Review Article
Volume 1 Issue 5 - 2017
Novel Structure-Activity Relationship and Quantitative Data for Phenolic Drugs Involving Alzheimer’s and Parkinson’s disease: Antioxidants, Oxidative Stress, and Selectivity.
1Department of Chemistry and Biochemistry, San Diego State University, San Diego, CA, USA
2Library and Information Access, San Diego State University, San Diego, CA, USA
2Library and Information Access, San Diego State University, San Diego, CA, USA
*Corresponding Author: Peter Kovacic, Department of Chemistry and Biochemistry, San Diego State University, San Diego, CA, USA.
Received: October 17, 2017; Published: October 23, 2017
Abstract
This report presents a novel structure-activity relationship (SAR) for phenolic drugs involving Alzheimer’s disease (AD) and Parkinson’s disease (PD). The drugs function as antioxidants in combating harmful reactive oxygen species (ROS) and oxidative stress (OS). Substituents, such as alkyl, vinyl, imine, and carbonyl, para to the hydroxyl, stabilize the para position radical and block harmful quinone formation which would induce ROS and OS. For the catechol type drugs, generation of O-monomethyl ether avoids harmful formation of a quinone while maintaining the substituent para to hydroxyl.
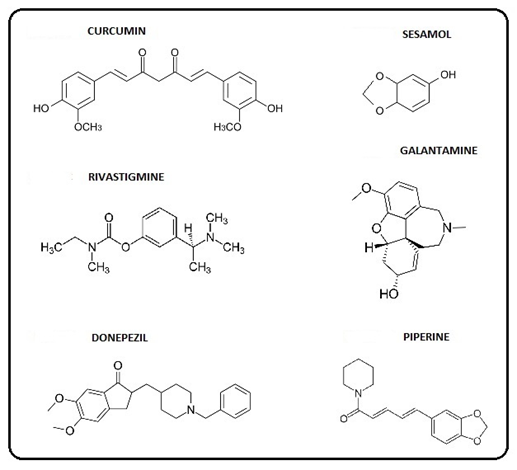
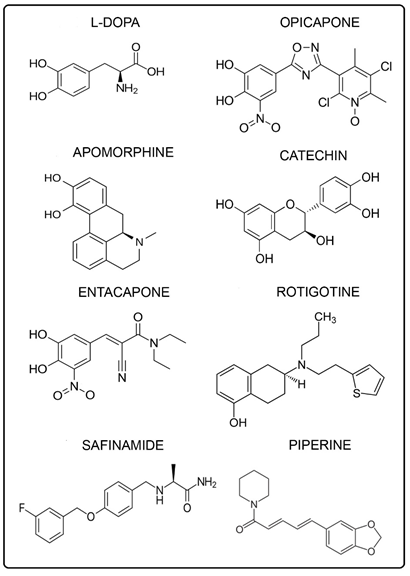
There is uncertainty concerning conflicting results from some phenols, such as vitamin E, which can be rationalized selectively by the dual property of anti- and pro- oxidant. The quantitative data and the SAR model indicate that the SAR theme may have wider applicability suggesting a broad unifying aspect. The phenolic AD drugs involved are curcumin, seasamol, rivastigmine, galantamine, donepezil, and piperine and the PD drugs are L-dopa, opicapone, apomorphine, catechin, entacapone, rotigotine, safinamide, and piperine.
Keywords: Structure-activity relationship (SAR); phenolic antioxidant drugs; Alzheimer’s disease; Parkinson’s disease; oxidative stress
Abbreviations: ET: Electron transfer; ROS: Reactive oxygen species; OS: Oxidative stress; AO: Antioxidant; SAR: Structure-activity relationship; QSAR: Quantitative structure-activity relationship; COMT: Catechol- O-methyltransferase; AD: Alzheimer’s disease; and PD: Parkinson’s disease
Introduction
Structure-activity relationship (SAR) studies are commonly made by altering the structure of an existing drug in order to improve activity and reduce toxicity [1]. The alteration usually involves size and shape of the skeleton, nature and degree of substitution, and stereochemistry. Another approach entails testing a drug with activity in one area for desirable properties in another. A basic aspect would involve similar mechanisms in both cases. Initially, research was based on beneficial changes of naturally occurring drugs. Many potential drugs are examined in computer studies involving SAR.
SAR plays an important part in modern day design involving both chemical and physical features. Quantitative aspects may also play a role. This report addresses SAR in phenolic drugs for treatment of Alzheimer’s disease (AD) [2] and Parkinson’s disease [3] based on oxidative stress (OS) and antioxidant (AO) action. Quantitative data are included. The SAR favors aspects that maintain AO action while eliminating or suppressing unwanted features that lead to generation of toxic reactive oxygen species (ROS) that promote OS.
The Quantitative structure-activity relationship (QSAR) plays an important role in drug discovery and development [1]. The method involves mathematical relationship between physiological activity and physicochemical parameters. The various properties measured include electron distribution; one of the electronic effects determines how readily and how long the drug adheres to the receptor molecule.
The electronic aspect examined in the present study is quite different. Support for formation of Figure 2 from Figure 1 is provided by generation of analogous Figure 3 from oxidation by ozone of 3, 4, 5 – trimethylphenol [4]. Although the two oxidations produce very similar products, the oxidizing agents are quite different. Ozone is not an important in vivo oxidant. Oxygen is a principal one in vivo entailing conversion to superoxide which is a precursor for other oxidants in the ROS class.
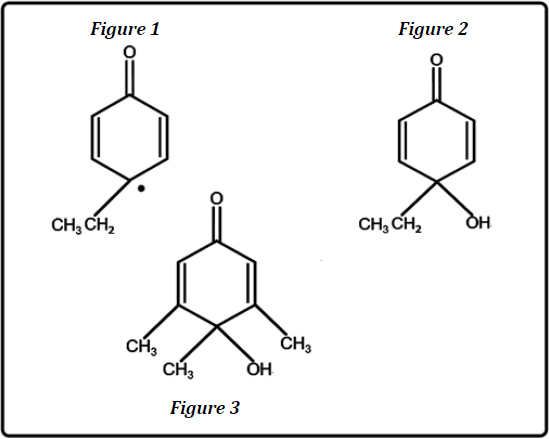
Figure 1: Resonance form of peroxy radical from p-ethylphenol.
Figure 2: Cyclohexadienone from oxidation of p-ethylphenol.
Figure 3: Cyclohexadienone from oxidation of 3,4,5-trimethylphenol.
Figure 2: Cyclohexadienone from oxidation of p-ethylphenol.
Figure 3: Cyclohexadienone from oxidation of 3,4,5-trimethylphenol.
Discussion
The SAR proposal is mainly composed of two themes. The principal one is based on the substituent in the para position of the phenoxy radical derived from the AO phenolic. The substituent performs several functions, one being to block formation of the p-quinone, an ET entity which generates harmful ROS and OS. Another property is to stabilize the phenoxy radical which is delocalized into the para position. Various groups posses this function, such as alkyl, vinyl, imine, and carbonyl. The alkyl-like group provides a tert-radical which is more stable than the sec-radical. In the other cases, stabilization is performed by delocalization via conjugation.
The AD drugs with the alkyl group are: galantamine, and donepezil and the PD drugs are: L-dopa, apomorphine, catechin, and safinamide. The AD drugs with the vinyl group are: curcumin and piperine and the PD drugs are: entacapone and piperine. An imine, a vinyl analog, is the para substituent present in opicapone, a PD drug [5]. In the AD case, the carbonyl substituent is present in the drug, donepezil. Phenolic ethers which can be dealkylated to phenols can be found in the AD (Figure 4) and PD (Figure 5) figures. However, it should be noted that there is one drug, namely rotigatine (Figure 5), which has no substituent in the para position.
Quantitative
QSAR provides insight from various perspectives to a complicated picture. Quantitative data for hydrocarbon radicals relevant to SAR are available [6]. The material relative to the para –ethyl and para –vinyl (allylic type) models (Table 1) contains bond dissociation energies (∆H) for the radicals involved, based on C-H bond cleavage. The order of stability for the radicals is allylic > tert > sec > pri.
QSAR provides insight from various perspectives to a complicated picture. Quantitative data for hydrocarbon radicals relevant to SAR are available [6]. The material relative to the para –ethyl and para –vinyl (allylic type) models (Table 1) contains bond dissociation energies (∆H) for the radicals involved, based on C-H bond cleavage. The order of stability for the radicals is allylic > tert > sec > pri.
| Hydrocarbon radical | ∆H (Kcal) |
| Pri | 98 |
| SEc | 94 |
| TERT | 91 |
| Allylic | 88 |
Table 1: Bond dissociation energies for hydrocarbon radicals.
The models represent various phenolic AO drugs in the AD and PD classes. Less energy is required to cleave the C-H bond in formation of more highly substituted radicals. Delocalization involving resonance is an important factor for the allylic type. The saturated hydrocarbon radicals may be stabilized by the electron donating effect of alkyl groups making for the order of stability shown in Table 1. Apparently, greater stability is associated with lower reactivity. There are SAR studies with some of the phenolic AD and PD drugs, but the subject matter is quite different from that for the present case which is multifaceted.
Another factor, a physical one, has received little or no attention. The much bulkier methyl groups would exert more blockage or steric hindrance to reaction than the smaller hydrogens of H3C. In the planar radicals. Atoms and groups in molecules and reactive intermediates are in continual motion. The planar tert –butyl radical is not a stationary entity. The methyl substituents are in rapid vibratory movement, thereby increasing the scope of steric hindrance. This physical effect is somewhat similar to the influence of reactant concentration on reactivity in solution.
In relation to SAR, it is relevant to discuss the role of catechol monomethyl ethers, e.g. from L-dopa and dopamine. Efficient catalysis is provided by the catechol- O-methyltransferase (COMT) enzyme. There are several important aspects involved, one being blockage of o-quinone formation which otherwise would lead to toxic ROS and OS. Another factor entails the remaining hydroxyl group with an alkyl entity in the para position. The importance of that structure is treated in detail in an earlier section. An appreciable number of the various brain drugs display the catechol or catechol ether structure. In the AD case, they comprise donepezil, piperine, curcumin, sesamol, and galantamine. For the PD drugs, they are L-dopa, opicapone, apomorphin, catechin, entacapone, and piperine.
In the case of PD, most of the SAR studies have been based on dopamine and substances with similar structural features, e.g. apomorphine [7]. Also, monohydroxy derivatives are less potent than the dihydroxy form of apomorphine. Monomethylation of each of the hydroxyl groups with catalysis by COMT is treated. The two rings attached to the catechol structure are highlighted. As in the case of L-dopa, the gold standard, and dopamine, structural features are in accord with the present SAR approach. There is an alkyl-like substituent para to a hydroxyl group which prevents p-quinone formation and stabilizes the phenoxy radical.
There are prior reports dealing with SAR in brain diseases and related areas. Additional material is presented on apomorphine [7] which was briefly treated in an earlier section. Among the key structural features is hydrogen bonding involving catechol hydroxyls and nitrogen. The protonated form appears to play an important role in electrostatic interactions. Other aspects are also addressed in relation to SAR [8]. Another article provides a structural model for central nervous system drugs which play an important part in various aspects of brain chemistry [9]. The structural model consists primarily of an aromatic group and a nitrogen atom, applied to various drugs including apomorphine. Hydrophobic interactions occur between the aromatic entity and the receptor, which is an aspect of focus.
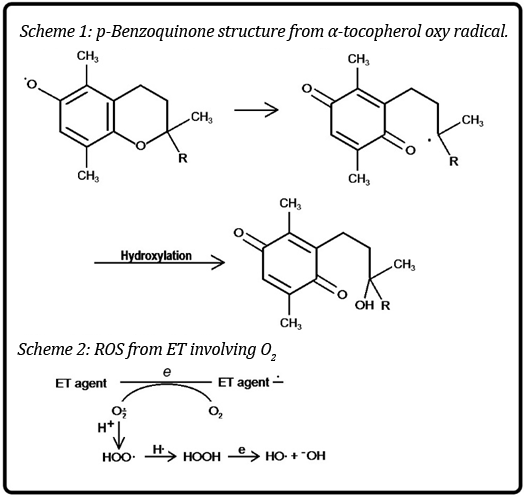
A 2016 review provides a recent update on vitamin E (α-tocopherol) [10]. Vitamin E, a widely used phenolic AO, scavenges ROS, thereby decreasing lipid peroxidation and OS in general. In PD, results with animals were mostly contradictory. Controversial findings were obtained from clinical trials. However, progress of the disease may be slowed by the AO. Also, vitamin E in the diet reduces the risk of PD, but vitamin E supplements apparently did not provide favorable results.
Beneficial effects in PD were not observed in controlled trials. Similarly, α-tocopherol did not provide evidence for benefits. In another study with the same AO, the life of the patient was not increased. Similar conflicting findings were obtained in AD [11]. There is no evidence that α-tocopherol prevents progression to dementia or that it improves cognitive function in AD [12]. In another report vitamin E did not prevent AD [13]. Vitamin E had no cognitive benefits, but could delay functional decline in AD patients [14].
There is scare discussion of rationale for the contradictory results. An explanation is based on the molecule acting both as an AO and a generator of ROS-OS [15]. In Scheme 1, the oxy radical formed by AO action, can undergo cleavage of the ether link leading to generation of a p-benzoquinone, an ET agent that is known to give rise to harmful ROS-OS (Scheme 2). Thus, the two conflicting forces are at play with variable results based on conditions. The dual character is found in other cases in a recent review [16].
There are two recent reviews that are quite relevant to the SAR approach. In the 2017 one [17] 75% of the nutrients were in accord with SAR. The list consists of curcumin, genistein, luteolin, ellagic acid, punicalagin, cyanidin-3-glucoside, caffeic acid, urolithin, resveratrol, epigallocatechin, and piperine. In the 2015 one [14], 56% of natural monophenols fell in line with SAR. The compounds involved are eugenol, tyrosine, vanillin, capsaicin, 5-hydroxytryptamine, psilocin, sesamol, estradiol, etoposide, morphine, platensimycin, vancomycin, tetarimycin, and curcumin [17].
In both cases, the substances exhibited various types of physiological and drug activity. A magazine article addressed testing of a flaxseed component, secoisolariciresinol diglucoside, for preventive action against mesothelioma cancer from asbestos [18]. The aromatic core of the compound consists of a catechol monomethyl ether whose structure closely resembles that of an L-dopa metabolite. Another example is the isoapocodeine metabolite from apomorphine [7].
These results suggest broad applicability of SAR. An exhaustive literature search was performed; although other SAR and related materials are available, as noted herein, the present SAR appears to be novel. This SAR report should be of interest to the drug industry and to appropriate academicians. It is important to recognize that various factors are involved in drug action. Among the contributors are chemical and physical properties, electrochemistry, receptor binding, and cell signaling.
Future Research
A rational approach would be based upon SAR presented herein, in addition to other factors. One method is to place multiple AO phenolic groups into a single substance. Thus, theoretically, in this manner, a single molecule with three AO hydroxyls might be expected to function as well or better than three separate AO compounds. This suggestion avoids the hazards encountered by each of the multiple molecules on the path to the active site.
A rational approach would be based upon SAR presented herein, in addition to other factors. One method is to place multiple AO phenolic groups into a single substance. Thus, theoretically, in this manner, a single molecule with three AO hydroxyls might be expected to function as well or better than three separate AO compounds. This suggestion avoids the hazards encountered by each of the multiple molecules on the path to the active site.
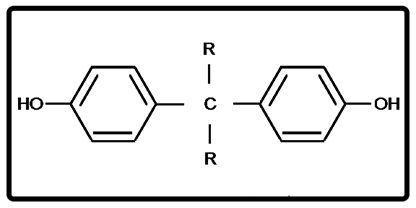
The various factors to be considered in designing SAR are addressed in a reference book [1], in addition to the blood-brain barrier. As a result, a candidate molecule to be tested is represented in Figure 7. The R groups take into account other important features of SAR. Other substituents could be introduced onto the aromatic rings. All incorporate an alkyl entity para to hydroxyl which is part of SAR. A series based on Figure 7 of analogs containing 3 and 4 phenolic groups in place of R group (Figure 7) might be considered.
Studies could be carried out that introduce other desirable groups para to hydroxyl based on SAR. One such substituent is - SO2NH2 which would be related to the well-known antibacterial sulfonamide category. The biphenyl class is a possibility consisting of HOC6H4 – C6H4R with para links. Another approach involves aromatic heterocyclic groups containing 1-3 atoms comprising O, N, and S. The aromatic structures provide delocalization via conjugation for stabilization of the para radical. Also, a possibility is a fused ring system involving the heterocyclic and the meta-para positions. Another is a naphthalene phenolic, such as 1,8-dihydroxynaphthalene with R groups in the para positions. Multiple phenols are found naturally within a single molecule in the flavonoid class, such as catechin (Figure 5), a Parkinson’s drug, which displays various activities based on anti- and pro- oxidant effects. A second m-dihydroxy could be substituted for the catechol.
Acknowledgement
Helpful discussions with Dr. Andrew Cooksy are appreciated. Assistance by Thelma Chavez is acknowledged.
Helpful discussions with Dr. Andrew Cooksy are appreciated. Assistance by Thelma Chavez is acknowledged.
References
- Thomas G. Medicinal Chemistry. Wiley, Hoboken, NJ (2007): 76-110.
- Kovacic P and Weston W. “Phenolic antioxidants as drugs for Alzheimer’s disease: oxidative stress and selectivity”. 2.5 (2017).
- Kovacic P and Weston W. “Treatment of Parkinson’s disease with phenolic antioxidant drugs: Oxidative stress, reactive oxygen species and selectivity”. Chronicles of Pharmaceutical Science 1.4: 193-198.
- Carreño MC., et al. “Oxidative de-aromatization of para-alkyl phenols into para-peroxyquinols and para-quinols mediated by oxone as a source of singlet oxygen”. Angewandte Chemie International Edition in English 45.17 (2006): 2737-2741.
- Kovacic P and Somanathan R. “Mechanism of conjugated imine and iminium species, including marine alkaloids: Electron transfer, reactive oxygen species, therapeutics and toxicity”. Current Bioactive Compounds 6.1 (2010): 46-59.
- Wade LG. Organic Chemistry. Prentice-Hall, Englewood Cliffs, NJ (1991): 154-155 & 641.
- Subramony JA. “Apomorphine in dopaminergic therapy”. Molecular Pharmacology 3.4 (2006): 380-385.
- Jiang H., et al. “Advances in the structure-activity relationship of anti-dementia drugs”. Chinese Journal of New Drugs 14.5 (2005): 535-539.
- Lloyd EJ and Andrews PR. “A common structural model for central nervous system drugs and their receptors”. Journal of Medicinal Chemistry 29.4 (1986): 453-462.
- Filograna R., et al. “Anti-Oxidants in Parkinson’s disease therapy: A critical point of view”. Current Neuropharmacology 14.3 (2016): 260–271.
- Grimm MO., et al. “Vitamin E: Curse or benefit in Alzheimer's disease? A systematic investigation of the impact of α-, γ- and δ-tocopherol on Aß generation and degradation in neuroblastoma cells”. The Journal of Nutrition Health and Aging 19.6 (2015): 646-56.
- Farina N., et al. “Vitamin E for Alzheimer's dementia and mild cognitive impairment”. The Cochrane Database of Systematic Reviews – Journals 1 (2012).
- Kryscio RJ., et al.“Association of antioxidant supplement use and dementia in the prevention of Alzheimer's disease by vitamin E and selenium trial (PREADViSE)”. JAMA Neurology 74.5 (2017): 567-573.
- Adalier N and Parker H. (2016) “Vitamin E, turmeric and saffron in treatment of Alzheimer's disease”. Antioxidants (Basel) 5.4 (2016): 40.
- Kovacic, P., et al. “Natural monophenols as therapeutics, antioxidants and toxins; electron transfer, radicals and oxidative stress”. The Natural Products Journal 5.3 (2015): 142-151.
- Kovacic P and Somanathan R. “Beneficial effects of antioxidants in relation to carcinogens, toxins and various illnesses. In: Panglossi, H.V., editor. Antioxidants: New Research”. NY: Nova Science Publishers Inc (2006): 1-38.
- Kovacic P and Somanathan R. “Unifying mechanism for nutrients as anticancer agents: Electron transfer, reactive oxygen species and oxidative stress”. Global Journal of Health Science 9.8 (2017): 66-83.
- Arnaud CH. “Attacking asbestos from all angles”. Chemical & Engineering News 94.47 (2016): 32-33.
Citation:
Peter Kovacic and Wil Weston. “Novel Structure-Activity Relationship and Quantitative Data for Phenolic Drugs Involving
Alzheimer’s and Parkinson’s disease: Antioxidants, Oxidative Stress, and Selectivity.” Chronicles of Pharmaceutical Science 1.5 (2017):
299-306.
Copyright: © 2017 Peter Kovacic and Wil Weston. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.