Research Article
Volume 1 Issue 2 - 2017
Biochemical and Ultrasonographic Prediction of Placenta Accreta in First Trimester of Pregnancy
1Department of Obstetrics and Gynecology, Faculty of Medicine, Alexandria University, Egypt
2Department of Clinical and Chemical Pathology, Faculty of Medicine, Alexandria University, Egypt
2Department of Clinical and Chemical Pathology, Faculty of Medicine, Alexandria University, Egypt
*Corresponding Author: Tamer Mamdouh Abdel dayem, Department of Obstetrics and Gynecology, Faculty of Medicine, Alexandria University, Egypt.
Received: November 09, 2017; Published: November 29, 2017
Abstract
Background: Placental development requires the coordination of antigenic growth factors and their receptors. These protein factors include vascular endothelial growth factor (VEGF), placental growth factor (Pig). Excessive trophoblast invasion is a frequent occurrence in pregnancies complicated by complete placenta prevail (CPP). This may be related to differences in oxygen tension in the lower uterine segment or in the uterine scar. Women with CPP have an abnormal serum antigenic profile.
Aim: The aim of this study was to evaluate the effectiveness of ultrasound as screening tool of placenta accreta in cases of previous caesarean deliveries and low lying anterior placenta in the first trimester and to clarify the relation between Pig and placenta accreta attending the obstetric clinic and ultrasound unit in El-Shatby Maternity University Hospital.
Methods: This study included 80 singleton pregnant women aged 20-40 years, study group of 40 cases at the first trimester with low lying placenta and history of previous caesarean section (CS), and control group of 40 pregnant women at the first trimester with fundal placenta who had previous history of normal vaginal delivery and had not history of previous CS. All patients were subjected to history taking, clinical and ultrasonographic examination. Serum placental growth factor (SPlGF) was measured by enzyme-linked immunosorbent assay (ELISA) at 11-14 week of gestation.
Results: Women with prevail and invasive placentation accreta (n = 5) had lower serum Pig (invasive prevail: median4.75 [IQR]: [4.5–5] vs. control: 5.4 [5–5.8] pg. /mL, P=0.001). Incidence of placenta prevai increased with low cord insertion near internal cervical os. Incidence of placenta accreta increased in cases with placental lacunae and Doppler signs of increased retroplacental vascularity.
Conclusion: According to this study, first trimester localization of placenta by ultrasonography could predict placenta prevail in cases with a history of previous caesarean section when diagnose low-lying placenta. Low cord insertion to the placenta has a value in predicting placenta prevail. Reduced serum level of Pig in the first trimester predicts placenta prevail.
Keywords: Low lying placenta; Pregnant women; Serum placental growth factor; Placenta accreta
Introduction
The term morbidly adherent placenta implies an abnormal invasion of the placenta into the uterine wall and has been used to describe placenta accreta, increta, and percreta. [1] The overall incidence of placenta accreta is around 3 per 1000 deliveries [2].
The pathogenesis of placenta accreta is not clear however, there have been several theories proposed [3]. Abnormal vascularization resulting from the scarring process after surgery with secondary localized hypoxia leading to both defective decidualization and excessive trophoblastic invasion [4]. Pig is a member of the VEGF sub-family, a key molecule in angiogenesis and vasculogenesis, in particular during embryogenesis. The main source of Pig during pregnancy is the placental trophoblastic process. [5]
Placenta prevail is the strongest risk factor for placenta accreta [6,7]. There has been a considerable increase in the incidence of placenta accreta over the past several decades. The main reason for this increase is the substantial rise in the rates of caesarean deliveries [8,9].
The risk of placenta accreta in the presence of placenta prevail increases dramatically with the number of previous CS, with a 25% risk for one prior CS, and more than 40% for two prior CS [10,11]. In this same patient, the risk of the presence of placenta accreta decreases to less than 1% if there is no placenta prevail in the at-risk pregnancy [11].
Most patients with placenta accreta are asymptomatic. Symptoms related to placenta accreta may include vaginal bleeding and cramping. These findings are largely seen in relation to placenta prevail. Although rare, a potentially catastrophic presentation is that of acute abdominal pain and hypotension due to hypovolemic shock from uterine rupture secondary to placenta percreta [6,7].
Complications of placenta accreta are many and include damage to local organs, postoperative bleeding, amniotic fluid embolisms, consumptive coagulopathy, transfusion related complications, acute respiratory distress syndrome, postoperative thromboembolisms, infectious morbidities, multisystem organ failure and maternal death [12]. Genital ureteral complications are common and include cystotomy and ureteral injury [13,14]. There is a high incidence of perinatal mortality and morbidity due to preterm delivery and its related complications like low birth weight, birth asphyxia and neonatal sepsis [15].
Ultrasonography should be the primary tool for the diagnosis of placenta accreta and should be used exclusively in most cases [16]. In addition, 2-dimensional and 3-dimensional sonography with power Doppler imaging has been used to assess placental adherence [17]. The evaluation of placental vessel architecture with 3-dimensional power Doppler imaging may help differentiate placenta accreta from placenta percreta [18].
The sonographic findings that suspect placenta accreta in the first trimester, include the following: A gestational sac that is located in the lower uterine segment, multiple irregular vascular spaces, and cord insertion into the lower third of the uterus [19,20].
The sonographic findings of placenta accreta in the third trimester include the following: Multiple vascular lacunae within the placenta, loss of the normal hypoechoic retroplacental zone, abnormalities of the uterine serosa–bladder interface, extension of the villi into the myometrium, serosa, or bladder, retroplacental myometrial thickness of less than1 mm and turbulent blood flow through the lacunae [21,22]. There are four basic options for management of placenta accreta: the extirpative method, the caesarean hysterectomy, conservative treatment and the one-step conservative surgery [23,24].
Aim of the work
This study was conducted to evaluate the effectiveness of ultrasound as screening tool of placenta accreta in cases of previous caesarean deliveries and low lying anterior placenta in the first trimester and to clarify the relation between Pig and placenta accrete.
This study was conducted to evaluate the effectiveness of ultrasound as screening tool of placenta accreta in cases of previous caesarean deliveries and low lying anterior placenta in the first trimester and to clarify the relation between Pig and placenta accrete.
Patients and Methods
Study design: This study was a prospective case–control design.
Setting: The patients were selected from those attending the obstetric clinic and ultrasound unit in El-Shatby Maternity University Hospital from December 2015 to June 2017.
Inclusion criteria: Pregnant women at the first trimester with low lying placenta, who had previous history of caesarean sections
Exclusion criteria: Prior uterine surgery, prior uterine irradiation, endometrial ablation, Asherman syndrome, uterine leiomyomas, uterine anomalies, hypertensive disorders in pregnancy and smoking.
Patient groups
Group A [Study group]: 40 pregnant women at the first trimester with low lying placenta, who had previous history of caesarean sections.
Group A [Study group]: 40 pregnant women at the first trimester with low lying placenta, who had previous history of caesarean sections.
Group B [Control group]: 40 pregnant women at the first trimester with fundal placenta who had previous history of normal vaginal delivery.
All patients were subjected to the following:
- History taking: Complete history taking including: Age, parity, prior uterine surgery, irradiation and smoking.
- Clinical Examination including: General, abdominal and pelvic examination.
- Laboratory investigation: Serum placental growth factor was measured at 11-14 weeks gestation. These tests were performed by enzyme-linked immunosorbent assay [25].
- Imaging Study: Transvaginal and transabdominal ultrasonographic examination was performed at 11- 14 weeks, with follow-up at 24- 32 weeks to detect placental migration.
Statistical analysis of the data
Statistical analysis was done using SPSS software, version 18 (SPSS Inc., Chicago, IL, USA). Qualitative data were described using number and percent. Comparisons between groups for categorical variables were assessed using Chi-square test (Fisher’s Exact). The diagnostic sensitivity, specificity and predictive values were calculated by 2 × 2 tables. Significance of the obtained results was judged at the 5% level.
Statistical analysis was done using SPSS software, version 18 (SPSS Inc., Chicago, IL, USA). Qualitative data were described using number and percent. Comparisons between groups for categorical variables were assessed using Chi-square test (Fisher’s Exact). The diagnostic sensitivity, specificity and predictive values were calculated by 2 × 2 tables. Significance of the obtained results was judged at the 5% level.
Results
| No. | % | |
| Migration | ||
| Non- migrated placenta | 5 | 12.5 |
| Migrated placenta | 35 | 87.5 |
Table 1: Distribution of the studied cases according to migration in study group (n = 40).
Out of 40 cases with low lying placenta in first scan at 11-14 weeks, 35 cases (87.5%) migrated upwards away from the internal cervical os and 5 cases (12.5%) remained in the lower uterine segment in the second scan as placenta previa at 24-32 weeks of gestation (Table 1).
| Study group (n = 40) |
Control (n = 40) |
χ2 | FEp | |||
| No. | % | No. | % | |||
| SPLGF | ||||||
| Low (<5) | 6 | 15.0 | 2 | 5.0 | 2.222 | 0.263 |
| Normal (5-5.8) | 34 | 85.0 | 38 | 95.0 | ||
| Sensitivity | 15.0 | |||||
| Specificity | 95.0 | |||||
| PPV | 75.0 | |||||
| NPV | 52.78 | |||||
| Accuracy | 55.0 | |||||
χ2, p: χ2 and p values for Chi square test for comparing between the two categories
FEp: p value for Fisher Exact for Chi square test for comparing between the two categories
*: Statistically significant at p ≤ 0.05
Table 2: Comparison between the two studied groups according to SPLGF.
FEp: p value for Fisher Exact for Chi square test for comparing between the two categories
*: Statistically significant at p ≤ 0.05
Table 2: Comparison between the two studied groups according to SPLGF.
Table 2 shows the level of serum Pig in study and control groups, it was normal at almost all control and in the cases which placenta became normal site at the second scan, and low in only 5% of control and 15% of cases which placenta became placenta previa.
| Migration | χ2 | FEp | ||||
| Non- migrated Placenta (n = 5) |
Migrated placenta (n = 35) |
|||||
| No. | % | No. | % | |||
| SPLGF | ||||||
| Low (< 5) | 4 | 80.0 | 2 | 5.7 | 18.936* | 0.001* |
| Normal (5-5.8) | 1 | 20.0 | 33 | 94.3 | ||
| Sensitivity | 94.29 | |||||
| Specificity | 80.0 | |||||
| PPV | 97.06 | |||||
| NPV | 66.67 | |||||
| Accuracy | 92.50 | |||||
χ2, p: χ2 and p values for Chi square test for comparing between the two categories
FEp: p value for Fisher Exact for Chi square test for comparing between the two categories
*: Statistically significant at p ≤ 0.05
Table 3: Sensitivity, specificity and accuracy for SPLGF in cases group (n = 40).
FEp: p value for Fisher Exact for Chi square test for comparing between the two categories
*: Statistically significant at p ≤ 0.05
Table 3: Sensitivity, specificity and accuracy for SPLGF in cases group (n = 40).
Table 3 shows that serum Pig level was low in cases which became placenta previa in the second scan while normal in cases with migrated placenta with sensitivity 94.29%, specificity 80%, PPV 97.06%, NPV 66.67% and accuracy 92.5%% with significant value (p = 0.001).
| Migration | χ2 | FEp | ||||
| Non- migrated Placenta (n = 5) |
Migrated placenta (n = 35) |
|||||
| No. | % | No. | % | |||
| Site first scan | ||||||
| Low anterior | 3 | 60.0 | 33 | 94.3 | 5.714 | 0.069 |
| Over IO | 2 | 40.0 | 2 | 5.7 | ||
| Cord Insertion | ||||||
| Near IO | 2 | 40.0 | 2 | 5.7 | 5.714 | 0.069 |
| Away IO | 3 | 60.0 | 33 | 94.3 | ||
χ2, p: χ2 and p values for Chi square test for comparing between the two categories
FEp: p value for Fisher Exact for Chi square test for comparing between the two categories
Table 4: Relation between migration with site of placenta first scan and cord insertion in cases group (n = 40).
FEp: p value for Fisher Exact for Chi square test for comparing between the two categories
Table 4: Relation between migration with site of placenta first scan and cord insertion in cases group (n = 40).
All cases had low lying placenta at first scan; 4 of them had placenta covering the internal os. Four cases had very low cord insertion; 2 of them became placenta prevail (p = 0.069) (Table 4).
| Migration | Sensitivity | Specificity | PPV | NPV | Accuracy | ||||
| Migrated placenta (n = 35) |
Non- migrated Placenta (n = 5) |
||||||||
| No. | % | No. | % | ||||||
| An irregular placental-myometrium interface | |||||||||
| Negative | 35 | 100.0 | 4 | 80.0 | 20.0 | 100.0 | 100.0 | 89.74 | 90.0 |
| Positive | 0 | 0.0 | 1 | 20.0 | |||||
| c2(FEp) | 7.179(0.125) | ||||||||
| Irregular or absent retro-placental vascular spaces | |||||||||
| Negative | 35 | 100.0 | 4 | 80.0 | 20.0 | 100.0 | 100.0 | 89.74 | 90.0 |
| Positive | 0 | 0.0 | 1 | 20.0 | |||||
| c2(FEp) | 7.179(0.125) | ||||||||
| Placental lacunae | |||||||||
| Negative | 35 | 100.0 | 3 | 60.0 | 40.0 | 100.0 | 100.0 | 92.11 | 92.50 |
| Positive | 0 | 0.0 | 2 | 40.0 | |||||
| χ2(FEp) | 14.737*(0.013*) | ||||||||
| Disruption of bladder line | |||||||||
| Negative | 35 | 100.0 | 5 | 100.0 | 0.0 | 100.0 | - | 87.50 | 87.50 |
| Positive | 0 | 0.0 | 0 | 0.0 | |||||
| χ2(FEp) | - | ||||||||
χ2, p: χ2 and p values for Chi square test for comparing between the two categories
FEp: p value for Fisher Exact for Chi square test for comparing between the two categories
*: Statistically significant at p ≤ 0.05
Table 5: Relation between migration with 1st scan gray scale ultra sound criteria of abnormal placental invasion in cases group (n = 40).
FEp: p value for Fisher Exact for Chi square test for comparing between the two categories
*: Statistically significant at p ≤ 0.05
Table 5: Relation between migration with 1st scan gray scale ultra sound criteria of abnormal placental invasion in cases group (n = 40).
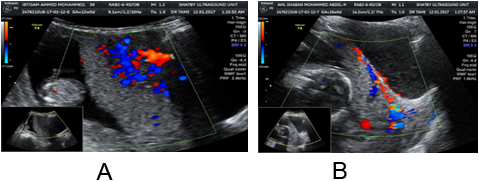
The gray scale criteria of abnormal placental invasion had very low sensitivity for prediction of placenta previa when investigated for during first trimester in cases with history of previous caesarean section while having high specificity value with no statistical significant (Table 5). Only placental lacunae had statistical significance in prediction of placenta previa in the first trimester (p =0.013), it has been detected in 2 cases from the 5 prevail cases.
Irregular or absent retro-placental spaces, irregular placental myometrial interface was detected only in one case, while disruption of the bladder line was not detected in the 5 non-migrated cases during the first trimester.
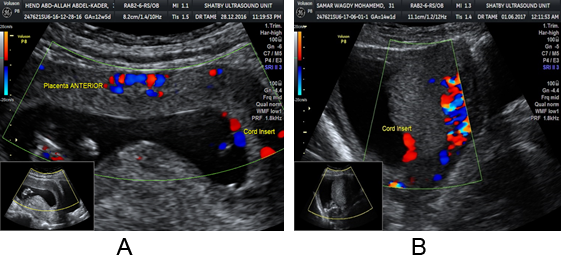
Figure 2A and B: TVU at 13-14 weeks shows anterior low lying placenta crossing
the internal os. The placenta was reported as migrated during the second scan.
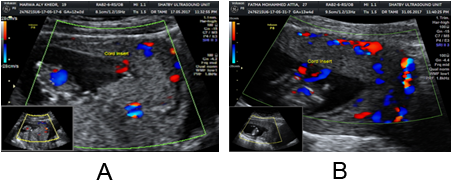
Figure 3A and B: Figure 21.a show low lying placenta at 13 week of gestation
with normal hypoechoic retroplacental zone between the placenta and uterine
wall. This placenta became fundal att3week of gestation.
| Migration | Sensitivity | Specificity | PPV | NPV | Accuracy | ||||
| Migrated placenta (n = 35) |
Non- migrated Placenta (n = 5) |
||||||||
| No. | % | No. | % | ||||||
| Loss of the normal hypo echoic retro placental zone | |||||||||
| Negative | 35 | 100.0 | 3 | 60.0 | 40.0 | 100.0 | 100.0 | 92.11 | 92.50 |
| Positive | 0 | 0.0 | 2 | 40.0 | |||||
| c2(FEp) | 14.737*(0.013*) | ||||||||
| Multiple vascular lacuna within the placenta | |||||||||
| Negative | 35 | 100.0 | 3 | 60.0 | 40.0 | 100.0 | 100.0 | 92.11 | 92.50 |
| Positive | 0 | 0.0 | 2 | 40.0 | |||||
| χ2(FEp) | 14.737*(0.013*) | ||||||||
| Abnormal uterine serosa bladder interface | |||||||||
| Negative | 35 | 100.0 | 4 | 80.0 | 20.0 | 100.0 | 100.0 | 89.74 | 90.0 |
| Positive | 0 | 0.0 | 1 | 20.0 | |||||
| χ2(FEp) | 7.179(0.125) | ||||||||
| Turbulent blood flow through the lacuna | |||||||||
| Negative | 35 | 100.0 | 4 | 80.0 | 20.0 | 100.0 | 100.0 | 89.74 | 90.0 |
| Positive | 0 | 0.0 | 1 | 20.0 | |||||
| c2(FEp) | 7.179(0.125) | ||||||||
| Retro placental thickness < 1 mm | |||||||||
| Negative | 35 | 100.0 | 4 | 80.0 | 20.0 | 100.0 | 100.0 | 89.74 | 90.0 |
| Positive | 0 | 0.0 | 1 | 20.0 | |||||
| c2(FEp) | 7.179(0.125) | ||||||||
χ2, p: χ2 and p values for Chi square test for comparing between the two categories
FEp: p value for Fisher Exact for Chi square test for comparing between the two categories
*: Statistically significant at p ≤ 0.05
Table 6: Relation between migration with 1st scan Doppler criteria abnormal placental invasion in cases group (n = 40).
Table 6: Relation between migration with 1st scan Doppler criteria abnormal placental invasion in cases group (n = 40).
Table 6 showed the Doppler criteria of abnormal placental invasion showed very low sensitivity for prediction of placenta prevail, the specificity was 100.0%. Multiple vascular lacunae within the placenta with color Doppler and loss of the normal hypoechoic retroplacental zone showed statistical significance in prediction of placenta prevail. (p = 0.013). The remaining criteria were detected only in one case from the 5 non-migrated cases.
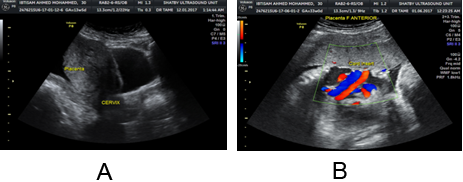
Figure 4A and B: Thickening and irregularities of the uterine serosa–bladder interface
line in a pregnancy with placenta previa marginalis at 16 and 24 week of gestation.
Discussion
Morbidly adherent placenta is strongly associated with massive peripartum hemorrhage. Prenatally, a combination of gray-scale and color Doppler ultrasonography is used to establish the diagnosis [26]. Although the pathogenesis of placenta accreta is characterized at the microscopic (i.e., poor decidualization with intramyometrial infiltration of the villous tissues) and macroscopic (i.e., vigorous uteroplacental neovascularization in the region of interest) levels, the molecular mechanism of this disorder remains unknown [27].
In our study 40 cases had previous history of caesarean section and diagnosed to have low lying anterior placenta at 11-14 weeks of gestation, 5 cases diagnosed as placenta accreta at term. It was shown that 20% had history of one caesarean section, compared to 40% with two caesarean sections, 40% with three sections. Our study showed that number of caesarean section increase risk of placenta prevail with significant value (P = 0.036). This is similar to a case series study done by Silver., et al. [28], who reported on over 30 000 women of whom 723 had placenta prevail, 143 had placenta accreta. 11% had one caesarean section had chance of placenta accreta if they had placenta prevail, compared to 40% had two caesarean sections, 61% had three sections, 67% had four caesarean sections.
Our study reported increased incidence of placenta accreta with advancing maternal age that 60% of cases over 30 years had placenta prevail and 40%less than 30 years had placenta prevail. This observation is similar to a study done by Ojha N., et al. Wu S., et al. [29] on 16361 deliveries shown nearly one fourth of women are above 30 years of age. In our study, 40 cases with low lying placenta in first scan at 11-14 weeks of gestation, 35 cases migrated upwards and became fundal placenta with migration rate 87.5% and 5 cases remained in the lower uterine segment in the second scan (24-32 weeks) as placenta prevail (12.5%).
In comparison with other case-control study design on 90 women enrolled prospectively at Yale-New-Haven Hospital [30] between May 2005 and January 2010. The study group consisted of 45 consecutive singleton patients mean gestational age 31 weeks who were admitted with a diagnosis of complete placenta previa, and 45 healthy women 31 weeks of gestation Placenta previa diagnosed in 13 women of cases (29%).The difference in migration rate between the two studies may related to the time of Ultrasonographic scan.
Normal level of circulating serum Pig in control is (5-5.8 pg./ml) and it was normal level in 85% of cases whom the placenta became fundal at the second scan and low in 15% of cases whom the placenta still in the lower uterine segment and became placenta accreta with sensitivity 94.29%, specificity 80%, PPV 97.06%, NPV 66.67% and accuracy 92.5% with significant value (p= 0.001),In other case control study analysed maternal blood serum samples from 90 women enrolled prospectively at Yale-New-Haven Hospital between May 2005 and January 2010.Khaliq A [31] showed also decreased serum PlGF in women with complete placenta previa. PlGF (CPP: 421vs. control: 492 pg. /mL, P = 0.710). Other case control study by the Medical Research Council of Taichung Veterans General Hospital [32] (Pig 74.6 G 29.6 in cases vs. 137.9 G 85.5 pg. /mL, P Z 0.149), Pig level did not show any significance in study groups. All study reported decrease serum level of Pig in cases with placenta prevail but the other previous two studies did not show any significance, the difference between our study and other studies may be due to time of sample 11-14weeks in our study and third trimester in other studies.
In our study, all cases had low lying placenta at first scan 4 cases had low cord insertion 2 of them (40%) became placenta prevail p = (0.069). The Incidence is different as Monteaudo., et al. [33] did a prospective cohort case control study performed at the Showa University School of Medicine between June 2003 and January 2005. The cord insertion in the lower third of the uterus during the first trimester was detected in 35/340 (10.3%) cases, nine cases (25.7%) were confirmed to have low cord insertion at delivery, the incidence of low-lying placenta at delivery was 8/35 (23%) for low cord insertion. this difference may be due to the differences in the number of cases.
Our study showed that, gray scale criteria of abnormal placental invasion had very low sensitivity for prediction of placenta accreta at the first trimester scan while having high specificity value with no statistical significant (Sensitivity 40%, Specificity 100%, PPV 100% and NPV 89%). Only placental lacunae had statistical significance in prediction of placenta accreta in the first trimester (p = 0.013).Irregular or absent retro-placental spaces, irregular placental myometrial interface was detected only in one case, while disruption of the bladder line was not detected in the 5 non-migrated cases during the first trimester.
Doppler criteria of abnormal placental invasion showed sensitivity for prediction of placenta prevail 40%, the specificity was 100.0%, PPV 100% and NPV 92% with accuracy 90%. Multiple vascular lacunae within the placenta with color Doppler and loss of the normal hypoechoic retroplacental zone showed statistical significance in prediction of placenta prevail. (p = 0.013). The remaining criteria were detected only in one case from the 5 non-migrated cases.
Shih., et al. [34,35] did prospectively study Imaging at the second trimester on 170 women of whom 72 had had a previous caesarean section. Thirty-eight of the women with a previous caesarean section had placenta accreta identified at delivery. Considering just the 72 women with previous caesarean section, Gray scale US (Sensitivity 95%, Specificity 76%, PPV 82%, Risk 93%), Color Doppler US (Sensitivity 92%, Specificity 68%, PPV 76%, Risk 89%) and Three-dimensional power Doppler (Sensitivity 100%, Specificity 85%, PPV 88%, Risk 100%).
Other prospective study included one hundred pregnant women presenting with placenta prevail with and without abnormal placentation. US sensitivity and specificity in diagnosing abnormal placentation, who were examined in the Department of Radiology, Hamad Medical Corporation in Doha, Qatar [36] between January 1, 2011 and March 31, 2014. Out of 100 pregnant women diagnosed with placenta prevail, 66 were diagnosed as having placenta prevail with no abnormal placentation, 34 were diagnosed with US as having associated abnormal placentation. The sensitivity of US was 94%, the specificity 97%, Positive predictive value (PPV) of US was 94% and negative predictive value (NPV) of US was 97%.
Conclusion
- First trimester localization of placenta by ultrasonography could predict placenta prevail in cases with a history of previous cesarean section when diagnose low-lying placenta.
- Low cord insertions to the placenta and sonographic criteria of abnormal placental invasion have a value in predicting placenta prevail.
- Reduced serum level of Pig in the first trimester predicts placenta prevail.
- Ultrasonography in late first trimester can suspect abnormal placental invasion but it does not definitively diagnose placenta accreta because it is histopathological diagnosis.
Recommendation
Patients with a prior caesarean delivery and placenta prevail are recommended to be screened for accreta with antenatal sonography.
Patients with a prior caesarean delivery and placenta prevail are recommended to be screened for accreta with antenatal sonography.
Overall, gray scale sonography is an excellent tool for the prenatal diagnosis of placenta accreta in women at risk for this abnormality, but not in the first trimester.
References
- Hegazy AA. “Clinical Embryology for Medical Students and Postgraduate Doctors”. Berlin: Lap Lambert Academic Publishing (2014):
- Wortman AC and Alexander JM. “Placenta accreta, increta, and percreta”. Obstetrics & Gynecology Clinics of North America 40.1 (2013):137-154.
- Wehrum MJ., et al. “Accreta complicating complete placenta prevail is characterized by reduced systemic levels of vascular endothelial growth factor and by epithelial-to-mesenchymal transition of the invasive trophoblast”. American Journal of Obstetrics & Gynecology 204.5(2011):411.
- Knöfler M. “Critical growth factors and signalling pathways controlling human trophoblast invasion”. The International Journal of Developmental Biology 54.2-3 (2010): 269–280.
- Khalil A., et al. “Effect of antihypertensive therapy with alpha methyldopa on levels of antigenic factors in pregnancies with hypertensive disorders”. PLoS ONE 3.7 (2008): e2766.
- Jang DG., et al. “Placenta percreta–induced uterine rupture diagnosed by laparoscopy in the first trimester: case report”. International Journal of Medical Sciences 8.5(2011):424-427.
- Chen CH., et al. “Uterine rupture secondary to placenta percreta in a near-term pregnant woman with a history of hysterotomy”. Journal of Obstetrics and Gynaecology Research 37.1 (2011):71-74.
- Belfort MA. “Placenta accrete”. American Journal of Obstetrics & Gynecology 203 (2010): 430-439.
- Hull AD and Resnik R. “Placenta accreta and postpartum hemorrhage”. Clinical Obstetrics and Gynecology 53.1 (2010): 228-236.
- Clark SL., et al. “Placenta prevail/accreta and prior caesarean section”. Obstetrics & Gynecology 66.1 (1985): 89–92.
- Silver RM., et al. “Maternal morbidity associated with multiple repeat cesarean deliveries”. Obstetrics & Gynecology 107.6 (2006): 1226–1232.
- Oppenheimer L., et al. “Diagnosis of low-lying placenta: can migration in the third trimester predict outcome?” Ultrasound in Obstetrics & Gynecology 18.2 (2001):100–102.
- Eshkoli T., et al. “Placenta accreta: Risk factors, perinatal outcomes, and consequences for subsequent births”. American Journal of Obstetrics & Gynecology 208.3 (2013): 219.
- O’Brien JM., et al. “The management of placenta percreta: conservative and operative strategies”. American Journal of Obstetrics & Gynecology 175.6 (1996):1632–1638.
- Salihu HM., et al. “Placenta previa: neonatal deaths after live births in the United States”. American Journal of Obstetrics & Gynecology 188.5 (2003): 1305-1308.
- Esakoff TF., et al. “Diagnosis and morbidity of placenta accreta”. Ultrasound in Obstetrics & Gynecology 37.3 (2011): 324–327.
- Shih JC., et al. “Role of three-dimensional power Doppler in the antenatal diagnosis of placenta accreta: comparison with gray-scale and color Doppler techniques”. Ultrasound in Obstetrics & Gynecology 33.2 (2009): 193-203.
- Cali G., et al. “Morbidly adherent placenta: evaluation of ultrasound diagnostic criteria and differentiation of placenta accreta from percreta”. Ultrasound in Obstetrics & Gynecology 41.4 (2013):406–412.
- Berkley EM and Abuhamad AZ. “Prenatal Diagnosis of Placenta Accreta”. Journal of Ultrasound in Medicine 32 (2013): 1345–1350.
- Oyelese Y., et al. “Vasa prevail: the impact of prenatal diagnosis on outcomes”. Obstetrics & Gynecology 103.5 (2004): 937–942.
- Comstock CH., et al. “Sonographic detection of placenta accreta in the second and third trimesters of pregnancy”. American Journal of Obstetrics & Gynecology 190 (2004):1135–1140.
- Comstock CH. “Antenatal diagnosis of placenta accreta: a review”. Ultrasound in Obstetrics & Gynecology 26.1 (2005): 89–96.
- Kayem G., et al. “Conservative versus extirpative management in cases of placenta accreta”. Obstetrics & Gynecology 104.3 (2004): 531–536.
- Eller AG., et al. “Optimal management strategies for placenta accreta”. BJOG: An International Journal of Obstetrics & Gynaecology 116.5 (2009): 648-654.
- Buhimschi CS., et al. “Fractional excretion of antigenic factors in women with severe preeclampsia”. Obstetrics & Gynecology 107 (2006): 1103–1113.
- Cunningham FG., et al. “Obstetric haemorrhage. In: Cox S, Werner C, Hoffman B (eds). Williams Textbook of Obstetrics. 22nded. New York: McGraw-Hill (2005) 809-823.
- Martin JA., et al. “Births : final data for 2001”. National Vital Statistics Reports 51.2 (2002): 1-102.
- Knight M. “Peripartum hysterectomy in the UK: management and outcomes of the associated haemorrhage”. BJOG: An International Journal of Obstetrics & Gynaecology 114.11(2007): 1380–1387.
- Wu S., et al. “Abnormal placentation: twenty-year analysis”. American Journal of Obstetrics & Gynecology192.5 (2005):1458-1461.
- Oyelese Y and Smulian JC. “Placenta prevail, placenta accreta, and vasa prevail”. Obstetrics & Gynecology 107.4 (2006): 927–941.
- Khaliq A., et al. “Hypoxia down–regulates placenta growth factor, whereas fetal growth restriction upregulates placenta growth factor expression: molecular evidence for "placental hyperoxia" in intrauterine growth restriction”. Laboratory Investigation 79.2(1999): 151–170.
- Ebos JM., et al. “A naturally occurring soluble form of vascular endothelial growth factor receptor 2 detected in mouse and human plasma”. Molecular Cancer Research 2.6 (2004): 315-326.
- Lauria MR., et al. “The use of second-trimester transvaginal sonography to predict placenta prevail”. Ultrasound in Obstetrics & Gynecology 8.5 (1996): 337–340.
- Paterson-Brown S and Singh C. “Developing a care bundle for the management of suspected placenta accreta”. Obstetrics & Gynecology12 (2010): 21–27.
- Megier P., et al. “Picture of the month. Antenatal diagnosis of placenta percreta using gray-scale ultrasonography, color and pulsed Doppler imaging”. Ultrasound in Obstetrics & Gynecology 15.3 (2000): 268.
- Elsayes KM., et al. “Imaging of the placenta: a multimodality pictorial review”. Radiographics 29 (2009): 1371–1391.
Citation:
Tamer Mamdouh Abdel dayem., et al. “Biochemical and Ultrasonographic Prediction of Placenta Accreta in First Trimester of
Pregnancy”. Gynaecology and Perinatology 1.2 (2017): 100-110.
Copyright: © 2017 Tamer Mamdouh Abdel dayem., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.