hand and Reconstructive Microsurgery Cluster National University Health System, Singapore.
Received: October 24, 2016; Published: November 01, 2016
Abstract
Introduction: While many articles exist on wound healing, little has been written on the healing of diabetic foot wounds. In a diabetic foot wound, in contrast to other wounds, special factors are at play including the elements of the ‘diabetic foot triad’ and underlying pathology involved. The latter must first be addressed and treated if the wound is to heal.
Impact of Basic Science of wound healing on healing of diabetic foot wounds: The basic science of wound healing included its four phases of Hemostasis, Inflammation, Proliferation and Remodeling. To promote healing chronic wounds resistant to healing, chronic wounds are converted by debridement into acute wounds to trigger acute healing process by releasing useful growth factors directing the phase of wound healing again. Wounds in the inflammatory phase with more exudate require dressings to be changed more frequently than wounds in the proliferation phase.
Impact of Community Factors on Healing of Diabetic Foot Wounds: Patients must be able to go to a nearby polyclinic or hospital as an outpatient for dressing of wounds. When no clinic is available nearby, arrangement must be made for a nurse to go to the patient’s home to perform the dressings (from a Home Nursing Foundation). Alternatively, patients and caregivers are taught to do their own dressings.
Conclusions: Because of the complexity and multiplicity of factors at work in the healing of diabetic foot wounds, a holistic approach is required including control of diabetes, control of nutrition, selection of dressings, choice of antibiotics and the need for early and adequate surgical debridement. Adequate surgical debridement is often the key to good wound healing. Wound bed preparation is best performed using the TIME concept. The dressing to be selected can also be done following the TIME Guide. Off-loading is required for neuropathic ulcers. The treatment of the diabetic foot wound is therefore best performed by an inter-disciplinary diabetic foot team which discusses jointly all issues and efficiently promotes the healing of diabetic foot wounds.
Introduction
Whilst much has been written on the healing of wounds in general, very little has been written specifically on the healing of diabetic foot wounds. Doctors and nurses are aware that diabetic wounds are one of the most difficult wounds to treat. Indeed, it is often wise to refer such wounds to a specialist trained for the management of such wounds.
There are several factors at work in diabetic wounds which are not seen with other wounds. These require attention if we are to succeed in healing the wound. Systemic factors which require special attention include the control of diabetes itself and also the nutritional status of the patient.
Control of Diabetes
With diabetes wounds, it is important to make sure that diabetes is well controlled. The wound would not heal with unless the blood sugar is under control. Endocrinologist and dietitian play a very important role. Hb1Ac must be controlled to less than 7%.
With diabetes wounds, it is important to make sure that diabetes is well controlled. The wound would not heal with unless the blood sugar is under control. Endocrinologist and dietitian play a very important role. Hb1Ac must be controlled to less than 7%.
Nutritional Control
The albumin level must also be normal to ensure that enough proteins are available as building blocks to heal the wound. Hemoglobin must be above 10g/dl to ensure there is enough oxygen perfusing the wound and enough adenosine triphosphates to provide energy for healing the wound. Local factors at play in a diabetic foot wound include the “diabetic foot triad” and underlying pathology present.
The albumin level must also be normal to ensure that enough proteins are available as building blocks to heal the wound. Hemoglobin must be above 10g/dl to ensure there is enough oxygen perfusing the wound and enough adenosine triphosphates to provide energy for healing the wound. Local factors at play in a diabetic foot wound include the “diabetic foot triad” and underlying pathology present.
Diabetic Foot Triad
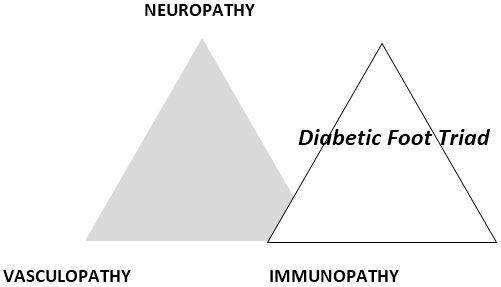
In the diabetic foot, the 3 components of the Diabetic Foot Triad [1], namely Neuropathy, Immunopathy and Vasculopathy (Figure 1) play a big role in determining the outcome of healing of diabetic foot wounds.
In the diabetic foot, the 3 components of the Diabetic Foot Triad [1], namely Neuropathy, Immunopathy and Vasculopathy (Figure 1) play a big role in determining the outcome of healing of diabetic foot wounds.
In each wound, the contribution by each component may be different. In some, vascular components predominate. In others, the problem is mainly infection due to immunopathy.
Underlying Pathology
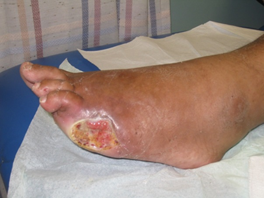
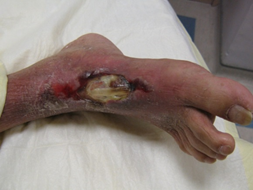
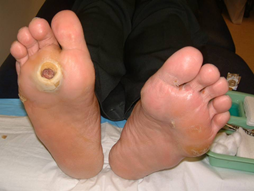
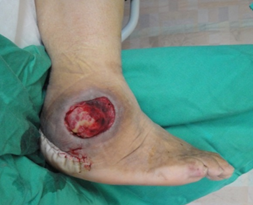
In addition, one must pay attention to the underlying pathology prevailing in each wound. The pathology includes infection, ischemia, infection and ischemia, neuropathy or decubitus. An infective wound (Figure 2) is usually found on the dorsum of the foot. An ischemic ulcer (Figure 3) results when there is a lack of blood flow to the feet. Patients suffering from atherosclerosis have a higher chance of developing ischemic ulcers. A neuropathic ulcer (Figure 4) is usually found on the sole of the foot. A Decubitus ulcer (Figure 5) is usually found on the over the lateral malleolus, the lateral aspect of base of 5th metatarsal as well as over the heel.
In addition, one must pay attention to the underlying pathology prevailing in each wound. The pathology includes infection, ischemia, infection and ischemia, neuropathy or decubitus. An infective wound (Figure 2) is usually found on the dorsum of the foot. An ischemic ulcer (Figure 3) results when there is a lack of blood flow to the feet. Patients suffering from atherosclerosis have a higher chance of developing ischemic ulcers. A neuropathic ulcer (Figure 4) is usually found on the sole of the foot. A Decubitus ulcer (Figure 5) is usually found on the over the lateral malleolus, the lateral aspect of base of 5th metatarsal as well as over the heel.
Impact of Underlying Pathology on Healing of Diabetic Foot Wound
Before a diabetic wound will heal the underlying pathology must be addressed and treated. In an infected wound, adequate surgical debridement must be performed to remove all devitalised and infected tissue and the appropriate antibiotics administered. In an ischaemic ulcer, the foot needs to be revascularised. Duplex ultrasound is performed to identify blockages in the anterior tibial, posterior tibial or peroneal artery and angioplasty performed to improve the blood flow supplying the foot and the wound. Only then can the wound heal. In neuropathic ulcers mechanical factors must be addressed. Off-loading the ulcer is mandatory in addition to dressing of wounds. In decubitus ulcers, in addition to the debridement and antibiotics, supportive measures must be instituted including 2 hourly turning of patient and the use of ankle wraps to help off-load the ulcer.
Before a diabetic wound will heal the underlying pathology must be addressed and treated. In an infected wound, adequate surgical debridement must be performed to remove all devitalised and infected tissue and the appropriate antibiotics administered. In an ischaemic ulcer, the foot needs to be revascularised. Duplex ultrasound is performed to identify blockages in the anterior tibial, posterior tibial or peroneal artery and angioplasty performed to improve the blood flow supplying the foot and the wound. Only then can the wound heal. In neuropathic ulcers mechanical factors must be addressed. Off-loading the ulcer is mandatory in addition to dressing of wounds. In decubitus ulcers, in addition to the debridement and antibiotics, supportive measures must be instituted including 2 hourly turning of patient and the use of ankle wraps to help off-load the ulcer.
Importance of Team Approach in Healing Diabetic Wounds
Because of the complexity and multitude of factors at play in the healing of diabetic foot wounds, the latter is best treated by an inter-disciplinary diabetic foot team including endocrinologist, orthopaedic surgeon, vascular surgeon, infectious disease consultant, podiatrist, wound care nurse, education nurse, dietitian and medico-social workers.
Because of the complexity and multitude of factors at play in the healing of diabetic foot wounds, the latter is best treated by an inter-disciplinary diabetic foot team including endocrinologist, orthopaedic surgeon, vascular surgeon, infectious disease consultant, podiatrist, wound care nurse, education nurse, dietitian and medico-social workers.
Anatomy of the Skin
It is also important to understand the anatomy and functions of the skin in order to better comprehend the basic science of wound healing. The skin is composed of 2 major layers: the epidermis and the dermis. Both skin layers contain cells and extracellular structures. The epidermis is a multi-layered sheet of cells with little extracellular matrix. It is the outermost, continuously renewing part of the skin which is composed of several layers and which includes several cell types. (Figure 6)
It is also important to understand the anatomy and functions of the skin in order to better comprehend the basic science of wound healing. The skin is composed of 2 major layers: the epidermis and the dermis. Both skin layers contain cells and extracellular structures. The epidermis is a multi-layered sheet of cells with little extracellular matrix. It is the outermost, continuously renewing part of the skin which is composed of several layers and which includes several cell types. (Figure 6)
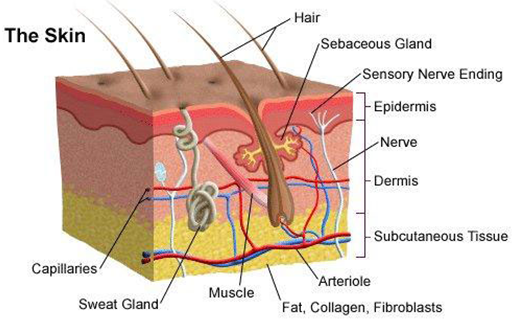
Figure 6: (from http://hospitals.unm.edu/burn/skin_anatomy.shtml): Cross section of the skin
Different depths of wound heal differently. A Wagner 1 wound is a partial thickness wound which heals by epithelialisation. A Wagner 2 wound is a full thickness wound which heals by scar formation.
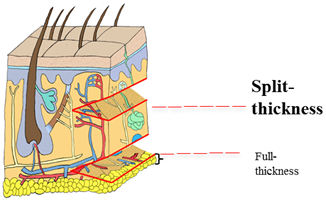
Figure 7: (Adapted from Pearson Education Inc 2004): Difference in amount of dermis harvested for split and full thickness skin grafts.
The Wagner-Meggitt Wound Classification [2] (Figure 8) is a useful six-grade system to classify ulcers according to the depth and extent of wound.
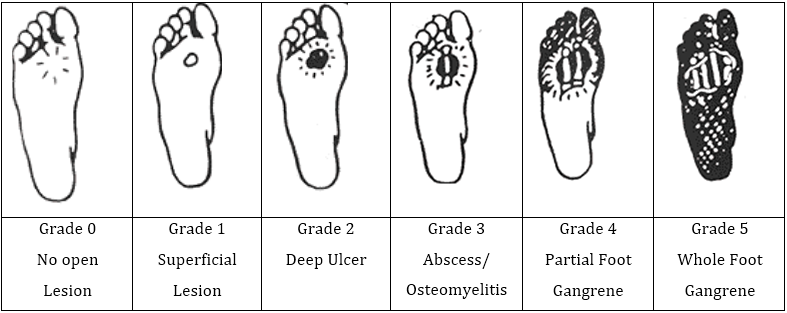
Figure 8: (Adapted from http://www.oandp.org/): The Wagner-Meggitt Wound Classification
Advantages of the Wagner-Meggitt Wound classification include its simplicity in usage. It also provides a useful guide for practitioners to plan treatment.
Basic Science of Wound Healing
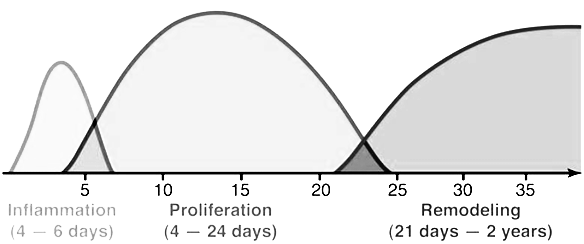
Wounds have the intrinsic capacity to heal. It is a complex process. The process occurs in 4 phases, namely hemostasis, inflammation, proliferation and remodeling (Table 1, Figure 9).
Wounds have the intrinsic capacity to heal. It is a complex process. The process occurs in 4 phases, namely hemostasis, inflammation, proliferation and remodeling (Table 1, Figure 9).
| Phase | Time After Injury | Cells Involved | Activity |
| Hemostasis | Immediately | Platelets | Clotting |
| Inflammation | Day 1 to 4 | Neutrophils Macrophages |
Phagocytosis |
| Proliferation | Day 4 to 21 | Fibroblasts Myofibroblasts Angiocytes Lymphocytes Keratinocytes Macrophages |
Collagen secretion Wound contraction Angiogenesis Epithelialisation Forms new granulation tissue |
| Remodeling | Day 21 to 2 years | Fibrocytes | Develop tensile strength |
Table 1: (Adapted from Orsted., et al3.): 4 phases of wound healing.
Hemostasis
Hemostasis takes place immediately after injury [3,4]. During this phase, adenosine diphosphate (ADP) from damaged tissues cause platelets to adhere to the exposed Type I Collagen. This activates the platelets and stimulates the clotting cascade, leading to the formation of a clot to seal damaged blood vessels [3,5]. The activated platelets also secrete platelet-derived growth factor (PDGF) which recruits neutrophils and monocytes [3].
Hemostasis takes place immediately after injury [3,4]. During this phase, adenosine diphosphate (ADP) from damaged tissues cause platelets to adhere to the exposed Type I Collagen. This activates the platelets and stimulates the clotting cascade, leading to the formation of a clot to seal damaged blood vessels [3,5]. The activated platelets also secrete platelet-derived growth factor (PDGF) which recruits neutrophils and monocytes [3].
Inflammation
The inflammation phase occurs from day 1 to day 4 following injury [3,5]. Platelets continue to release PDGF and transforming growth factor-beta (TGF-b) from their alpha granules to attract neutrophils and macrophages [5]. The extracellular matrix interacts with the integrin receptors of cells, resulting in platelet activation, epithelial cell migration and fibroblast movement. Neutrophils and macrophages phagocytose bacteria to prevent infection [3]. The macrophages, fibroblasts and other cells involved in wound healing secrete various growth factors. Key growth factors include the fibroblast growth factor, the epidermal growth factor, transforming growth factor-beta, and interleukins. These play an important role in directing the next phase of wound healing [3,5]. Table 1 shows some of the functions of important growth factors.
The inflammation phase occurs from day 1 to day 4 following injury [3,5]. Platelets continue to release PDGF and transforming growth factor-beta (TGF-b) from their alpha granules to attract neutrophils and macrophages [5]. The extracellular matrix interacts with the integrin receptors of cells, resulting in platelet activation, epithelial cell migration and fibroblast movement. Neutrophils and macrophages phagocytose bacteria to prevent infection [3]. The macrophages, fibroblasts and other cells involved in wound healing secrete various growth factors. Key growth factors include the fibroblast growth factor, the epidermal growth factor, transforming growth factor-beta, and interleukins. These play an important role in directing the next phase of wound healing [3,5]. Table 1 shows some of the functions of important growth factors.
Proliferation
Proliferation can take place from day 4 to day 21 [3]. Several processes occur during this phase. Fibroblasts migrate to the wound site under the influence of growth factors such as TGF-beta and PDGF [4], and secrete mainly Type III Collagen [6]. Pericytes are involved in angiogenesis, regenerating the outer layers of capillaries and the endothelial cells. Keratinocytes are responsible for epithelialization and differentiate to form the protective outer layer of the skin [3].
Proliferation can take place from day 4 to day 21 [3]. Several processes occur during this phase. Fibroblasts migrate to the wound site under the influence of growth factors such as TGF-beta and PDGF [4], and secrete mainly Type III Collagen [6]. Pericytes are involved in angiogenesis, regenerating the outer layers of capillaries and the endothelial cells. Keratinocytes are responsible for epithelialization and differentiate to form the protective outer layer of the skin [3].
MMPs and TIMPs
During proliferation, there is a balance between two types of enzymes, namely Matrix Metalloproteases (MMPs) and Tissue Inhibitors of Metalloproteases (TIMPs). This balance is vital in ensuring that there is a net production of new tissue to allow for proper wound healing. MMPs are zinc-dependent endopeptidases made up of 3 domains, namely the pro-peptide domain, the catalytic domain and the haemopexin-like domain. They are secreted by macrophages, neutrophils, fibroblasts and other cell types during wound healing. TIMPs are proteases which inactivate MMPs by reversibly binding to them [3].
During proliferation, there is a balance between two types of enzymes, namely Matrix Metalloproteases (MMPs) and Tissue Inhibitors of Metalloproteases (TIMPs). This balance is vital in ensuring that there is a net production of new tissue to allow for proper wound healing. MMPs are zinc-dependent endopeptidases made up of 3 domains, namely the pro-peptide domain, the catalytic domain and the haemopexin-like domain. They are secreted by macrophages, neutrophils, fibroblasts and other cell types during wound healing. TIMPs are proteases which inactivate MMPs by reversibly binding to them [3].
23 human MMPs have been identified, with MMP-1, MMP-2, MMP-8 and MMP-9 being the particular focus of research in wound healing. MMPs play several roles throughout the process of normal wound healing. These include the removal of damaged tissue and bacteria during inflammation, facilitating epidermal cell migration during proliferation and the contraction of scar extracellular matrix during remodeling.
Although controlled levels of MMPs are important for normal wound healing, elevated levels of MMPs can result in the degradation of essential growth factors and their receptors, as well as extracellular matrix proteins [6]. During the normal wound healing process, the levels of proteases (including MMPs) peak at about day 3 and begin to fall by about day 5 [7]. An excess of MMPs and a decrease in the level of TIMPs has been identified as a cause of impaired healing [8,9,10]. When wound healing is impaired, it is due to an imbalance between the levels of MMPs and TIMPs. The reason for the imbalance is not fully understood [8].
Collagen
Collagen plays a key role in wound repair and is a major protein component of the extracellular matrix. The collagen molecule is composed of three alpha chains that collectively form a triple helix structure. Collagen fibrils consist of collagen molecules linked by intermolecular cross-links [11]. These give collagen high tensile strength and thermal stability [12].
Collagen plays a key role in wound repair and is a major protein component of the extracellular matrix. The collagen molecule is composed of three alpha chains that collectively form a triple helix structure. Collagen fibrils consist of collagen molecules linked by intermolecular cross-links [11]. These give collagen high tensile strength and thermal stability [12].
Collagen in the skin are predominantly Type I and Type III. Both types of collagen are found in all layers of the dermis. However, type III collagen is concentrated in the outer layers of the skin [13,14]. Collagen in the skin is about 80% Type I and 20% Type III [4,5]. During wound healing, the fibroblasts primarily produce Type III Collagen which is subsequently replaced by Type I Collagen in the next stage of wound healing [5].
Remodeling
The remodeling phase begins at day 21, and can continue for up to 2 years after wounding [3]. During this phase, the initial Type III Collagen is replaced by Type I Collagen until a Type I: Type III ratio of 4:1 is reached. This is similar to the ratio found in normal skin [5,16].
The remodeling phase begins at day 21, and can continue for up to 2 years after wounding [3]. During this phase, the initial Type III Collagen is replaced by Type I Collagen until a Type I: Type III ratio of 4:1 is reached. This is similar to the ratio found in normal skin [5,16].
Collagen is reorganised along lines of tension and crosslinks to give added strength [6]. The strength of collagen eventually reaches 80% of that of unwounded tissue [4,15]. Cell density and vascularity decrease as a result of apoptosis [17]. Fibroblasts differentiate into myofibroblasts which express smooth muscle alpha-actin and actin-myosin stress fibers. These myofibroblasts are responsible for wound contraction that leads to wound closure [18].
Impact of Basic Science of Wound Healing on Healing of Diabetic Foot Wounds
Several diabetic wounds are chronic and resistant to heal. Debridement of such wounds can play a role in promoting the healing of such chronic “anergic” wounds by converting such chronic wounds into acute wounds. This will trigger the acute healing process by releasing the useful growth factors which direct the phases of wound healing again.
Several diabetic wounds are chronic and resistant to heal. Debridement of such wounds can play a role in promoting the healing of such chronic “anergic” wounds by converting such chronic wounds into acute wounds. This will trigger the acute healing process by releasing the useful growth factors which direct the phases of wound healing again.
The frequency of dressing changes required can also be determined by the phase of the wound healing the wound is in. In wounds undergoing inflammatory phase of healing, with much inflammation and exudate occurring, dressings need to be changed more frequently than in wounds undergoing the proliferation phase.
Factors Influencing Healing of Diabetic Foot Wound
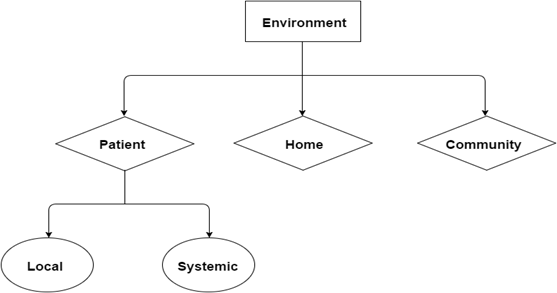
Several factors affect wound healing, namely the patient, his home and his community (Figure 10).
Several factors affect wound healing, namely the patient, his home and his community (Figure 10).
Patient factors include local and systemic factors. Local factors include the 3 components of the diabetic foot triad (Figure 1), namely neuropathy, vasculopathy and immunopathy. Systemic factors include endocrine control, haemoglobin level and level of nutrition. For wounds to heal, it is important to control the blood sugar level. The level of haemoglobin must also be adequate – more than 10 g/dL. This is necessary to carry sufficient amount of oxygen via haemoglobin to the tissue. HbA1C should preferably by < 7%. Oxygen is needed for aerobic respiration to generate adenosine triphosphate (ATP). ATP is a source of energy for cell proliferation and collagen synthesis [18]. Nutrition also plays a key role in influencing wound healing. Albumin and other proteins from foods are needed to support wound healing.
Home factors include sufficient space in the home to provide for patient mobility, and cleanliness of the home to provide a clean environment for wound healing. Thirdly, family support must be available to promote foot hygiene and to dress the wounds of the patient. Where no family member is present, a caregiver is useful.
Community factors play an important role in influencing wound healing. These include the accessibility of the community to the patient. Walking aids and wheelchairs should be made available to them to facilitate their movement in the community. It is also useful to have a polyclinic and a hospital in close proximity to the home. This facilitates healing of the patient’s wound.
Impact of Community Factors on Healing of Diabetic Foot Wounds
It is therefore important to ensure that the patient with diabetic foot wound should be able to visit a polyclinic or a hospital nearby for the dressing of wound as an outpatient. If there is no clinic available nearby, arrangement must be made for a nurse to go to the patient’s home to do the dressing. Such a nurse need to be contracted from a Home Nursing Foundation providing such nursing.
It is therefore important to ensure that the patient with diabetic foot wound should be able to visit a polyclinic or a hospital nearby for the dressing of wound as an outpatient. If there is no clinic available nearby, arrangement must be made for a nurse to go to the patient’s home to do the dressing. Such a nurse need to be contracted from a Home Nursing Foundation providing such nursing.
Nowadays, we advocate teaching our patients and caregivers to do their own dressings in their home. Our experience shows that better results are obtained when patients perform their own dressings. However, for this to succeed patients and caregivers must be carefully trained by our nurses how to perform their own dressings.
Concepts in Management of Diabetic Foot Wound
Holistic Approach
Wound healing is both an art and a science [19]. It requires a holistic approach. One has to treat the whole patient and not just the wound alone.
Holistic Approach
Wound healing is both an art and a science [19]. It requires a holistic approach. One has to treat the whole patient and not just the wound alone.
General Assessment
General assessment of the patient must be performed. This includes assessment of endocrine control, nutritional assessment of the patient and choice of antibiotics to be given.
General assessment of the patient must be performed. This includes assessment of endocrine control, nutritional assessment of the patient and choice of antibiotics to be given.
Local Assessment
The foot and wound must then be examined methodically to assess for all three components of the diabetic foot triad (Figure 1) – vasculopathy, neuropathy and immunopathy1. The underlying pathology must also be recognized and treated (Figure 2,3,4,5). The wound is classified according to the Wagner-Meggitt classification [2] for better management.
The foot and wound must then be examined methodically to assess for all three components of the diabetic foot triad (Figure 1) – vasculopathy, neuropathy and immunopathy1. The underlying pathology must also be recognized and treated (Figure 2,3,4,5). The wound is classified according to the Wagner-Meggitt classification [2] for better management.
Medical Treatment
Medical treatment of the wound must be instituted first. Blood sugar level must be controlled. Antibiotics needed is started after tissue is sent for culture and sensitivity.
Medical treatment of the wound must be instituted first. Blood sugar level must be controlled. Antibiotics needed is started after tissue is sent for culture and sensitivity.
Surgical Treatment
The need for surgical debridement is made by the clinician after assessing the wound.
The need for surgical debridement is made by the clinician after assessing the wound.
Wound Bed Preparation
This is performed following the TIME Concept (Table 2) [20]: T for Tissue, I for Infection and Inflammation, M for Moisture and E for Epidermal Margin.
This is performed following the TIME Concept (Table 2) [20]: T for Tissue, I for Infection and Inflammation, M for Moisture and E for Epidermal Margin.
| Clinical Observations | Proposed Pathophysiology | WBP clinical actions | Effect of WBP actions | Clinical outcome |
| Tissue non-viable or deficient | Defective matrix and cell debris impair healing | Debridement (episodic or continuous):
|
Restoration of wound base and functional extracellular matrix proteins |
Viable wound base |
| Infection or Inflammation | High bacterial counts or prolonged inflammation Inflammatory cytokines Protease activity Growth factor activity | Remove infected foci Topical/systemic:
|
Low bacterial counts or controlled inflammation: Inflammatory cytokines Protease activity Growth factor activity |
Bacterial balance and reduced inflammation |
| Moisture imbalance | Desiccation slows epithelial cell migration Excessive fluid causes maceration of wound margin | Apply moisture- balancing dressings Compression, negative pressure or other methods of removing fluid | Restored epithelial cell migration, desiccation avoided Oedema, excessive fluid controlled, maceration avoided | Moisture balance |
| Edge of wound — non-advancing or undermined |
Non-migrating keratinocytes
Non-responsive wound cells and abnormalities in extracellular matrix or abnormal protease activity |
Re-assess cause or consider corrective therapies:
|
Migrating keratinocytes and responsive wound cells. Restoration of appropriate protease profile | Advancing epidermal margin |
Table 2: TIME Concept [20].
The wound dressing used is selected according to the TIME Guide (Table 3).
| Aim of care | Exudate | Dressing | ||
| Contact layer | Outer dressing | |||
| Tissue Necrotic |
If vascular supply is good, debride eschar and promote moisture balance | Dry or Low | Hydrogel | Hydrocolloid or foam |
| Moderate hydrocolloid | Hydrocolloid | Gauze or film | ||
| Heavy Alginate, foam or hydrofibre |
Gauze or foam with pad | |||
| If vascular supply is compromised, keep eschar dry | Dry | Tulle gauze, film | Film or gauze | |
| Sloughy | Deslough, provide moisture balance | Low | Hydrocolloid, hydrogel |
Gauze or film |
| Moderate | Alginate | Hydrocolloid with pad or gauze | ||
| Heavy | Foam, hydrofibre | Foam with pad or gauze | ||
| Negative Pressure Wound Therapy(NPWT) | ||||
| Granulating | Provide moisture balance | Low Non adherence material | Non adherence | Film or gauze |
| Moderate | Hydrocolloid, foam | Gauze or contact layer with pad | ||
| NPWT | ||||
| Epithelializing | Provide moisture balance | Low | Non adherence material |
Film or gauze |
| Moderale | Hydrocolloid or foam |
Gauze | ||
| Infection | Get rid of infection (biofilm) | Low Nano-crystalline or ion silver containing material, Iodine cream | Hydrocolloid with pad or gauze | |
| Moderate Silver containing, iodine containing material |
Foam with pad or gauze | |||
| Moisture balance | Maintain moist environment | Low Film, hydrogel | Gauze | |
| Moderate Hydrocolloid, Alginate |
Contact layer with pad or foam | |||
| Heavy Foam, hydrofibre, NPWT | Contact layer with pad or gauze | |||
| Edge | Promote advance of wound edge | Low Film, hydrogel | Gauze | |
| Moderate Hydrocolloid, Alginate, NPWT |
Contact layer with pad or foam | |||
| Heavy Foam, hydrofibre, NPWT | Contact layer with pad or gauze | |||
Table 3: TIME Guide [21].
New Trends in Wound Healing
Over the last few years, new trends have emerged in the healing of wounds. These include the use of silver dressings, Platelet-Rich Plasma, Mesenchymal Stem Cells, Matrix Metalloproteinases and the Protease Test. It is important to evaluate whether these new developments are effective in promoting the healing of wounds.
Over the last few years, new trends have emerged in the healing of wounds. These include the use of silver dressings, Platelet-Rich Plasma, Mesenchymal Stem Cells, Matrix Metalloproteinases and the Protease Test. It is important to evaluate whether these new developments are effective in promoting the healing of wounds.
So far, there is no evidence that silver dressings can promote wound healing compared to conventional non-silver dressings [22]. With regards to the role of Platelet-Rich Plasma (PRP), there is also no evidence that PRP promotes wound healing [22]. Conflicting evidence exists in literature. With regards to Mesenchymal Stem Cells, present evidence does not support its role for wound healing [22].
Literature suggests that Matrix Metalloproteinases (MMP) level can be an accurate marker for wound healing if it can be measured. At present, it is very hard to measure MMP activity in wound fluids or biopsies [23]. A new diagnostic tool is needed to measure MMP activity in wounds. Such a device is still not available [7].
Conclusions
In a diabetic foot wound, special factors are at play include the elements of the ‘diabetic foot triad’ and underlying pathology present. The latter must first be addressed and treated before the wound can heal. Because of the complexity and multiplicity of factors at work in the healing of diabetic foot wounds, a holistic approach is required including control of diabetes, control of nutrition, selection of dressings, choice of antibiotics and the need for early and adequate surgical debridement when indicated. Adequate surgical debridement is often the key to good wound healing. Wound bed preparation is best performed using the TIME concept. The dressing to be selected can also be done following the TIME Guide. Off-loading is required for neuropathic ulcers. The treatment of diabetic foot wounds is therefore best performed by an inter-disciplinary diabetic foot team which discusses jointly all issues involved and efficiently promotes the healing of such wounds.
References
- Nather A and Tan TF. “Diabetic Foot Wounds- Types of Wounds and Classification Systems. In:Surgery for Diabetic Foot”. World Scientific (2016): 1-9.
- Wagner FW. “The Dysvascular foot: A system for diagnosis and treatment”. Foot & Ankle International 2.2 (1981): 64-122.
- Orsted HL., et al. “Basic Principles of Wound Healing”. Wound Care Canada 9.2 (2004): 4-12.
- Velnar T., et al. “The wound healing process: An overview of the cellular and molecular mechanisms”. Journal of International Medical Research 37.5 (2009):1528-1542.
- Gabriel A, Molnar J. Wound Healing and Growth Factors. Medscape.
- Gibson D., et al. “MMPs Made Easy”. Wound International. Published November 18, 2014. Accessed April 18, 2016.
- International consensus. The role of proteases in wound diagnostics. An expert working group review. London: Wounds International, 2011.
- Muller M., et al. “Matrix metalloproteinases and diabetic foot ulcers: The ratio of MMP-1 to TIMP-1 is a predictor of wound healing”. Diabetic Medicine 25.4 (2008):419-426.
- Schultz GS and Wysocki A. “Interactions between extracellular matrix and growth factors in wound healing”. Wound Repair and Regeneration 17.2 (2009):153-162.
- Yager DR., et al. “Wound fluids from human pressure ulcers contain elevated matrix Metalloproteinase levels and activity compared to surgical wound fluids”. Journal of Investigative Dermatology 107.5 (1996): 743-748.
- Moore K. “The Scientific Basis of Wound Healing. In: The Scientific Basis of Tissue Transplantation”. Ed. Nather A Singapore: World Scientific. (2001): 379-398.
- Shoulders MD and Raines RT. “Collagen structure and stability”. Annual Review of Biochemistry 78.1 (2009): 929-958.
- Meigel WN., et al. “Dermal architecture and collagen type distribution”. Archives for Dermatological Research 259.1 (1977):1-10.
- Ackerman AB., et al. “Embryologic, Histologic and Anatomic Aspects. In: Histologic diagnosis of inflammatory skin diseases: An Algorithmic method based on pattern analysis”. Ed. Ackerman AB, Boer A, Bennin B. 3rd ed. Ardor Scribendi, United States, 2005.
- Wang C., et al. “The content and ratio of type I and III collagen in skin differ with age and injury”. African Journal of Biotechnology 10.13 (2011):2524-2529.
- Broughton G and Rohrich R. “Wounds and Scars”. Selected Readings in Plastic Surgery 10.7 (2005): 1-54.
- Van De Water L., et al. “Mechanoregulation of the Myofibroblast in wound contraction, Scarring, and fibrosis: Opportunities for new therapeutic intervention”. Advances in Wound Care 2.4 (2013):122-141.
- Schreml S., et al. “Oxygen in acute and chronic wound healing”. British Journal of Dermatology 163.2 (2010): 257-268.
- Teo ZL., et al. “Basic Science of Wound Healing. In: The Diabetic Foot”. Ed. Nather A:World Scientific; 2012: 89-97.
- Schultz GS., et al. “Wound bed preparation: A systematic approach to wound management”. Wound Repair and Regeneration 11.s1 (2003): S1–S28.
- Nather A., et al. “Best practice guidelines for ASEAN Plus: Management of diabetic foot wounds”. Sri Lanka Journal of Diabetes Endocrinology and Metabolism 5.1 (2015): 1.
- The International Working Group on the Diabetic Foot. Prevention and Management of Foot Problems in Diabetes Guidance Documents and Recommendations 2015. IWGDF.
- Beidler SK., et al. “Multiplexed analysis of matrix metalloproteinases in leg ulcer tissue of patients with chronic venous insufficiency before and after compression therapy”. Wound Repair and Regeneration 16.5 (2008): 642-648.
Citation:
Aziz Nather., et al. “Healing of Diabetic Foot Wounds-The Role of Basic Science and Underlying Pathology in Management of Diabetic Foot Wounds”. Orthopaedic Surgery and Traumatology 1.2 (2016): 51-62.
Copyright: © 2016 Aziz Nather., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.



































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.