Case Report
Volume 1 Issue 2 - 2018
Outcome Lupus Nephritis in Pregnancy at Dr. Soetomo Hospital, Indonesia
1Department Obstetrics & Gynaecology, Universitas Airlangga, RSUD Dr. Soetomo, Surabaya, Indonesia
2Department Obstetrics & Gynaecology, Universitas Sebelas Maret/ RSUD. Dr. Moewardi, Surakarta, Indonesia
2Department Obstetrics & Gynaecology, Universitas Sebelas Maret/ RSUD. Dr. Moewardi, Surakarta, Indonesia
*Corresponding Author: Sri Sulistyowati, Department Obstetrics & Gynaecology, Universitas Sebelas Maret/ RSUD. Dr. Moewardi, Surakarta, Indonesia.
Received: December 20, 2017; Published: January 11, 2018
Abstract
Lupus nephritis is one of the most serious manifestations of SLE that occurs five years after diagnosis. This is a prospective case series of 16 lupus nephritis patients at RSUD Dr Soetomo, Surabaya (a tertiary Indonesian Hospital in East part of Indonesia) from 2013 to 2016. SLE was diagnosed using ACR criteria. The prevalence of lupus nephritis was 0.35%, while only 31.2% cases had a regular antenatal care in our hospital. Most of the case was diagnoses in pregnancy periods, highest in the second trimester (31%) and third trimester (25%).
The clinical manifestation found including renal involvement (100%), malar rash (31.2%), non erosive arthritis (47.3%), photosensitivities (31.2%), neurological symptoms (12.5%), and mouth ulcers (6.25%). The maternal death rate and flare rate was around 31%. The maternal complication of these series were mainly hypertension in pregnancy (43.7%), followed by cardiac problems (37.5%), infection (31.2%), pulmonary problems (25%), and kidney failure (25%). Mode of delivery were mainly vaginal (64.3%), followed by cesarean section (28.6%) and curretage.
Cesarean delivery was performed because of obstetric indication. The fetal-neonatal complication found were 3 cases (20%) of IUFD, 4 cases (26.7%) of IUGR, 1 cases of fetal distress, and 2 neonatal death (13.3%). In total the overall survival rate in this series is around 77.7%. Lupus nephritis in pregnancy poses a great challenge since it is associated with high maternal-fetal mortality and morbidity, especially in active states/flares. Good preconceptional counseling, close multidisciplinarry surveilance, tight monitoring, early diagnosis and management of the complication is a keypoint to improve maternal fetal outcome in this cases.
Keywords: Lupus Nephritis; SLE; Pregnancy
Introduction
Systemic Lupus Erythematosus (SLE) is a severe autoimmune disease, which is characterized by development immune complexes in maternal circulation, with the periods of relapse and remission. This disease has a complex pathogenesis between susceptible genes and environmental factors that may induce an abnormal immune response [1]. SLE is usually experienced by young women with a ratio of male: female 1:9 and reproductive onset peak of 25-35 years [2]. SLE prevalence is about 3.3-4.8 cases per 100,000 people per year [3] There is no SLE epidemiological data covering all parts of Indonesia.
SLE prevalence in the community based on a survey conducted by P. Handono Kalim., et al. in Malang shows a figure of 0.5% of the total population (1,250,000 people). Data from the Association of SLE Indonesia (PESLI) in 2016, the average incidence of new cases of SLE from the data of eight hospitals is 10.5% [4] In RSUD Dr Soetomo in the period January 2009 to December 2016 there were 105 cases of pregnancy with SLE with prevalence of 0.59% (105 cases of SLE from 17,834 pregnancies) [5].
Lupus nephritis is one of the most serious manifestations of SLE that often occurs after five years of diagnosis. More than 70% of SLE patients experience kidney involvement throughout the course of the disease [6]. Estimates of the prevalence of clinical renal involvement in SLE patients ranged from 30-90% in published studies. The prevalence of nephritis is significantly higher in African-American and Hispanic races than in whites, and is higher in males than in females. Overall, survival rates for SLE patients were about 95% within five years after diagnosis, and 92% within 10 years after diagnosis was established [7].
Clinical features of patients with lupus nephritis vary widely, ranging from asymptomatic or only mild proteinuria or hematuria to severe clinical features, ie, nephrotic syndrome or or glomerulonephritis accompanied by progressive decline in renal function, ending with end-stage renal disease. Clinical heterogeneity is related to differences in histological patterns of glomerular injury found from renal biopsy [8]. Pregnancy change the endocrinological and immunological status that greatly affects the course of SLE disease, especially in lupus nephritis.
In a meta-analysis of 37 studies conducted in 1984 and 2009 involving 1000 women with lupus nephritis, showed the frequency of most common complications were flares 26% and renal disease activation of about 16%. A study in Italy showed that lupus nephritis that occurred before conception or at the time of conception was associated with reactivation of severe lupus nephritis during pregnancy or the puerperium [9]. Nephritis remains one of the most horrific complications of lupus, with the incidence of end-stage renal disease due to lupus increased between 1982 and 1995, with no decrease in 2004.
These poor outcomes occurred despite the availability of new drug regimens. Diagnosis of lupus nephritis is a special challenge because the physiological conditions of pregnancy and preeclampsia may appear similar. So a careful examination of the diagnosis of lupus nephritis based on the American College of Rheumatology (ACR) criteria or using the recommendations of the Indonesian Rheumatology Society early on is essential. SLE diagnosis preconceptionally plays an important role because of remission conditions both clinically and laboratory 6-12 months before conception, lowering the rate of flares in pregnancy [5].
Treatment of lupus nephritis (SLE) also faces challenges since no drug is absolutely safe to use in pregnancy. The notification of general characteristics, maternal and fetal outcomes of pregnant patients with lupus nephritis in Dr. Soetomo and discussions on diagnosis, pretest management and management during pregnancy is expected to be able to provide insight into the management of lupus nephritis in pregnancy in order to provide better outcomes for mothers and fetuses in the future.
Materials and Methods
This was a prospective case series, collected in Dr. Soetomo Hospital, Surabaya (a tertiary hospital, and refferal center in east part of Indonesia) between January 2013-December 2016. SLE was diagnosed using ACR criteria, and the management was done multidicipllinary involved maternal fetal medicine consultant, rheumatologist, obstetricians, and intensivist. All of the women diagnosed with lupus nephritis in those periods was recruited for this study. The patient was followed until delivery with regular antenatal care, and the maternal-fetal outcome was collected.
The data collected including: characteristic of the patients (age, parity), antenatal care location, time the disease first diagnose, clinical manifestation, laboratory data, and maternal-fetal outcome. The disease activity was monitored by worsening of the clinical symptoms, laboratory examination, including: urinalysis, serum albumin, serum creatinin and BUN level, serum levels of C3 and C4, and anti dsDNA. Lupus was diagnosed as "flares" when some or all of the above examination went out of normal limits or tend to be worsening.
Result
During these period, 16 cases of lupus nephritis were found in pregnancies with a prevalence of 0.35% (16 cases of 4592 pregnancies, where all cases were established using clinical and laboratory findings that met the American Criteria Rheumatology (ACR). Based on the antenatal care location,only five cases (31.2%) had a regular antenatal care in our obstetric outpatient clinic (booked case), while 11 cases (75%) were referral cases.
But the trend showed that in the last year we had more booked cases than the refferal cases (3 vs 1 patients). Based on age group, most cases were found in the 20-24 years range ie five cases (31.2%). Only 3 cases (19%) patients was found in high risk pregnancy age group (> 35 years old). The youngest age of pregnant patients with lupus nephritis is 21 years old, where the oldest age is 39 years, with the average age 28.6 + 5.6 years. The distribution of parity in pregnant patients with lupus nephritis is: three-case primigravida (35.38%), and 13 cases (64.62%) multigravida consist of 9 cases gravida two, 2 cases gravida three, and 2 cases of gravida three.
Based on the time the disease was first diagnosed, most of the patients were diagnosed while pregnant as many as 10 cases (62.5%). The rest were diagnosed in the puerperium period, ie 1 case (6%), and 5 cases (32%) before pregnancy. The range of cases detected before pregnancy were from 1 to 11 years ago, with a median of 4 years. Diagnosis during pregnancy is highest in the second trimester (5 cases/31%) followed by third trimester (4 cases/25%).
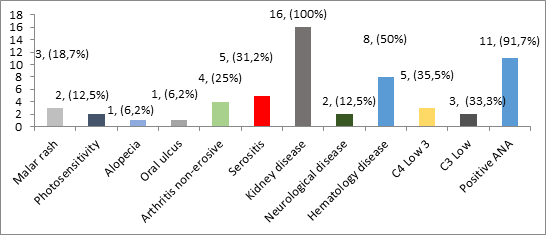
Clinical manifestations in patients with lupus nephritis were based on ARA criteria, found to be renal manifestation in all cases (100%). Other clinical manifestations: malar rash were found in 5 cases (31.2%), 7 cases with non-erosive arthritis, 5 cases (31.2%) accompanied by photo sensitiveness, 2 cases (12.5%) with neurological disorders, and 1 case (6.25%) with mouth ulcers and alopecia. The distribution of clinical manifestations in lupus nephritis patients based on ARA criteria is shown in Figure 1.
Figure 1: Distribution of clinical manifestations according to ARA criteria in lupus nephritis patients of all cases of lupus nephritis treated in RSUD Dr. Soetomo during the four-year period, we found five cases (31.2%) who experienced flare/exacerbation.
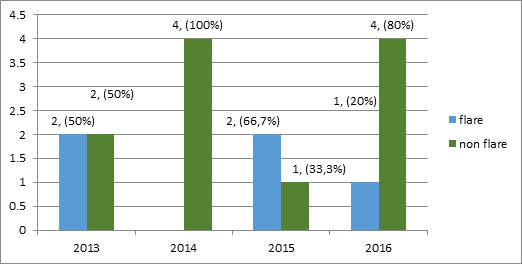
The all flares consist of renal manifestation, and 2 cases with added extra renal manifestation (cerebral lupus and pneumonitis lupus). Unfortunately 4 from these 5 cases (80%) flares ended in maternal death. Distribution of flare event and no flare per year is shown in Figure 2.
We have obtained laboratory data on 16 cases of pregnancies with lupus nephiritis, with most results indicating a decrease in hemoglobin and albumin as well as increased renal function test and renal activity. On urinalysis, proteinuria > +3 (dipstick) is found in 15 patients (93.7%). Similiarly, on the results of urine sediment examination, there are 15 patients (93.7%) with hematuria and pyuria. In term of ANA test, only 11 cases (68.7%) were found with positive test, 1 case (6.25%) with negative result, and the examination was not performed in 4 cases.
We got the result of complement (C3 and C4) examination in only 10 cases. On C3 examination, we obtained 3 cases (12.5%) with low C3 result and 6 cases with normal C3. Meanwhile, as many as 5 cases obtained a low C4 complement and 4 cases with normal C4. We only get anti dsDNA data in only 2 cases with both negative results.
| Laboratorium | Minimum | Maximum | Mean + SD | Normal Value |
| Hb | 5.80 | 12.80 | 8.83 +1.97 | 11,0-14,7 |
| Leukocyte | 3430 | 26200 | 13205.62 + 8347.26 | 4000-10.000 |
| Thrombocyte | 66900 | 434000 | 199618.75 + 106446.94 | 150.000-450.000 |
| GDA | 45.00 | 155.00 | 89.87 + 32.14 | <120 |
| SGOT | 8.00 | 108.00 | 32.12 + 29.43 | 0-35 |
| SGPT | 4.00 | 48.00 | 20.75 + 12.69 | 0-35 |
| Albumin | 1.78 | 3.80 | 2.99 + 0.68 | 3,4-5,0 |
| BUN | 4.00 | 234.00 | 50.60 + 63.70 | 7-18 |
| Creatini Serum | 0.60 | 24.80 | 3.77 + 5.97 | 0,6-1,3 |
Table 1: Distribution of values in laboratory data of lupus nephritis patients.
| Urinalysis | Lupus nephritis (N : 16) |
Percentage (%) |
| Proteinuria (> 0.5 g/24 hour or > 3 + dipstick) Hematuria (> 5 RBC/lpb) Pyuria (> 5 WBC/lpb) |
15 | 93,7% |
| 15 | 93,7% | |
| 15 | (3,7% | |
| Laboratorium | Lupus nephritis (N : 16) |
Percentage (%) |
| ANA Test positive | 11/12* | 99,7 |
| C3 low | 3/9* | 33,3 |
| C4 low | 5/9* | 55,5 |
*The amount of case with available data
In these cases series there were 15 pregnancies with only 14 delivered, while 1 case came to us in the puerperium period, and the other one died prior to curettage for the incomplete abortion. The most frequent labor method was vaginal delivery in 9 cases (64.3%), 4 cases (28.6%) with cesarean delivery, and 1 case (7.1%) with curettage. Cesarean delivery was indicated because of obstetric indication (3 cases) and one case because of maternal worsening condition.
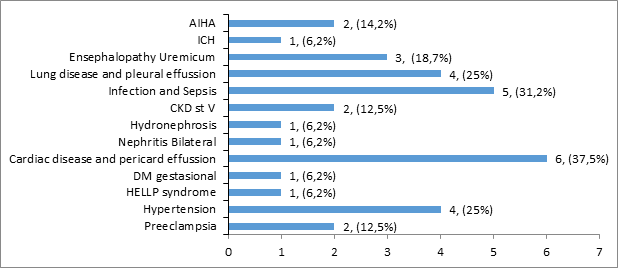
The most common maternal complications of lupus nephritis were infection (septic) in 5 cases (31.2%) and cardiac problems in 6 (37.5%) cases. The complications of gestational hypertension and chronic hypertension were in 4 cases (25%). Preeclampsia in 2 cases (12.5%), Auto Immune Hemolytic Anemia (AIHA) 2 cases (12.5%) and gestational diabetes and Hemolysis-Elevated Liver Enzymes-Low Platelet (HELLP) syndrome each in 1 case (6.2%).
Other complications include lung disease and pleural effusion four cases (25%), uremic encephalopathy three cases (18.7%), and stage IV or V CKD two cases (12.5%). Hydronephrosis, nephritis and intra cranial haemorage (ICH), each in one case (6.2%). The distribution of maternal complications in lupus nephritis patients can be seen in Figure 3.
For the maternal outcome, we got 5 maternal death (31%) from 16 cases of lupus nephritis in pregnancy. A total of 11 patients (69%) were discharged from hospital with stable condition. The most common cause of death was septic shock (100%), uremic encephalopathy in two cases (40%), and cardiogenic shock, one case (20%), 4 cases already in state of chronic kideney disease, even though 2 of the lupus just diagnose less than 1 years. A total of 4 cases of maternal death (80%) in lupus nephritis were obtained under flare conditions.
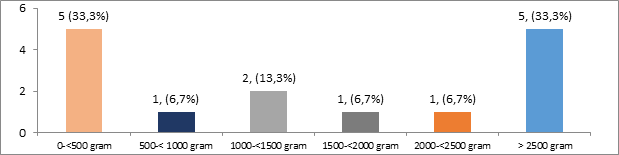
1 of the case was complicated by severe manifestation of preeclampsia, HELLP syndrome, and acute pulmonary edema. Two other case was complicated by extra renal manifestation of SLE, 1 with cerebral lupus and the other one with pulmonary lupus. A total of 15 pregnancies (93.7%) in this case series, while 1 patient came in post partum periods. 5 patients delivered aterm (33.3%), 5 patients preterm (33.3%), and 5 patients experienced abortion (Figure 4). One case of incomplete abortion was found, one case was curettage for indication of abortion provocatus medicinalis and one case of infant born outside hospital.
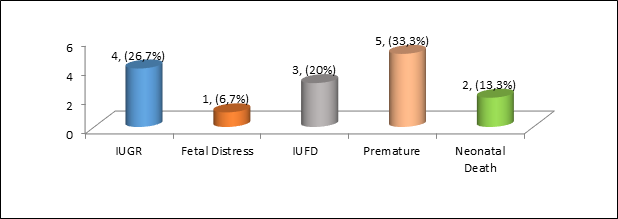
Most infants were born with a mild to normal condition of asphyxia, ie Apgar Score (AS) above seven in five cases (33.3%), followed by moderate asphyxia (AS 4-6) and severe asphyxia (AS 0-3), three cases (20%) and five cases (33.3%), respectively. The fetal-neonatal complication found were 3 (20%) of IUFD, 4 cases (26.7%) of IUGR, 1 cases of fetal distress, and 2 neonatal death (13.3%). In total there was 5 still birth in this case series (33.3%). The distribution of complications in the infant outcome of lupus nephritis patients is shown in.
Discussion
The number of lupus nephritis patients in our hospital tends to increase annualy while the total prevalence is quite low (0.35%). Most patients were first diagnosed as lupus nephritis in pregnancy (62.5%), while the others in preconception and post partum periods. More than 70% of patients with SLE will developed renal involvement [1] In our study the mean duration since diagnosis of SLE to the presence of kidney organ involvement and established diagnosis of lupus nephritis is 2.3 years with the shortest 7 months and the longest 10 years. In the United States, about 35% of adults with SLE have clinical signs of nephritis at diagnosis, with an estimated 50-60% developing into nephritis within the first 10 years [1,7].
Diagnosis of lupus nephritis was confirmed with the ARA criteria. Clinical and laboratory manifestations to be met from the American College of Rheumatology 1997 (ACR) criteria to diagnose lupus nephritis were persistent proteinuria > 0.5 g/day or > 3+ with dipstick, and/or cellular cast including red blood cells, hemoglobin, granular, tubular, or mixtures) [8]. For the diagnosis of SLE, it needs minimal 4 positive ARA criteria from total 11 criteria. 8,13 As the main criteria for the diagnosis of lupus nephritis on is a renal biopsy that describes glomerulonephritis associated with immune complexes, accompanied by positive anti dsDNa or ANA test [7,11].
In this study the majority of patients (62.5%) were diagnosed at the time of pregnancy, while only 32% diagnosed before preganncy. In term of clinical manifestation, all cases have a renal symptoms, followed by malar rash, non-erosive athritis, and mouth ulcer. Distinguishing normal pregnancy symptoms with preeclampsia and lupus nefrtitis is not easy, unless a history of lupus nephritis or treatment is already existed [11,19] In this report, five cases were diagnosed with lupus nephritis prior to pregnancy. The essentials needed in diagnosing lupus nephritis and distinguishing it from both normal pregnancy and preeclampsia are complete examination of clinical or laboratory and immunological markers.
One of the keypoint to diagnose lupus nephritis is the existence of abnormal renal function from blood or urine examination. Hematuria, active urine sediment, high serum creatinine, accompanied by extra renal manifestation, low complement and positive anti dsDNA, is highly suggestive of lupus nephritis [1,10,19,24]. From the laboratory examination, ANA test can be used as a screening tools for SLE in pregnancy. From the 12 cases that have an ANA test result, 11 showed a positive result (91.7%). This finding was in line with other study show the high prevalence of positive ANA test in SLE patients (96%) [11].
Complement examination in the other hand can be used as a marker of disease activity. Low complement is associated with SLE disease activity and cause premature delivery before 37 weeks [10,12]. In this study we found low C3 and C4 results was suitable with the flare rates of 31.2%. So the complement level need to be examined in every SLE patients in order to evaluate the disease activity and distinguished between lupus or preeclampsia. Distinguishing the appearance of active lupus nephritis from preecampsia becomes challenging because both diseases have the same manifestations, such as hypertension and increased proteinuria.
In such situations, or in patients which the diagnosis is diffcult to established during pregnancy, kidney biopsy may be considered as an option [13,14]. Biopsy can be performed safely in patients with adequate blood pressure and normal coagulation parameters to obtain a definitive diagnosis. The other option is to measure the complement level, anti dsDNA, active urine sediment, and prescence of extra renal SLE manifestation as a differential markers between those conditions. Differentiating preelampsia and lupus nephritis is very important, because both condition are managed in different ways [13,14].
Lupus nephritis significantly increased the risk of maternal-fetal mortality and morbidity. In our study, there were 5 maternal death (31%) and 5 stillbirth (31%) consist of 3 IUFD and 2 neonatal death. The flares of lupus was happened in only 31.2% cases, and unfortunately 3 of 5 flare cases (60%) ended with maternal death, indicate the high risk of maternal death in state of lupus nephritis flare. Even the existence of lupus nephritis it self is increasing the risk of maternal death, as mention by systematic review of 13 studies finding 17 maternal death in patients within first 6 weeks postpartum [15].
All death in this review occured in women with active lupus nephritis, and mainly due to infections (41%) or SLE activity it self [15]. This was difference with our findings that all of the maternal death cases is complicated by infection-sepsis. The existence of infections in active/flares of lupus made such a dillema in the management, the high dose steroid need to be given to control the disease eventhough it possesed the risk of worsening infections because steroid effect. In this series we found various complication in maternal side, from infection, cardiac problems, gestational hypertension, preeclampsia, HELLP syndrome, gestational diabetes, lung disease, and renal complication.
This findings was in line with the first prospective publication about outcome of lupus nephritis, included 71 pregnancies, with the result of 19.7% lupus flared, 8.4% preeclampsia and 2 cases of HELLP syndrome. 16 The condition of flares, hypertension, preeclampsia, infection and diabetes mellitus is the most common maternal complication in patients with SLE [16]. The incidence of preeclampsia in our study was slighly higher than Moroni G (12.5%) [16]. Lupus nephritis and active SLE are associated with high risk of preeclampsia [18].
From the fetal side, the stillbirth rates is still high in this series, followed by preterm birth. The fetal loss rate is difference with the Moroni G study, which only have 8.4% stillbirth rates, while the preterm birth rates is similiar with theirs. But in the others study published, the fetal loss rate ranged between 13-35% [16]. The high stillbirth rates in our hospital is related to the late diagnosis of the disease in primary health care, late refferal, lack of optimal early management, the appearence of the disease in the very early stage of pregnancy, and the severity of the disease [3] of the cases came to our hospital has already in IUFD states and severe maternal condition, indicating the late refferal of the disease.
Lupus nephritis, flare, high activity if the disease is associated with fetal death [19,20,21]. In a cohort study of 408 SLE patients from Latin America, with a median follow-up duration of 11 years, 60% of patients met the criteria of lupus nephritis. Lupus nephritis increases 2.37 times the associated mortality rate of SLE.7 Active lupus nephritis in pregnancy is increasing the risk of maternal death and hypertensive disorders [20]. Active disease 6 month before conception, and history of nephritis increased the risk of flares during pregnancy, while flares increase the adverse outcome risk.
PROMISSE study also suggested that patients with previous kidney disease more often experienced reactivation of nephritis than those without [21]. From the fetal side, lupus nephritis significantly increase stillbirth rates and preterm delivery, compared to SLE without renal involvement. The 30-60% preterm delivery rate can be expected from the active lupus nephritis state. Low birth weight or SGA complicated around 24-27 cases in lupus nephritis, this is comparable with our result [22]. Active state of lupus nephritis is an important factor related to the increased risk adverse maternal-fetal outcome.
Early diagnosis of lupus nephritis becomes very important even before conception, it is necessary to have a collaboration between obstetricians, maternal fetal medicine consultant, rheumatologist, and intensivist [1]. Clinicians should be able to explore the various organ systems as carefully as possible based on ARA criteria particularly in reproductive women. All SLE patients expecting pregnancy should be given pre-conceptional counseling [13]. Pregnancy is planned in the optimal condition, to reduce the risk of flare and improving obstetric outcomes [14]. The SLE activities should be assessed with one of the criteria/index, and special attention was required to possible involvement of the kidneys.
Lupus nephritis that has recently experienced flares before preganancy is a significant risk factor for lares reccurence [16]. As a consequence, renal function should be stable without any signs of lupus flare nephritis for a minimum of 6 months with a drug regimen that is safe to continue during pregnancy. Remission of clinical and laboratory parameters for six months to 12 months before conception can reduce the risk of prematurity and flares [24]. Risk of flares is 50-60% when the diseases is active, whereas in the remission state the risk of flares is only 7-10% [23]. Prevention of lupus nephritis flares in SLE during pregnancy may decrease the risk of abortion, preterm birth, and flares [16,23].
Good cooperation between obstetricians and rheumatologist is required in order to optimize lupus nephritis state before conception. During pregnancy careful monitoring of both clinical maternal-fetal conditions is necessary, to detect early sign of increased SLE activity and prevention of adverse fetal outcome. The development and well-being of the fetus is closely monitored. Doppler ultrasound examination is used to monitor and evaluate the growth and development of the fetus in the womb. Pregnancy may be continued until aterm, termination of pregnancy is indicated if maternal condition is deteriorate or there is a sign of maternal hypertension or evidence of impaired fetal growth or fetal distress [1,13,14,19].
The use of medications to prevent lupus flares will need to be evaluated individually. If patients have been maintaned on medications throughout pregnancy, these should be continued until delivery [24]. In our case, all of the patients consume steroid regularly, and about 53% got the immunosuppresants (azathioprine) with indication of flare suspicion. High dose steroid was added in the flare groups. Women who receive glucocorticoid therapy such as prednisone to control SLE, during pregnancy require increased stress dose during labor[1]. Stress dose are indicated if the patients regularly consumed prednisone > 20 mg daily [24].
Most of the cases was delivered with vaginal delivery, and only small percentages delivered by cesarean section. The indication for cesarean delivery was mostly obstetrics, only one cases delivered because of maternal worsening condition. Labor should be performed in hospitals with pediatric care facilities and even neonatal intensive care units [1]. Indications of cesarean section is mainy from the obstetrics such as cephalopelvic disproportion, transverse lie, fetal distress, and abnormal NST [25]. In this case series we got 5 flares cases (31.2%), based on worsening clinical/laboratory examination.
Management in the event of lupus flares is aimed primarily at maternal condition stabilization. Agressive therapy with a high pulse doses steroids in the form of methylprednisolone dose 1000 mg/day intravenously for 3 days followed prednisone per oral 0.5 to 1 mg/kg per day should be started immediately. Flare is not an indication of pregnancy termination because there is no clear evidence showed an remission of the disease after termination. When aggressive therapy is necessary for the mother, termination is recommended as early as 32 weeks of gestation [19].
However, if pregnancy at the time of the flare is still far from aterm and the mother needs cytotoxic therapy such as cyclophosphamide, azathioprine and methotrexate, therapy is still given and pregnancy can be continued with good counseling and close monitoring of the fetal development [7]. Signs of infection and disease exacerbations need to be monitored after delivery. Most drugs are excreted in breast milk. However, prednisone therapy < 30 mg/day, warfarin and cyclosporine with standard doses and chloroquine weekly malaria doses are considered safe for breastfeeding mothers.
Breastfeeding is compatibel eventhough the mother consumes azathioprine, hydroxychloroquine, ciclosporine, tacrolimus, and IVIG. Safe contraception need to be start as eary as possible to avoid unwanted pregnancy. Preconceptional counseling is a mandatory in the patients with SLE in order to prepare the optimal state to pregnant [1,11,13,14,19].
Conclusion
Lupus nephritis in pregnancy poses a great challenge since it is associated with high maternal-fetal mortality and morbidity, especially in active states/flares. Despite the aggresive therapy, the maternal death rate is still very high, because often lupus complicated with infections and extra renal severe manifestations. And the rate of fetal loss and preterm birth is also still very high. The best management to reduce the high mortality and morbidity in this cases is to prevent the flares during pregnancy by good preconceptional plan.
The pregnancy should be started only in the remission state of lupus and the medication need to be continued regularly until delivery. During pregnancy, close multidisciplinary surveilance, tight monitoring, early diagnosis and treatment of complication, delivery in the tertiary care center is the keypoint to improve the outcome of the mother and baby.
References
- Cunningham FG., et al. “Systemic lupus erythematosus”. In Williams Obstetrics 24th ed United State: McGraw-Hill (2014): 2438-2453.
- Dall'era, M., et al. “Clinical features of systemic lupus erythematosus”. Kelley's textbook of rheumatology 9th ed ition Philadelphia: WB Saunders Elsevier. (2013): 1283-1301.
- Crow MK., et al. “Systemic lupus erythematosus and related syndromes”. In G. S. Firestein, R. C. Budd, S. E. Gabriel, I. B. McInnes, & J. R. O'Dell, Kelley's textbook of rheumatology 9th edition Philadelphia: Elsevier Inc (2013): 1443-1455.
- Kementrian Kesehatan RI. “Situasi Lupus Di Indonesia”. Jakarta: Infodatin (2017):
- Akbar MIA and Yustinasari. “Characteristic and maternal-fetal outcome of SLE in pregnancy, in Dr. Soetomo Hospital January 2009 - Desember 2016”. (2017):
- Kasjmir YI., et al. “Diagnosis dan Pengelolaan Lupus Eritematosus Sistemik”. Rekomendasi Perhimpunan Reumatologi Indonesia.
- Hahn BH., et al. “American College of Rheumatology Guidelines for screening, treatment, and management of lupus nephritis”. Arthritis Care & Research (2012): 797-808.
- Dooley M., et al. “Review of ACR renal criteria in systemic lupus erythematosus”. Lupus 13.11 (2004): 857-860.
- Lazzaroni MG., et al. “A comprehensive review of the clinical approach to pregnancy and systemic lupus erythematosus”. Journal of Autoimmunity 74 (2016): 106-117.
- Lv J., et al. “Clinical outcomes and predictors of fetal and maternal consequences of pregnancy in lupus nephritis patients”. International Urology and Nephrology 47.8(2015): 1379-1385.
- Cervera R., et al. “Systemic Lupus Erythematosus: Pathogenesis, clinical manifestation, and diagnosis”. EULAR online course on Rheumatic Diseases - module no 17, 2007-2009. (2009):
- Deguchi M., et al. “Factors associated with adverse pregnancy outcomes in women with systemic lupus erythematosus”. Journal of Reproductive Immunology 125(2018): 39-44.
- Giraldo-Isaza MA. "Chapter 25: Systemic Lupus Erythematosus" in book: Maternal-Fetal Evidence Based Guidelines, 3rd edition (2017): 246-253.
- Lazzaroni MG., et al. A comprehensive review of the clinical approach to pregnancy and systemic lupus erythematosus. Journal of Autoimmunity 74 (2016): 106-117.
- Ritchie J., et al. “Maternal deaths in women with lupus nephritis: a review of published evidence". Lupus 21.5(2012):534-541.
- Moroni G., et al. “Maternal outcome in pregnant women with lupus nephritis A prospective multicenter study”. Journal of Autoimmunity 74 (2016): 194-200.
- Carvalheiras G., et al. “Fetal outcome in autoimmune disease”. Autoimmunity Reviews 11.6-7 (2011): 520-530.
- Koh JH., et al. “Pregnancy and patiens with preexisting lupus nephritis: 15 years of experience at a single center in Korea”. Lupus 24.7 (2015): 764-772.
- Betz RF and Specker C. “Pregnancy in lupus erythematosus and antiphospholipid syndrome”. Best Practice & Research Clinical Rheumatology 31(2017): 397-414.
- Huong DLT., et al. “Pregnancy in past or present lupus nephritis: a study of 32 pregnancies from a single center”. Annals of the Rheumatic Diseases 60.6 (2001): 559-604.
- Buyon JP., et al. “Kidney outcomes and risk factors for nephritis (flare/de novo) in a multiethnic cohort of pregnant patient with lupus”. Clinical Journal of the American Society of Nephrology 12.6 (2017): 940-946.
- Imbasciati E., et al. “Pregnancy in women with pre-existing lupus nephritis: predictors of fetal and maternal outcome”. Nephrology Dialysis Transplantation 24.2 (2009): 519-525.
- Alarfaj AS and Khalil N. “Fertility, ovarian failur, and pregnancy outcome in SLE patients treated with intavenous cyclophosphamide in Saudi Arabia”. Clinical Rheumatology33.12(2014): 1731-1736.
- Berghella V. “Maternal-Fetal Evidence Based Guidelines”. CRC Press, Taylor&Francis Group (2017): 246-253.
- Sree Roy J., et al. “SLE in pregnancy”. BSMMU 3.1(2010): 54-59.
Citation:
Sri Sulistyowati., et al. “Outcome Lupus Nephritis in Pregnancy at Dr. Soetomo Hospital, Indonesia”. Gynaecology and Perinatology
1.2 (2018): 120-129.
Copyright: © 2018 Sri Sulistyowati., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.