Mini Review
Volume 2 Issue 6 - 2018
Dementia – Unifying Mechanism Involving Antioxidant Therapy: Reactive Oxygen Species, Oxidative Stress, and Phenolics.
1Department of Chemistry and Biochemistry, San Diego State University, San Diego, CA, USA
2Library and Information Access, San Diego State University, San Diego, CA, USA
2Library and Information Access, San Diego State University, San Diego, CA, USA
*Corresponding Author: Peter Kovacic, Department of Chemistry and Biochemistry, San Diego State University, San Diego, CA, USA.
Email: pkovacic@sdsu.edu
Received: July 16, 2018; Published: July 28, 2018
Abstract
Reactive oxygen species (ROS) and oxidative stress (OS) play roles in dementia, as also in Alzheimer’s (AD), Parkinson’s (PD) disease
and Schizophrenia (SCZ). Various sources, including oxidases, serve as generators of ROS-OS, such as mitochondria, NADPH,
cytochromes P450, monoamines, ET metal complexes, G72 gene, and microglia. Diverse types of antioxidants (AOs) exert a positive
influence on the harmful effects. A unifying mechanism based on ET-ROS-OS-AO is involved. Drugs for treatment of dementia are
discussed in relation to the unifying theme.
Keywords: Dementia; Radicals; Oxidative stress; Reactive oxygen species; Antioxidants
Abbreviations: ET: Electron transfer; ROS: Reactive oxygen species; OS: Oxidative stress; AO: Antioxidant; SCZ: Schizophrenia; AD:
Alzheimer’s disease; PD: Parkinson’s disease
Introduction
Symptoms
Dementia is a term of medieval origin from the Latin demens meaning out of one’s mind [1]. Contemporary usage of the term is a syndrome of progressive and persistent decline in multiple cognitive domains with associated changes in behavior, personality, and/or social functioning, produced by brain impairment. Senile dementia is the most common cause of dementia syndrome in a rapidly aging world. Memory impairment is the key cognitive domain, followed by frontal executive, language, and spatial dysfunctions. The most common dementia type associated with aging remains Alzheimer’s disease, followed by dementia with Lewy bodies and vascular dementia. Clinical features suggesting dementia with Lewy bodies include hallucinations, deficits in attention with fluctuations in cognition, mild Parkinsonism with progressive gait disorder and falls, and early visuospatial deficits; memory complaint or impairment is common, but less severe. Clinical features are mild memory impairment and mild behavioral changes. The diagnosis of vascular dementia is assisted by the presence of vascular risk factors and clinical and/or imaging evidence of strokes. Senile dementia is often a mix of these common dementia types. The dementia syndrome may also be caused by external insults (such as vasculitis, toxins, drugs, infectious agents, brain trauma, systemic organ failure) and caused by other systemic disorders, including disturbance in thyroid, vitamin, and calcium metabolism, although rarely.
Dementia is a term of medieval origin from the Latin demens meaning out of one’s mind [1]. Contemporary usage of the term is a syndrome of progressive and persistent decline in multiple cognitive domains with associated changes in behavior, personality, and/or social functioning, produced by brain impairment. Senile dementia is the most common cause of dementia syndrome in a rapidly aging world. Memory impairment is the key cognitive domain, followed by frontal executive, language, and spatial dysfunctions. The most common dementia type associated with aging remains Alzheimer’s disease, followed by dementia with Lewy bodies and vascular dementia. Clinical features suggesting dementia with Lewy bodies include hallucinations, deficits in attention with fluctuations in cognition, mild Parkinsonism with progressive gait disorder and falls, and early visuospatial deficits; memory complaint or impairment is common, but less severe. Clinical features are mild memory impairment and mild behavioral changes. The diagnosis of vascular dementia is assisted by the presence of vascular risk factors and clinical and/or imaging evidence of strokes. Senile dementia is often a mix of these common dementia types. The dementia syndrome may also be caused by external insults (such as vasculitis, toxins, drugs, infectious agents, brain trauma, systemic organ failure) and caused by other systemic disorders, including disturbance in thyroid, vitamin, and calcium metabolism, although rarely.
Symptoms of dementia emerge slowly and get worse over time [2]. There may be difficulty with complex mental tasks as well as with mood and behavior. There are problems with speaking and personal care, as well as hallucinations and delusions. They may not recognize their surroundings or other people and there is difficulty with language and performing acts. Dementia is a pattern of mental decline caused by different diseases or conditions, such as when brain cells (neurons) die, and connections between neurons are interrupted, which usually cannot be reversed. Alzheimer’s disease causes over 60% of all dementia. Vascular disease, such as stroke, is the second most common cause. In the developed nations, about 15% of people older than 65 are thought to have dementia.
The strongest risk factor for dementia is age [1]. Other neurodegenerative diseases progress to age-related dementia including dementia with Lewy bodies and Parkinson disease dementia; less common frontotemporal dementias are cortico-basal degeneration and progressive supranuclear palsy, motor neuron disease, and other rarer types of dementia. Mid-life vascular factors, causing later-life small vessel vascular brain disease, have also come to be regarded more as risk factors for mixed pathology senile dementia than as common predictors of vascular dementia. Much of the recent focus has been on memory-defined mild cognitive impairment, predicting progression to Alzheimer’s disease.
Unifying Mechanism
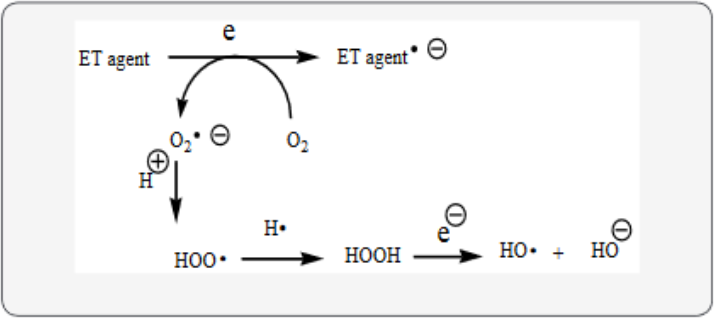
Dementia fits into the unifying mechanism which has been widely applied, previously in an article involving electron transfer (ET), reactive oxygen species (ROS) and oxidative stress (OS) [3]. This unifying mechanism argues that the preponderance of bioactive substances, usually as the metabolites, incorporate ET functionalities. We believe these ET-metabolites play an important role in physiological responses. The main group includes quinones (or phenolic precursors), metal complexes (or complexors), aromatic nitro compounds (or reduced hydroxylamine and nitroso derviatives), and conjugated imines (or iminimum species). Resultant redox cycling is illustrated in Scheme 1. In vivo redox cycling with oxygen can occur, giving rise to OS through generation of ROS, such as hydrogen peroxide, hydroperoxides, alkyl peroxides, and diverse radicals (hydroxyl, alkoxyl, hydroperoxyl, and superoxide) (Scheme 1). Cellular and mitochrondrial enzymes can also perform cataytically in the reduction of O2.
Dementia fits into the unifying mechanism which has been widely applied, previously in an article involving electron transfer (ET), reactive oxygen species (ROS) and oxidative stress (OS) [3]. This unifying mechanism argues that the preponderance of bioactive substances, usually as the metabolites, incorporate ET functionalities. We believe these ET-metabolites play an important role in physiological responses. The main group includes quinones (or phenolic precursors), metal complexes (or complexors), aromatic nitro compounds (or reduced hydroxylamine and nitroso derviatives), and conjugated imines (or iminimum species). Resultant redox cycling is illustrated in Scheme 1. In vivo redox cycling with oxygen can occur, giving rise to OS through generation of ROS, such as hydrogen peroxide, hydroperoxides, alkyl peroxides, and diverse radicals (hydroxyl, alkoxyl, hydroperoxyl, and superoxide) (Scheme 1). Cellular and mitochrondrial enzymes can also perform cataytically in the reduction of O2.
In some cases, ET results in involvement with normal electrical effects (e.g. neurochemistry). Generally, active entities possessing ET groups display reduction potentials in the physiologically responsive range. Hence, ET in vivo can occur resulting in production of ROS, which can be beneficial in cell signaling at low concentrations, but produce toxic results at high levels. Electron donors consist of phenols, N-heterocycles or disulfides in proteins, which produce relatively stable radical cations. ET, ROS and OS have been increasingly implicated in the mode of action of drugs and toxins, e.g. anticancer drugs [4], carcinogens [5], cardiovascular toxins [6], toxins [7], ototoxins [8] and various other catagories [9].
In addition to the above, there is a plethora of experimental evidence supporting the theoretical framework. This evidence includes generation of the common ROS, lipid peroxidation, degradation products of oxidation, depletion of AOs, effect of exogenous AOs, and DNA oxidation and cleavage products, as well as electrochemical data [10]. This comprehensive, unifying mechanism is consistent with the frequent observation that many ET substances display a variety of activities (e.g. multiple-drug properties), as well as toxic effects. It is important to recognize that mode of action in the biodomain is often involved with many physiological actions and is multifaceted. In addition to ET-ROS-OS in relation to mechanism, much attention in the literature is paid to AO action entailing physiological action.
ROS-OS
ROS can be beneficial, but at high levels toxic effects often predominate. There are various sources for these species [11]. NAPDH oxidase is an important producer of the ROS in various organs. The G72 gene increased radical generation in cells. The gene acts as an activator of oxidase. ROS generated by NO synthase have been implicated in an array of harmful behaviors. Mitochondia provide another source of ROS-OS which appears to contribute to aging. Leakage of electrons occurs in the ET chain which react with oxygen to produce superoxide, a precursor of other ROS. Other examples of ROS producers are cytochrome P450, metal complexes, monoamine oxidase and microglia.
Drugs Used in Dementia Treatment
The major associate of dementia is AD, both of which are neurodegenerative disorders [12]. AD is the common most reason for dementia in the elderly [13]. There are various other reports that link the two illnesses [14-24]. In addition to these articles, there are assorted others that associate dementia with the unifying theme of ROS-OS-AO [25-30]. The literature deals with various drugs that are used for treatment of dementia. OS is believed to be the main risk for dementia and several of these studies deal with oxidative and AO markers in dementia patients [27-31]. Higher levels of AO power counter increased OS. Phenolics or their derivatives play important roles due to their AO and neuroprotective potential in the prevention of dementia, as in the case of PD and AD. Another important reference is a recent review concerning novel structure-activity relationship and quantitative data for phenolic drugs involving Alzheimer’s and Parkinson’s disease [32], which is applicable to dementia, as in importance of the substituent para to phenolic hydroxyl. Another factor is the presence of more than one phenolic group in the drugs discussed.
The major associate of dementia is AD, both of which are neurodegenerative disorders [12]. AD is the common most reason for dementia in the elderly [13]. There are various other reports that link the two illnesses [14-24]. In addition to these articles, there are assorted others that associate dementia with the unifying theme of ROS-OS-AO [25-30]. The literature deals with various drugs that are used for treatment of dementia. OS is believed to be the main risk for dementia and several of these studies deal with oxidative and AO markers in dementia patients [27-31]. Higher levels of AO power counter increased OS. Phenolics or their derivatives play important roles due to their AO and neuroprotective potential in the prevention of dementia, as in the case of PD and AD. Another important reference is a recent review concerning novel structure-activity relationship and quantitative data for phenolic drugs involving Alzheimer’s and Parkinson’s disease [32], which is applicable to dementia, as in importance of the substituent para to phenolic hydroxyl. Another factor is the presence of more than one phenolic group in the drugs discussed.
Phenols and Phenolic derivatives
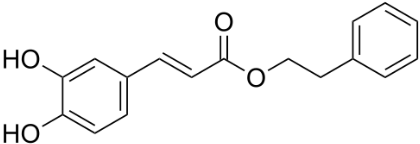
Caffeic acid phenethyl ester (CAPE) (Figure 1) is a natural polyphenolic substance attributed with AD immunomodulatory and neuroprotective properties [25]. The drug arrested development of cognitive defecits in rats with dementia. Glutathione (GSH) content was enhanced, accompanied by decreased measure in rat
Caffeic acid phenethyl ester (CAPE) (Figure 1) is a natural polyphenolic substance attributed with AD immunomodulatory and neuroprotective properties [25]. The drug arrested development of cognitive defecits in rats with dementia. Glutathione (GSH) content was enhanced, accompanied by decreased measure in rat
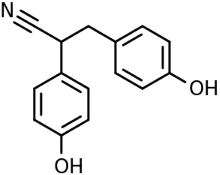
Diarylpropionitrile (Figure 2), a diphenolic, is a synthetic, nonsteroidal, estrogen receptor ligand that protects neurons in dementia through various mechanisms, including anti-oxidation, anti-apoptosis and anti-inflammation [17].
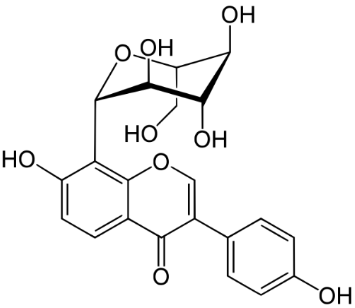
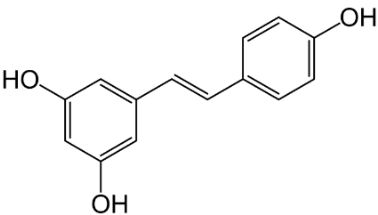
Puerarin (Figure 3) exerts various positive effects in vascular dementia, such as, decrease in malondialdehyde levels; GSH and total thiol levels were restored; apoptosis was reduced, and scavenging of ROS occurred [33]. Similar results were observed with Gastrodin (Figure 5) which is further discussed with the phenolic derivatives. [34], the polyphenol AO resveratrol (Figure 4) in nanoparticles attenuates mitochondrial OS in vascular dementia [35]. The agent improved redox rates and superoxide dismutase (SOD) activity.
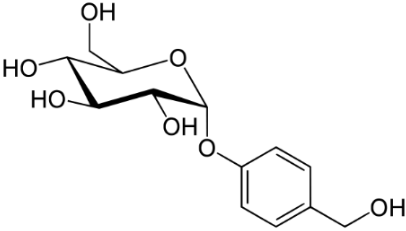
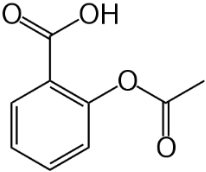
Gastrodine (Figure 5), a phenolic ether, exerts various positive effects in vascular dementia, decreasing MDA levels and restoring total thiol levels [34]. Scavenging of ROS occurred and a reduction of apoptosis. Aspirin (Figure 6), a phenolic ester that on hydrolysis yields the phenolic salicylic acid, was studied for its effect on OS and homocysteine [36]. Aspirin was effective in reducing factors associated with cognitive impairment in people at risk of dementia.
Disulfide and Thiolsulfinate
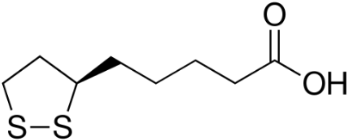
A 2014 study reported beneficial effects of α-lipoic acid (Figure 7) complex on rats with dementia [36]. The supplementation of α-lipoic acid improved their AO status. Garlic, which contains sulfur AOs, such as allicin (Figure 8), has been studied extensively for health benefits [30]. In a number of studies, garlic preparations protect neuronal cells against β -amylod toxicity and apoptosis [32]. The broad range of AO and anti-apoptotic effects afforded by garlic also provides protection against dementia.
A 2014 study reported beneficial effects of α-lipoic acid (Figure 7) complex on rats with dementia [36]. The supplementation of α-lipoic acid improved their AO status. Garlic, which contains sulfur AOs, such as allicin (Figure 8), has been studied extensively for health benefits [30]. In a number of studies, garlic preparations protect neuronal cells against β -amylod toxicity and apoptosis [32]. The broad range of AO and anti-apoptotic effects afforded by garlic also provides protection against dementia.
Other Drugs
Simvastatin (Figure 9) exerts benefits in the progression of senile dementia [37]. The drug regulates SOD, ROS, catalase and GSH in OS. Additionally, there is prevention of memory lapses. Hu-Yi-Neng, a diet supplement, including the AO ginko biloba, exerts a protective effect against OS [31]. Non-drugs such as acupuncture [38,39] and certain vitamins [40] are also reported to exert a positive effect on dementia.
Simvastatin (Figure 9) exerts benefits in the progression of senile dementia [37]. The drug regulates SOD, ROS, catalase and GSH in OS. Additionally, there is prevention of memory lapses. Hu-Yi-Neng, a diet supplement, including the AO ginko biloba, exerts a protective effect against OS [31]. Non-drugs such as acupuncture [38,39] and certain vitamins [40] are also reported to exert a positive effect on dementia.
Conclusion
Various drugs have proven effective in treatment of dementia. The major ones are phenolic, followed by sulfur containing ones, similar to AD and PD. Structure activity relationship (SAR) is applicable, as for AD and PD [41]. More work is needed on the important aspects of prevention and cure for the various brain illnesses.
References
- Howell T. “Behavioral and psychological symptoms of dementia. In E. Capezuti. The Encyclopedia of Elder Care: The Comprehensive Resource on Geriatric Health and Social Care (3rd ed.). New York, NY”. Springer Publishing Company (2013).
- Dementia. “In Harvard Medical School (Ed.), Health Reference Series: Harvard Medical School Health Topics A-Z. Boston, MA”. Harvard Health Publications (2017).
- Kovacic P. “Unifying mechanism for nutrients as anticancer agents: Electron transfer, reactive oxygen species and oxidative stress”. Global Journal of Health Science 9.8(2017): 66-83.
- Kovacic P., et al. “Mechanisms of anticancer agents: Emphasis on oxidative stress and electron transfer”. Current Pharmaceutical Design: Ingenta Connect Publication 6 (2000): 277-309.
- Kovacic P., et al. “Reproductive toxins. Pervasive theme of oxidative stress and electron transfer”. Current Pharmaceutical Design: Ingenta Connect Publication 8 (2001): 863-892.
- Kovacic P., et al. “Cardiovascular toxicity from the perspective of oxidative stress, electron transfer, and prevention by antioxidants”. Current Pharmaceutical Design: Ingenta Connect Publication 3 (2005): 107-117.
- Kovacic P., et al. “Mechanism of mitochondrial uncouplers, inhibitors, and toxins: Focus on electron transfer, free radicals, and structure-activity relationships”. Current Pharmaceutical Design: Ingenta Connect Publication 12.22 (2005): 2601-2623.
- Kovacic P., et al. “Ototoxicity and noise trauma: Electron transfer, reactive oxygen species, cell signaling, electrical effects, and protection by antioxidants: Practical medical aspects”. Medical Hypotheses 70 (2008): 914-923.
- Halliwell B., et al. “Free Radicals in Biology and Medicine (3rd ed., Oxford science publications) Oxford: New York: Clarendon Press”. Oxford University Press (1999): 192-194.
- Kovacic P., et al. “Various factors involving aging: Electron transfer, reactive oxygen species and oxidative stress”. Current Pharmaceutical Design: Ingenta Connect Publication 9.7 (2017): 53518-53528.
- Howell T., et al. “Behavioral and psychological symptoms of dementia. In E. Capezuti. The Encyclopedia of Elder Care: The Comprehensive Resource on Geriatric Health and Social Care (3rd ed.). New York, NY: Springer Publishing Company (2013).
- Dementia. “In Harvard Medical School (Ed.), Health Reference Series: Harvard Medical School Health Topics A-Z”. Boston, MA: Harvard Health Publications (2017).
- Kovacic P., et al. “Cause and treatment of schizophrenia: Electron transfer, reactive oxygen species, oxidative stress, antioxidants, and unifying mechanism”. Chronicles of Pharmaceutical Science 1.6 (2017): 332-340.
- Gugliandolo A., et al. “Role of vitamin E in the treatment of Alzheimer's disease: Evidence from animal models”. International Journal of Molecular Sciences 18.12 (2017).
- Dey A., et al. “Natural products against Alzheimer's disease: Pharmaco-therapeutics and biotechnological interventions”. Biotechnology Advances 35.2 (2016): 178-216.
- Bhatti A.B., et al. “Vitamin supplementation as an adjuvant treatment for Alzheimer's disease”. Journal of Clinical and Diagnostic Research 10.8(2016).
- Sarkar S., et al. “Nanocapsulated ascorbic acid in combating cerebral ischemia reperfusion - induced oxidative injury in rat brain”. Current Alzheimer Research 13.12 (2016): 1363-1373.
- Zuo L., et al. “The role of oxidative stress-induced epigenetic alterations in amyloid-β production in Alzheimer's disease”. Oxidative Medicine and Cellular Longevity (2015).
- Suwanna N., et al. “Neuroprotective effects of diarylpropionitrile against β-amyloid peptide-induced neurotoxicity in rat cultured cortical neurons”. Neuroscience Letters 578 (2016): 44-49.
- Gubandru M., et al. “Alzheimer's disease treated patients showed different patterns for oxidative stress and inflammation markers”. Food and Chemical Toxicology 61 (2013): 209-214.
- Ferreiro E., et al. “Mitochondrial- and endoplasmic reticulum-associated oxidative stress in Alzheimer's disease: from pathogenesis to biomarkers”. International Journal of Cell Biology (2012): 735206.
- Choi DY., et al. “Antioxidant properties of natural polyphenols and their therapeutic potentials for Alzheimer's disease”. Brain Research Bulletin 87.2 (2012): 144-153.
- Bayati S., et al. “Protective effects of 1,3-diaryl-2-propen-1-one derivatives against H₂O₂ -induced damage in SK-N-MC cells”. Journal of Applied Toxicology 31.6 (2011): 545-553.
- Di Bona D., et al. “Immune-inflammatory responses and oxidative stress in Alzheimer's disease: therapeutic implications”. Current Pharmaceutical Design 16.6 (2010): 684-691.
- Taupin P. “A dual activity of ROS and oxidative stress on adult neurogenesis and Alzheimer's disease”. Central Nervous System Agents in Medicinal Chemistry 10 (2010): 16-21.
- Bennett S., et al. “Oxidative stress in vascular dementia and Alzheimer's disease: a common pathology”. Journal of Alzheimer's Disease 179.2 (2009): 245-257.
- Kumar M., et al. “Caffeic acid phenethyl ester (CAPE) prevents development of STZ-ICV induced dementia in rats”. Pharmacognosy Magazine 13.1 (2017).
- Ajith TA., et al. “Effect of palladium α-lipoic acid complex on energy in the brain mitochondria of aged rats”. Alternative Therapies In Health And Medicine 20.3 (2014): 27-35.
- Gironi M., et al. “Oxidative imbalance in different neurodegenerative diseases with memory impairment”. Neurodegenerative Diseases 8.3 (2011): 129-137.
- Shih YT., et al. “Arecoline, a major alkaloid of the areca nut, causes neurotoxicity through enhancement of oxidative stress and suppression of the antioxidant protective system”. Free Radical Biology & Medicine 49.10 (2010): 1471-1479.
- Wu SB., et al. “Mitochondrial DNA mutation-elicited oxidative stress, oxidative damage, and altered gene expression in cultured cells of patients with MERRF syndrome”. Molecular Neurobiology 41.2 (2-010): 256-266.
- Mathew B., et al. “Neuroprotective effects of garlic a review”. Libyan Journal of Medicine 3.1 (2008): 23-33.
- Yang YH., et al. “Neuroprotective effects of Hu-Yi-Neng, a diet supplement, on SH-SY5Y human neuroblastoma cells”. The Journal of Nutrition Health and Aging 18.2 (2014): 184-190.
- Kovacic P., et al. “Novel structure-activity relationship and quantitative data for phenolic drugs involving Alzheimer’s and Parkinson’s disease: Antioxidants, oxidative stress, and selectivity”. Chronicles of Pharmaceutical Science 1.5 (2017): 299-306.
- Zhang J., et al. “Puerarin attenuates cognitive dysfunction and oxidative stress in vascular dementia rats induced by chronic ischemia”. International Journal of Clinical and Experimental Pathology 8.5 (2015): 4695-4704.
- Li Y., et al. “Gastrodin improves cognitive dysfunction and decreases oxidative stress in vascular dementia rats induced by chronic ischemia”. International Journal of Clinical and Experimental Pathology 8.11(20145): 14099-14109.
- Yadav A., et al. “Resveratrol loaded solid lipid nanoparticles attenuate mitochondrial oxidative stress in vascular dementia by activating Nrf2/HO-1 pathway”. Neurochemistry International 112 (2018): 239-254.
- Clarke R., et al. “Effect of vitamins and aspirin on markers of platelet activation, oxidative stress and homocysteine in people at high risk of dementia”. Journal of Internal Medicine 254.1 (2013): 67-75.
- Liu W., et al. “Simvastatin ameliorates cognitive impairments via inhibition of oxidative stress‑induced apoptosis of hippocampal cells through the ERK/AKT signaling pathway in a rat model of senile dementia”. Molecular Medicine Reports 17.1 (2018): 1885-1892.
- Du SQ., et al. “Acupuncture inhibits TXNIP-associated oxidative stress and inflammation to attenuate cognitive impairment in vascular dementia rats”. CNS Neuroscience & Therapeutics | Neuroscience 24.1 (2018): 39-46.
- Li H., et al. “Acupuncture reversed hippocampal mitochondrial dysfunction in vascular dementia rats”. Neurochemistry International 92 (2016): 35-42.
Citation:
Peter Kovacic and Wil Weston. “Dementia – Unifying Mechanism Involving Antioxidant Therapy: Reactive Oxygen Species,
Oxidative Stress, and Phenolics.” Chronicles of Pharmaceutical Science 2.6 (2018): 710-717.
Copyright: © 2018 Peter Kovacic and Wil Weston. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.