Review Article
Volume 2 Issue 1 - 2018
Endometriosis in the Adolescents
Discipline of Obstetrics, Gynaecology and Neonatology, Medical Foundation Building, the University of Sydney, Australia
*Corresponding Author: Frank Manconi, Medical Foundation Building, Discipline of Obstetrics, Gynaecology and Neonatology, the University of Sydney, 92 – 94 Parramatta Road, Camperdown, NSW, Australia.
Received: February 01, 2018; Published: February 13, 2018
Abstract
Endometriosis is an enigmatic disease of uncertain aetiology and pathogenesis, characterised by the presence of endometrial-like tissue in areas outside the uterine cavity. It is a chronic disease associated with pelvic pain and subfertility.
Endometriosis in adolescents presents a particular challenge of differential diagnosis and choice of treatment. The disease often begins in adolescence, but is most often recognised after years of dysmenorrhea. The combination of variable clinical presentation that is difficult to distinguish from primary dysmenorrhoea and the shortage of non-invasive methods for diagnosis has led to this delay.
More recently, studies have emerged with promises of markers for endometriosis with the prospect of using this method as a less invasive definitive diagnosis for endometriosis. These studies have demonstrated the expression of sympathetic, parasympathetic, and sensory nerve fibres in the functional layer of the endometrium and peritoneal lesions of women with endometriosis. Suggestive that these nerve fibres play a role in the pathophysiology of endometriosis-associated pelvic pain.
There currently exist no data that discusses the innervation of peritoneal lesions or eutopic endometrium of the adolescent. Facilitating earlier diagnosis and intervention for patients with endometriosis has potential to prevent disease progression, psychological pain and preserve future fertility.
Introduction
Endometriosis is an enigmatic disease of uncertain aetiology and pathogenesis characterised by the presence of endometrial glands and stroma histologically similar, although not identical to the inner lining of the uterus (endometrium) and at sites outside the uterus (Hudelist., et al. 2009). The inner menstruating layer of the uterus in women with endometriosis is termed the eutopic endometrium, whereas, the abnormal tissues outside the uterus are termed ectopic endometrium or ‘endometriotic lesions’. This a review of various aspects of endometriosis in the adolescent female.
General Perspectives
Endometriosis is a chronic oestrogen-dependent gynaecological disease affecting millions of women worldwide (Giudice and Kao, 2004). Despite the fact that endometriosis has been studied for nearly a century, it still remains an enigmatic condition with disputed aetiology amongst clinician and researchers with no cure (Rizk and Abdalla, 2003).
Endometriosis is a chronic oestrogen-dependent gynaecological disease affecting millions of women worldwide (Giudice and Kao, 2004). Despite the fact that endometriosis has been studied for nearly a century, it still remains an enigmatic condition with disputed aetiology amongst clinician and researchers with no cure (Rizk and Abdalla, 2003).
Endometriosis is frequently associated with debilitating pelvic pain i.e. pain during menstruation (dysmenorrhoea), sexual intercourse (deep dyspareunia), non-cyclic chronic pain with ovulation or bowl movements, and infertility (Goldstein., et al. 1980). However, the relationship between pain and endometriosis still remains unclear. A number of studies have suggested a link between nerve fibres and the pathophysiology of endometriosis and endometriosis related pain (Asante and Taylor, 2011, Tomita and Mah, 2014), with the prospect of using an increased expression of nerve fibres in endometrial biopsies as a definitive diagnosis for endometriosis (Al-Jefout., et al. 2009).
This disorder is most commonly diagnosed in women of reproductive age, although the time to actual diagnosis can be quite delayed with a mean of 11.7 years in the United States of America (USA), 8.0 years in the United Kingdom (UK) (Perloe, 2014). It has become progressively evident that the impediment is due to the fact that the extent of endometriosis is extremely variable and does not often correlate with the frequency and the severity of symptoms or with long term prognosis in terms of recurrence and contraception (Giudice and Kao, 2004).
For many women, endometriosis imposes a substantial burden in terms of well-being, personal relationships, time off work, and the need for surgery and expensive therapies. In addition, findings have suggested that these women also have an increased risk of ovarian, breast and skin cancer, as well as autoimmune diseases (Swiersz, 2002). Therefore, it is not only essential for greater awareness of the possible diagnosis of endometriosis in patients with pelvic pain, although it is becoming particularly important to do so in adolescent females for earlier and accurate therapy.
Endometriosis in adolescents
Endometriosis in the adolescent population has been particularly challenging in the field of gynaecology with differential diagnosis for pelvic pain and choice of treatment. The presentation of endometriosis in adolescents is variable, and it is often difficult to distinguish from primary dysmenorrhoea (Yang., et al. 2012). It has been speculated that this disease has different pathophysiology in adolescents, however there is little epidemiologic or molecular data that exists to either support or refute this concept (Shah and Missmer, 2011). The literature has not confirmed that intervention in the adolescent prevents long-term sequelae. Therefore, further studies to identify risk factors and treatment outcomes are needed for further understanding of endometriosis in the adolescent population (Laufer, 2008).
Endometriosis in the adolescent population has been particularly challenging in the field of gynaecology with differential diagnosis for pelvic pain and choice of treatment. The presentation of endometriosis in adolescents is variable, and it is often difficult to distinguish from primary dysmenorrhoea (Yang., et al. 2012). It has been speculated that this disease has different pathophysiology in adolescents, however there is little epidemiologic or molecular data that exists to either support or refute this concept (Shah and Missmer, 2011). The literature has not confirmed that intervention in the adolescent prevents long-term sequelae. Therefore, further studies to identify risk factors and treatment outcomes are needed for further understanding of endometriosis in the adolescent population (Laufer, 2008).
Epidemiology
There is currently no epidemiological data that is available for the true incidence or prevalence of endometriosis in adolescents, although data from the Endometriosis Association indicated that 66% of adult women with endometriosis have reported their symptoms commencing prior to the age of 20 years (American College of Obstetricians and Gynaecologists, 2005).
There is currently no epidemiological data that is available for the true incidence or prevalence of endometriosis in adolescents, although data from the Endometriosis Association indicated that 66% of adult women with endometriosis have reported their symptoms commencing prior to the age of 20 years (American College of Obstetricians and Gynaecologists, 2005).
Extent
The extent of endometriosis is extremely inconsistent due to the variability in the presentation of the disease and it does not often correlate with the severity of symptoms. Although, it is often associated with severe pain and infertility, it can also sometimes be asymptomatic. The complications in the diagnosis of endometriosis have led to long delays from onset of symptoms to the actual establishment of the diagnosis itself. Although endometriosis is popularly presented in women of reproductive age, there have been reports of symptomatic cases prior to menarche in girls who have some breast development and others soon after menarche (Goldstein., et al. 1979, Laufer, 2000, Yamamoto., et al. 1997).
The extent of endometriosis is extremely inconsistent due to the variability in the presentation of the disease and it does not often correlate with the severity of symptoms. Although, it is often associated with severe pain and infertility, it can also sometimes be asymptomatic. The complications in the diagnosis of endometriosis have led to long delays from onset of symptoms to the actual establishment of the diagnosis itself. Although endometriosis is popularly presented in women of reproductive age, there have been reports of symptomatic cases prior to menarche in girls who have some breast development and others soon after menarche (Goldstein., et al. 1979, Laufer, 2000, Yamamoto., et al. 1997).
There is evidence that some adolescents have a genetic predisposition to developing endometriosis. In one particular study involving patients with histologically confirmed endometriosis, first degree female relatives of affected patients were significantly more likely to have been diagnosed with endometrioses compared to relatives (Simpson., et al. 1980).
Prevalence
The true prevalence of endometriosis is not clear as there have been long term difficulties in its diagnosis. The estimates that have been reported in the literature are wide and vary depending upon the population studied (symptomatic or asymptomatic) and the method of diagnosis (clinical vs. surgical). Additionally, further complications of these estimates are caused by a small population of women who are symptomatic, although do not present themselves medically. Consequently, this cohort goes undetected in the population. Despite these impediments, Missmer (2004); ASRM: American Society for Reproductive Medicine (2012) suggested that the prevalence of endometriosis ranges from 30% to 80% in women with pelvic pain and 9% to 50% in women undergoing a laparoscopy for evaluation of infertility.
The true prevalence of endometriosis is not clear as there have been long term difficulties in its diagnosis. The estimates that have been reported in the literature are wide and vary depending upon the population studied (symptomatic or asymptomatic) and the method of diagnosis (clinical vs. surgical). Additionally, further complications of these estimates are caused by a small population of women who are symptomatic, although do not present themselves medically. Consequently, this cohort goes undetected in the population. Despite these impediments, Missmer (2004); ASRM: American Society for Reproductive Medicine (2012) suggested that the prevalence of endometriosis ranges from 30% to 80% in women with pelvic pain and 9% to 50% in women undergoing a laparoscopy for evaluation of infertility.
Common among adolescents as well as adults, endometriosis has been observed in females as young as the age of 10 (Gould, 2003). Published incidence rates vary among adolescents with chronic pelvic pain with reported rates of 25% to 38% (Kontoravdis., et al. 1999, Vercellini., et al. 1989) and 47% in those with chronic pelvic pain that have undergone laparoscopy (Goldstein., et al. 1980). Of these adolescents with pelvic pain uncontrolled by medical management with oral contraceptives (OCs) and non-steroidal anti-inflammatory drugs (NSAIDs), the incidence has been shown to be as high as 60% to 70% at time of laparoscopy (Laufer, 2012, Laufer., et al. 1997, Reese., et al. 1996).
Although these rates vary for chronic pelvic pain, one study estimated an incidence of 12% among girls between the age of 11-13 years (Propst and Laufer, 1999). In addition, another study from Boston Children’s Hospital found that teenagers typically presented with early stages of endometriosis, with 77% presenting with stage 1 and 23% at stage 2 (Propst and Laufer, 1999). In Australia studies have suggested that between 5-10% of menstruating women are affected by endometriosis. Most of these women have been diagnosed with endometriosis have reported that their symptoms commenced during their adolescent years (Braun, 2015).
Burden of Disease
Endometriosis has been linked to other burdens aside from the physical pain. Studies have concluded that it impairs health related quality of life, specifically in areas related to physical, psychological and social function. Endometriosis has often been described as a deliberating chronic disease. A study conducted by Oehmke (2009) demonstrated that pain in women aged 18-65, was a major cause of physical, psycho-social, emotional and professional or work-related impairment among women with endometriosis.
Endometriosis has been linked to other burdens aside from the physical pain. Studies have concluded that it impairs health related quality of life, specifically in areas related to physical, psychological and social function. Endometriosis has often been described as a deliberating chronic disease. A study conducted by Oehmke (2009) demonstrated that pain in women aged 18-65, was a major cause of physical, psycho-social, emotional and professional or work-related impairment among women with endometriosis.
The Global Study of Women’s Health investigated the care-seeking experience of affected women and examined in detail the impact of endometriosis on health-related quality of life and work productivity on a global scale, also concluding that endometriosis significantly affects women and societies world-wide (Nnoaham., et al. 2011). Indeed, chronic pelvic pain alone is associated with symptoms of depression, anxiety, low quality of life, low productivity, decreased energy, sexual dysfunction and relationship problems (Haggerty., et al. 2003, Mathias., et al. 1996). In addition, with its wide range of clinical presentation, endometriosis has been difficult to diagnose with high health care costs.
Adolescents have been noted to experience depression, fear or anxiety which may have resulted in increased use of medical resources and concomitant costs. Similarly, to adult women with work, adolescent endometriosis can affect school attendance (Laufer., et al. 1997). As mentioned earlier, pelvic pain and dysmenorrhoea, which are common symptoms of endometriosis, affect 45%-70% of adolescents. One survey of 2699 menarchal adolescent girls showed that 25% of all excessive school absences for these girls were due to dysmenorrhea or pelvic pain (Gao., et al. 2006, Klein and Litt, 1981). In particular, 50% of girls with severe dysmenorrhea or pelvic pain reported school absences due to their cramps (Klein and Litt, 1981).
Cost
Endometriosis is a very costly public health problem as it has high rates of hospital admissions, surgical procedures and incidences of comorbid conditions (Gao., et al. 2006). In effect, studies have shown that the yearly total (direct plus indirect) cost of endometriosis has been estimated at €30 billion in Europe and $22 billion in the USA, with direct costs still increasing steadily (Gao., et al. 2006, Simoens., et al. 2007).
Endometriosis is a very costly public health problem as it has high rates of hospital admissions, surgical procedures and incidences of comorbid conditions (Gao., et al. 2006). In effect, studies have shown that the yearly total (direct plus indirect) cost of endometriosis has been estimated at €30 billion in Europe and $22 billion in the USA, with direct costs still increasing steadily (Gao., et al. 2006, Simoens., et al. 2007).
Zhao (1998) studied the entire hospitalisation as compared to many of the other studies that focus on the procedural costs. This study included the hospitalisation rates, frequency of inpatient stay and related costs of endometriosis using the Healthcare Cost and Utilisation Project (HCUP). They found that the main inpatient charges per admission for endometriosis were $6,597 in 1991 and $7,449 in 1992. The mean length of stay for women with endometriosis as a principal diagnosis was 3.8 and 3.5 days in 1991 and 1992 respectively.
Social, indirect and intangible costs also contribute to the overall economic consequences of endometriosis. These costs include, although not limited to, loss in work productivity, loss of income, social withdrawal and psychological disorders such as depression (Gao., et al. 2006). Although endometriosis may be an economic burden to the health care systems, very few studies exist that quantify the indirect impact. In a multicentre study across ten countries, the impact of endometriosis on quality of life and work productivity was observed across ten different ethnicities (Nnoaham., et al. 2011).
In total, 1,486 of 1,669 eligible women agreed to participate, they found that affected women reported a greater number of absences from a duty or obligation and a higher number presented to work while sick when compared with symptomatic control women. Overall work productivity loss was 10.8 hours/week compared to 8.4 hours/week which rose with increasing disease severity (Nnoaham., et al. 2011).
Absenteeism-related costs ranged from US$1/week in Nigeria to US$250/week in the United States. Although reduced effectiveness at work is less frequently assessed and recorded than work absence, it accounted for nearly 60% of total work productivity loss. The annual cost per employed women of endometriosis associated work productivity loss varies from US$209 in Nigeria to US$23,712 in Italy (Nnoaham., et al. 2011).
The Endo Cost study has provided the best estimates of social costs of endometriosis. This study put the cost of endometriosis in terms of healthcare and loss of productivity in Europe in 2008 as €9570 per women per annum. Two-thirds of that cost was attributable to productivity loss (€6298) and the other third (€3113) made up health care costs (Simoens., et al. 2011). Estimating a 10% prevalence of endometriosis in women of reproductive age (15-49 years), these costs total almost $50 billion in North America per annum and almost €10 billion in the UK. An Australian study has estimated the direct costs in this country to be $6 billion per annum for medical and surgical treatments for women with endometriosis. Similarly, the estimated annual direct costs for adolescent girls with endometriosis is $12,000 per girl (Bush., et al. 2011).
Endometriosis-associated costs to society are considerable, as are the costs to the individual when disease symptoms interfere with day to day at work or at home (Bianconi., et al. 2007). The direct costs of endometriosis have increased steadily (Fourquet., et al. 2010). Diagnostics and surgical procedures, medicines, fertility treatments and involvement of healthcare professionals all contribute to these costs. However, social costs not only consist of costs of appropriate treatment of diagnosed endometriosis, however, also include costs of possible under-treatment as a result of delayed diagnosis and hit and miss treatments (Ballard., et al. 2006, Hadfield., et al. 1996, Hummelshoj., et al. 2006, Simoens., et al. 2007).
Theories of endometriosis more focused on adolescents
The aetiology of endometriosis is complex and not completely understood, with many proposed theories explaining the different mechanisms involved. To date no single theory has been able to explain all cases of endometriosis, particularly in the adolescent population although, all the proposed theories help to explain some aspects of the disease. Thus, the condition may arise from a combination of the major theories, consisting of the retrograde menstruation theory, the vascular/lymphatic dissemination theory, metaplasia of coelomic epithelium theory, the induction theory, and the embryonic rest theory (Seli., et al. 2003). The types and frequencies of pathogenic mechanisms involved in adolescent post pubertal/per menarche endometriosis may have different aetiologies compared to adult endometriosis (Laufer, 2012), (Song and Advincula, 2005).
The aetiology of endometriosis is complex and not completely understood, with many proposed theories explaining the different mechanisms involved. To date no single theory has been able to explain all cases of endometriosis, particularly in the adolescent population although, all the proposed theories help to explain some aspects of the disease. Thus, the condition may arise from a combination of the major theories, consisting of the retrograde menstruation theory, the vascular/lymphatic dissemination theory, metaplasia of coelomic epithelium theory, the induction theory, and the embryonic rest theory (Seli., et al. 2003). The types and frequencies of pathogenic mechanisms involved in adolescent post pubertal/per menarche endometriosis may have different aetiologies compared to adult endometriosis (Laufer, 2012), (Song and Advincula, 2005).
Retrograde Menstruation/Transplantation Theory
The most commonly accepted theory is that of Sampson (1927), who proposed the implantation or retrograde menstruation theory. This theory suggests that during menstruation, viable endometrial cells left from the shedding of the endometrium, reflux through the fallopian tubes, thereby gaining access to, and implanting on the surrounding pelvic viscera (Sampson, 1927). The disadvantage of this theory is that most normally menstruating women experience some retrograde menstruation with each cycle, thus, this theory lacks the explanation as to why certain women are predisposed while others are protected (Song and Advincula, 2005).
The most commonly accepted theory is that of Sampson (1927), who proposed the implantation or retrograde menstruation theory. This theory suggests that during menstruation, viable endometrial cells left from the shedding of the endometrium, reflux through the fallopian tubes, thereby gaining access to, and implanting on the surrounding pelvic viscera (Sampson, 1927). The disadvantage of this theory is that most normally menstruating women experience some retrograde menstruation with each cycle, thus, this theory lacks the explanation as to why certain women are predisposed while others are protected (Song and Advincula, 2005).
Furthermore, this theory is only supported assuming that endometriosis mostly occurs in the dependent portion of the pelvis. In the adolescent population, obstructive congenital anomalies of the female reproductive tract that encourages retrograde flow has been associated with endometriosis (Sanfilippo, 1997, Schifrin., et al. 1973). One study identified six adolescents with Müllerian (embryonic ducts which give rise to the genital passages in the female) anomalies (Schifrin., et al. 1973), where the youngest patient was a 12 year old female with vaginal atresia and bicornuate uterus with developed hematocolpos; an accumulation of menstrual blood in the vagina. This was likely followed by retrograde flow leading to her endometriosis. There has been evidence that reparation of this type of obstructive abnormality has been correlated with the resolution of endometriosis (Sanfilippo, 1997), however, this is not true in all cases.
The Induction/Coelomic Metaplasia Theory
The coelomic metaplasia theory was first proposed by Iwanoff and later propagated by Meyer (Metzger and Haney, 1989). Unlike most of the other theories, the induction/coelomic metaplasia theory is among those proposing a non-uterine origin of the disease. This theory suggests that the coelomic epithelium is the common ancestor of endometrial and peritoneal cells, thus allowing transformation of normal peritoneal tissue to ectopic endometrial tissue (Burney and Giudice, 2012). The ‘transformed’ peritoneal epithelium may be caused by chronic inflammation, chemical irritation from refluxed menstrual blood or hormonal stimuli (Iwanoff, 1898, Meyer, 1924). This is a theory based upon embryologic studies indicating that all pelvic organs, including the endometrium, are derived from cells that line that coelomic cavity (Vinatier., et al. 2001).
The coelomic metaplasia theory was first proposed by Iwanoff and later propagated by Meyer (Metzger and Haney, 1989). Unlike most of the other theories, the induction/coelomic metaplasia theory is among those proposing a non-uterine origin of the disease. This theory suggests that the coelomic epithelium is the common ancestor of endometrial and peritoneal cells, thus allowing transformation of normal peritoneal tissue to ectopic endometrial tissue (Burney and Giudice, 2012). The ‘transformed’ peritoneal epithelium may be caused by chronic inflammation, chemical irritation from refluxed menstrual blood or hormonal stimuli (Iwanoff, 1898, Meyer, 1924). This is a theory based upon embryologic studies indicating that all pelvic organs, including the endometrium, are derived from cells that line that coelomic cavity (Vinatier., et al. 2001).
Further, support for this theory developed from observations made from endometriosis in the adolescent population of premenarchal girls with some breast development (Batt and Mitwally, 2003, Laufer, 2000). The retrograde menstruation theory cannot explain the presence of endometriosis seen in case studies involving adolescents, as these girls have not yet had menarche and thus cannot have had retrograde menses. In addition, further strengthening of this theory was seen in a recent case study involving a 20-year old girl with Mayer-Rokitansky-Küster-Hauser syndrome (a condition causing the vagina and uterus to be under developed or absent), who presented with increasing pelvic pain and underwent laparoscopy (Mok-Lin., et al. 2010). Uterine, cervical, vaginal and tubal agenesis were confirmed and upon laparoscopy endometriosis was further identified and destroyed. This case of endometriosis in an adolescent with complete uterine agenesis supports the induction/coelomic metaplasia theory (Mok-Lin., et al. 2010).
Lesions in Adolescents
Adolescent lesions are similar to adults in such a way that they vary in presentation. However, they typically have clear, red, white and yellow brown lesions more frequently than black or blue lesions (Appelbaum and Nentin, 2010). Moreover, a study that compared endometriosis lesions in adolescents to those in adults found that red flame lesions were more common and powder burn lesions were less common (Davis., et al. 1993). These results are consistent with the presumption that powder burn lesions represent older, more advanced implants (Demco, 1998). This also correlates with the results in Konnickx study where they found that pain symptoms correlated with the depth of the lesions (Porpora., et al. 1999).
Adolescent lesions are similar to adults in such a way that they vary in presentation. However, they typically have clear, red, white and yellow brown lesions more frequently than black or blue lesions (Appelbaum and Nentin, 2010). Moreover, a study that compared endometriosis lesions in adolescents to those in adults found that red flame lesions were more common and powder burn lesions were less common (Davis., et al. 1993). These results are consistent with the presumption that powder burn lesions represent older, more advanced implants (Demco, 1998). This also correlates with the results in Konnickx study where they found that pain symptoms correlated with the depth of the lesions (Porpora., et al. 1999).
Together, this suggests that the more superficial red and atypical lesions present in adolescents may not be as painful. However, it has become clearer that there are other mechanisms responsible for the pain in endometriosis. There are cases of endometriomas in the adolescent populations that have been reported however, they are quite rare (Wright and Laufer, 2010). Though it is important to note that most adolescent cases of endometriosis are diagnosed on the basis of history and physical examination and not laparoscopic visualisations of lesions, there may be many presentations of lesions left undetected.
There are limited studies showing the frequent lesion sites for endometriosis in adolescents. However, a retrospective analysis of 63 cases of adolescent endometriosis in china found that of the 63 cases, 55 cases of lesions were found on the ovaries, 18 were found on the rectovaginal pouch, and 20 cases involved lesions of the uterosacral ligaments (Yang., et al. 2012).
Adolescents with endometriosis frequently have both acyclic and cyclic pain (severe, progressive dysmenorrhoea). However, isolated cyclic pain is the least commonly presented (Laufer., et al. 1997). Bowel symptoms inclusive of rectal pain, constipation, painful defecation that may be cyclic, and rectal bleeding are also common as well as bladder symptoms such as dysuria, urgency and hematuria. Whereas, ovarian endometriosis and infertility are rare in adolescents (Dessole., et al. 2012, Laufer., et al. 2003).
Diagnosis
The pain may commence at a young age, even prior to the onset of menstruation, however, the diagnosis by laparoscopy is almost postponed for several years by which time destructive lesions have affected the tubo-ovarian structures and severely compromised fecund ability (Brosens., et al. 2013). It has been speculated that with earlier diagnosis of endometriosis in the adolescent, there may be a decrease in the length of time between patient presentation and clinical diagnosis, which currently averages 6.7 years (Nnoaham., et al. 2011).
The pain may commence at a young age, even prior to the onset of menstruation, however, the diagnosis by laparoscopy is almost postponed for several years by which time destructive lesions have affected the tubo-ovarian structures and severely compromised fecund ability (Brosens., et al. 2013). It has been speculated that with earlier diagnosis of endometriosis in the adolescent, there may be a decrease in the length of time between patient presentation and clinical diagnosis, which currently averages 6.7 years (Nnoaham., et al. 2011).
Brosens (2013) reported that there are several important factors contributing to the establishment of lesions, one of the primary factors for the progression of the disease was most likely to be in the delay in diagnosis. In addition, earlier diagnosis and treatment of this disease will slow disease progression, possibly decreasing the adverse long-term effects of the disease such as chronic pain, endometriosis, and infertility, thus improving the quality of life of adolescents and women with the disorder (Nothnick, 2001).
Therefore, using established techniques such as transvaginal ultrasound also known as endovaginal ultrasound and transvaginal diagnostic imaging techniques which allow access with a less invasive needle endoscopy have been recommended for the exploration of the pelvis, diagnosis of endometriosis and treatment at an early stage prior to the development of severe lesions (Brosens., et al. 2013).
Method of Diagnosis
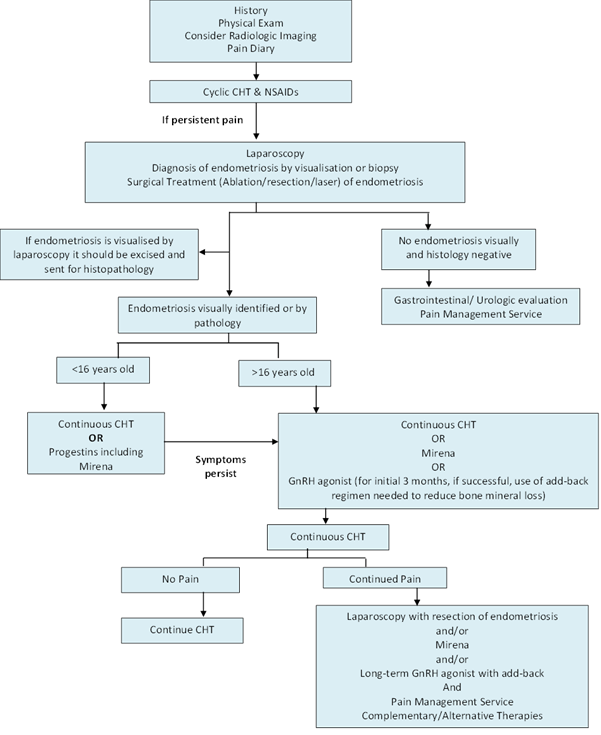
The gold standard for diagnosis of endometriosis is surgical assessment by laparoscopy or laparotomy, followed by a histopathological examination of biopsied or excised lesions (Farquhar, 2007). The severity is then scored using the revised classification of endometriosis system developed by the American Society of Reproduction Medicine (ASRM) (ASRM, 1997). However, it is difficult to perform laparoscopic procedures on all patients with suspected endometriosis, particularly with adolescents. Flow chart of methods commonly used for the diagnosis and treatment of endometriosis in adolescents (Figure 1).
The gold standard for diagnosis of endometriosis is surgical assessment by laparoscopy or laparotomy, followed by a histopathological examination of biopsied or excised lesions (Farquhar, 2007). The severity is then scored using the revised classification of endometriosis system developed by the American Society of Reproduction Medicine (ASRM) (ASRM, 1997). However, it is difficult to perform laparoscopic procedures on all patients with suspected endometriosis, particularly with adolescents. Flow chart of methods commonly used for the diagnosis and treatment of endometriosis in adolescents (Figure 1).
Figure 1: Diagnosis and treatment of adolescents with endometriosis. A flow chart of the method used. *CHT: combined hormonal therapy, NSAIDs: non-steroidal anti-inflammatory drugs, GnRH: gonadotropin releasing hormone agonist, Mirena: progesterone intrauterine device. Adapted from (Armstrong, 2011, Laufer., et al. 2003).
The diagnosis of patients by clinical patterns and detailed physical examination complemented with imagining methods is a fundamental step in the preliminary investigation of endometriosis, particularly in the adolescent. It provides the ability to identify adolescents at high risk and those that require further evaluation, reducing the need of invasive investigations (Kafali., et al. 2004). Obtaining a patient’s full medical history helps to determine whether their symptoms are due to other causes. Following patient history, a relevant physical examination is essential to determine the aetiology of the pain and rule out an ovarian tumour or anomaly of the reproductive tract (Laufer., et al. 2003).
Although it is important, it may not be possible to perform a complete pelvic examination in all adolescents. For adolescents who are not sexually active, a rectal-abdominal examination may be better tolerated than vaginal-abdominal. Imaging methods, such as transvaginal ultrasonography and magnetic resonance imaging (MRI) should be performed to help a limited physical examination and identify/exclude causes of abdominopelvic pain other than endometriosis.
Treatment
In adolescents, medical treatment of dysmenorrhoea is appropriate prior to consideration of surgical intervention for diagnosis of endometriosis. Particularly in adolescents with dysmenorrhea and/or have difficulty participating in normal activities, are missing school or avoiding extracurricular activities due to pelvic pain. Cyclic combination hormonal therapy (CHT) and nonsteroidal anti-inflammatory drugs (NSAIDS) are reasonable approaches when the pain evaluation suggests a non-acute gynaecological source. Hormonal therapy should be given with NSAIDS (American College of Obstetricians and Gynaecologists (2005)).
In adolescents, medical treatment of dysmenorrhoea is appropriate prior to consideration of surgical intervention for diagnosis of endometriosis. Particularly in adolescents with dysmenorrhea and/or have difficulty participating in normal activities, are missing school or avoiding extracurricular activities due to pelvic pain. Cyclic combination hormonal therapy (CHT) and nonsteroidal anti-inflammatory drugs (NSAIDS) are reasonable approaches when the pain evaluation suggests a non-acute gynaecological source. Hormonal therapy should be given with NSAIDS (American College of Obstetricians and Gynaecologists (2005)).
If the pain is unresolved with NSAIDS and hormonal treatment, further evaluation is needed to determine whether endometriosis is the aetiology of the pain. The use of GnRH agonist allows patients with chronic pelvic pain and a high probability of endometriosis to avoid a diagnostic surgical procedure. Empiric GnRH agonists are not used for adolescents 18 years of age or younger due to concerns of potential adverse long term effects on bone formation and bone mineral density (American College of Obstetricians and Gynaecologists (2005)).
Surgical approaches to relieve endometriosis related pain can be used as first-line therapy or initiated after failed medical therapies. A definitive diagnosis should be established before administering further treatment to adolescent with persistent pain after a period of three to six months with medicinal treatment (Armstrong, 2011). As mentioned previously, laparoscopy is the gold standard for the diagnosis of endometriosis.
Surgical approaches include excision, fulguration, or laser ablation of endometriomas, resection of rectovaginal nodules, lysis of adhesions, and interruption of nerve pathways (Giudice, 2010). It is essential that the gynaecologist performing the laparoscopy on the adolescent not only has experience operating on patients in this age group, although is also familiar with the appearance of endometriosis implants in adolescents. It is also important to achieve a good cosmetic result in adolescents minimising scarring as much as possible (Laufer, 2008).
Angiogenesis of Eutopic Endometrium in Endometriosis
Presently, there is limited data regarding angiogenesis specific to adolescent endometriosis. However, there are many studies with anti-angiogenic drugs that hold the promise of pelvic pain relief with the aim of successful achievement of pregnancy in infertile patient (Rocha., et al. 2013). Since the majority of adolescents with endometriosis suffer from dysmenorrhoea, it is logical that this method of treatment will target this population preventing onset symptoms such as infertility.
Presently, there is limited data regarding angiogenesis specific to adolescent endometriosis. However, there are many studies with anti-angiogenic drugs that hold the promise of pelvic pain relief with the aim of successful achievement of pregnancy in infertile patient (Rocha., et al. 2013). Since the majority of adolescents with endometriosis suffer from dysmenorrhoea, it is logical that this method of treatment will target this population preventing onset symptoms such as infertility.
Conclusion
There currently exist no data that discusses the innervation of peritoneal lesions or eutopic endometrium of the adolescent. Facilitating earlier diagnosis and intervention for patients with endometriosis has potential to prevent disease progression, psychological pain and preserve future fertility. Adolescents have less well developed lesions than in the adults which can makes the diagnosis more difficult and as a consequence the early management may be delayed.
References
- AL-JEFOUT M., et al. “Diagnosis of endometriosis by detection of nerve fibres in an endometrial biopsy: a double blind study”. Human reproduction 24.12 (2009): 3019-3024.
- APPELBAUM H and NENTIN F. “Endometriosis in Adolescents”. Adolescent Endometriosis 35(2010): 17-20.
- ARMSTRONG C. “ACOG Updates Guidelines on Diagnosis and Treatment of Endometriosis”. American Family Physician 83.1 (2011): 84-85.
- ASANTE A and TAYLOR RN. “Endometriosis: the role of neuroangiogenesis”. Annual review of physiology 73(2011): 163-182.
- American Society for Reproductive Medicine. “Revised American Society for Reproductive Medicine classification of endometriosis 1996”. Fertility and sterility 67.5 (1997): 817-821.
- BALLARD K., et al. “What’s the delay? A qualitative study of women’s experiences of reaching a diagnosis of endometriosis”. Fertility and Sterility 86.5 (2006): 1296-1301.
- BATT RE and MITWALLY MFM. “Endometriosis from thelarche to midteens: pathogenesis and prognosis, prevention and pedagogy”. Journal of Pediatric & Adolescent Gynecology 16.6 (2003): 337-347.
- BIANCONI L., et al. “Recognizing endometriosis as a social disease: the European Union-encouraged Italian Senate approach”. Fertility and Sterility 88.5 (2007): 1285-1287.
- BRAUN K. “Endometriosis in Adolescence”. Health Journey (2015): 6 -7.
- BROSENS I., et al. “Endometriosis in adolescents is a hidden, progressive and severe disease that deserves attention, not just compassion”. Human Reproduction 28.8 (2013): 2026-2031.
- BURNEY RO and GIUDICE LC. “Pathogenesis and pathophysiology of endometriosis”. Fertility and sterility 98.3 (2012): 511-519.
- BUSH B., et al. “The $6 billion women and the $600 million girl - The Pelvic Pain report”. Endorsed by Pain Australia and the Faculty of Pain Medicine (2011): 1-74.
- DAVIS GD., et al. “Clinical characteristics of adolescent endometriosis”. Journal of Adolescent Health 14 (1993): 362-368.
- DEMCO L. “Mapping the source and character of pain due to endometriosis by patient-assisted laparoscopy”. The Journal of the American Association of Gynecologic Laparoscopists 5.3 (1998): 241-245.
- DESSOLE M., et al. “Endometriosis in adolescence”. Obstetrics and gynaecology International (2012): 869191.
- FARQUHAR C. “Endometriosis”. BMJ (Clinical research Ed), 334 (2007): 249-53.
- FOURQUET J., et al. “Patients’ report on how endometriosis affects health, work, and daily life”. Fertility and Sterility 93.7 (2010): 2424-2428.
- GAO X., et al. “Economic burden of endometriosis”. Fertility and sterility 86.6 (2006): 1561-1572.
- GIUDICE LC. “Clinical practice. Endometriosis”. The New England Journal of Medicine 362.25 (2010): 2389-2398.
- GIUDICE LC and KAO LC. “Endometriosis”. The Lancet 364.9447 (2004): 1789-1799.
- GOLDSTEIN DP., et al. “Adolescent endometriosis”. Journal of adolescent health care: official publication of the Society for Adolescent Medicine 1 (1980): 37-41.
- GOLDSTEIN DP., et al. “New insights into the old problem of chronic pelvic pain”. Journal of Pediatric Surgery 14 (1979): 675-680.
- GOULD D. “Endometriosis”. Nursing standard (Royal College of Nursing (Great Britain): 1987), 17 (2003): 47-55.
- GYNAECOLOGISTS ACOOA. “ACOG Committee Opinion Number 310”. Obstetrics and Gynecology 105 (2005): 921.
- HADFIELD R., et al. “Delay in the diagnosis of endometriosis: a survey of women from the USA and the UK”. Human Reproduction 11.4 (1996): 878-880.
- HAGGERTY CL., et al. “Lower quality of life among women with chronic pelvic pain after pelvic inflammatory disease”. Obstetrics and Gynecology 102 (2003): 934-939.
- HUDELIST G., et al. “The migrating adenomyoma: past views on the etiology of adenomyosis and endometriosis”. Fertility and sterility 92.5 (2009): 1536-1543.
- HUMMELSHOJ L., et al. Update on Endometriosis. Womens Health 2 (2006): 53-56.
- IWANOFF NS. “Drusiges cysthaltiges uterusfibromyom compliziert durch sarcom und carcinom (Adenofibromyoma cysticum arcomatodes carcinomatosum)”. Monatsch Geburtsh Gyndkol 7 (1898): 295-300.
- KAFALI H., et al. “Use of CA125 fluctuation during the menstrual cycle as a tool in the clinical diagnosis of endometriosis; a preliminary report”. European Journal of Obstetrics & Gynecology and Reproductive biology 116.1 (2004): 85-88.
- KLEIN JR and LITT IF. “Epidemiology of adolescent dysmenorrhea”. Pediatrics 68 (1981): 661-664.
- KONTORAVDIS A., et al. “Laparoscopic evaluation and management of chronic pelvic pain during adolescence”. Clinical and Experimental Obstetrics and Gynecology 26.2 (1999): 76-77.
- LAUFER MR. “Current approaches to optimizing the treatment of endometriosis in adolescents”. Gynecologic and Obstetric Investigation 66.1 (2008): 19-27.
- LAUFER MR. “Dysmenorrhea, Acute and chronic Pelvic Pain, Endometriosis and Premenstrual Syndrome”. Pediatric and Adolescent Gynecology (2012):
- LAUFER MR., et al. “Prevalence of endometriosis in adolescent girls with chronic pelvic pain not responding to conventional therapy”. Journal of Pediatric & Adolescent Gynecology 10.4 (1997): 199-202.
- LAUFER MR., et al. “Adolescent endometriosis: diagnosis and treatment approaches”. Journal of Pediatric & Adolescent Gynecology 16 (2003): S3-11.
- LAUFER R. “Premenarcheal endometriosis without an associated obstructive anomaly: Presentation, diagnosis and treatment”. Fertility and sterility 74.3 (2000): S15.
- MATHIAS SD., “Chronic pelvic pain: prevalence, health-related quality of life, and economic correlates”. Obstetrics and Gynecology 87.3 (1996): 321-327.
- Practice Committee of the American Society for Reproductive Medicine. “Endometriosis and infertility: a committee opinion”. Fertility and sterility 98.3 (2012): 591-598.
- METZGER DA and HANEY AF. “Etiology of endometriosis”. Obstetrics and gynaecology clinics of North America 16 (1989): 1-14.
- MEYER RK. “Zur frage der heterotopen epithelwucherung, insbesondere des peritonealepithels und in die ovarien”. Virch Arch Path Anat Phys 250 (1924): 595-610.
- MISSMER SA., et al. “Incidence of laparoscopically confirmed endometriosis by demographic, anthropometric, and lifestyle factors”. American Journal of Epidemiology 160.8 (2004): 784-796.
- MOK-LIN EY., et al. “Endometriosis in a patient with Mayer-Rokitansky-Kuster-Hauser syndrome and complete uterine agenesis: evidence to support the theory of coelomic metaplasia”. Journal of Pediatric & Adolescent Gynecology 23.1 (2010): 35-37.
- NNOAHAM KE., et al. “Impact of endometriosis on quality of life and work productivity: a multicenter study across ten countries”. Fertility and sterility 96.2 (2011): 366-373.
- NOTHNICK W B. “Treating endometriosis as an autoimmune disease”. Fertility and sterility 76 (2001): 223 -231.
- OEHMKE F., et al. “Impact of endometriosis on quality of life: a pilot study”. Gynecological Endocrinology 25.11 (2009): 722-725.
- PERLOE M. Improving referral and speed of Diagnosis of endometriosis. (2014):
- PORPORA MG., et al. “Correlation between endometriosis and pelvic pain”. The Journal of the American Association of Gynecologic Laparoscopists 6.4 (1999): 429-434.
- PROPST AM and LAUFER MR. “Endometriosis in adolescents. Incidence, diagnosis and treatment”. The Journal of Reproductive Medicine 44.9 (1999): 751-758.
- REESE KA., et al. “Endometriosis in an adolescent population: the Emory experience”. Journal of Pediatric & Adolescent Gynecology 9.3 (1996): 125-128.
- RIZK B. and ABDALLA H. “Fast Facts: Endometriosis”. Abingdoh, United Kingdom, Health Press Limited (UK). (2003).
- ROCHA ALL., et al. “Angiogenesis and endometriosis”. Obstetrics and Gynecology International (2013): 859619.
- SAMPSON JA. “Peritoneal endometriosis due to the menstrual dissemination of endometrial tissue into the peritoneal cavity”. American Journal of Obstetrics and Gynecology 14.4 (1927): 422-469.
- SANFILIPPO JS. “Endometriosis. Laparoscopy--diagnostic and therapeutic approaches”. Annals of the New York Academy of Sciences 816 (1997): 310-319.
- SCHIFRIN BS., et al. “Teen-age endometriosis”. American journal of obstetrics and Gynecology 116 (1973): 973-980.
- SELI E., et al. “Pathogenesis of endometriosis”. Obstetrics and Gynecology Clinics of North America 30 (2003): 41-61.
- SHAH DK and MISSMER SA. “Scientific investigation of endometriosis among adolescents”. Journal of Pediatric & Adolescent Gynecology 24 (2011): S18-19.
- SIMOENS S., et al. “Endometriosis: cost estimates and methodological perspective”. Human reproduction update 13.4 (2007): 395-404.
- SIMOENS S., et al. “Endometriosis cost assessment (the EndoCost study): a cost of illness study protocol”. Gynecologic and Obstetric Investigation 71.3 (2011): 170 -176.
- SIMPSON JL., et al. “Heritable aspects of endometriosis. I. Genetic studies”. American Journal of Obstetrics & Gynecology 137.3 (1980): 327-331.
- SONG AH and ADVINCULA AP. “Adolescent chronic pelvic pain”. Journal of Pediatric & Adolescent Gynecology 18.6 (2005): 371-377.
- SWIERSZ LM. “Role of endometriosis in cancer and tumor development”. Annals of the New York Academy of Sciences 955, 281-92; discussion 293-5 (2002): 396-406.
- TOMITA T and MAH K. “Cyclic changes of nerve fibres in human endometrium”. Open Journal of Pathology 4.2 (2014): 68-78.
- VERCELLINI P., et al. “Laparoscopy in the diagnosis of chronic pelvic pain in adolescent women”. The Journal of Reproductive Medicine 34.10 (1989): 827-830.
- VINATIER, D., et al. “Theories of endometriosis”. European Journal of Obstetrics & Gynecology and Reproductive Biology 96.1 (2001): 21-34.
- WRIGHT KN and LAUFER MR. 2010. “Endometriomas in adolescents”. Fertility and sterility 94.4 (2010): 1529.e7-1529.e9.
- YAMAMOTO K., et al. “Tubal endometriosis diagnosed within one month after menarche: a case report”. The Tohoku Journal of Experimental Medicine 181.3 (1997): 385-387.
- YANG Y., et al. “Adolescent endometriosis in China: a retrospective analysis of 63 cases”. Journal of Pediatric & Adolescent Gynecology 25.5 (2012): 295-299.
- ZHAO SZ., et al. “The cost of inpatient endometriosis treatment: an analysis based on the Healthcare Cost and Utilization Project Nationwide Inpatient Sample”. The American Journal of Managed Care 4.8 (1998): 1127-1134.
Citation:
Amy T Nguyen., et al. “Endometriosis in the Adolescents”. Gynaecology and Perinatology 2.1 (2018): 181-191.
Copyright: © 2018 Amy T Nguyen., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.