Research Article
Volume 2 Issue 5 - 2019
The Role of Zinc in Pregnant Women Suffering from Anaemia
American Hospital, University Hospital “Mbreteresha Geraldine", Tirana, Albania
*Corresponding Author: Shpresa Thomaj, American Hospital, University Hospital “Mbreteresha Geraldine", Tirana, Albania.
Received: July 17, 2019; Published: July 27, 2019
Abstract
There is a Zinc insufficiency in the serum during pregnancy. Supplementary needs for Zinc are at least 15—20 mg in day and cannot be fulfilled from diet. As a consequence there is Zinc insufficiency real and it is manifested during pregnancy. Zinc insufficiency puts pregnant mother at risk of spontaneous aborts, preeclampsia, premature or extended delivery, prolonged labor amnionitis and postpartum infection.
The purpose of this study is too evident the changes of seric zinc and iron in pregnant women in their third trimester (i.e. pregnant women with congenital anemia Hb <10 gr%, HCT <30 % compared with the control group where Hb >10 gr. % and HTC >30%. Serum zinc level was determined by atomic absorption spectroscopy.
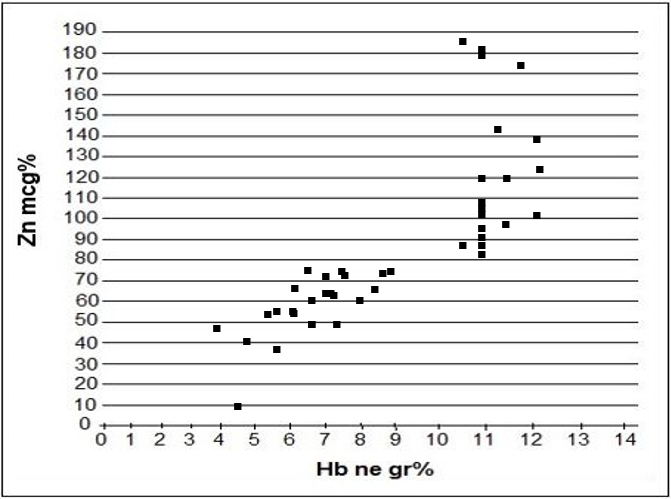
A measurement of serum zinc is made in 24 pregnant women in their third trimester, who suffered from anemia Zn=48mcg% ±15.4mcg%. Also, we measured the level of seric zinc in 20 pregnant normal women where Zn=112.5 mcg% ±30.2mcg%. When we compared the data, it resulted a lower serum zinc amount in pregnant anemic women than in normal pregnant women. There is a significant difference between them: P < 0.001, T= 6.6. Seric zinc is lower in anemic pregnant women than in normal pregnant women.
In addition, seric zinc is much lower in pregnant women which suffered from sickle cell disease - where seric zinc was < 40mcg%. The lower level of zinc in pregnant women which suffered from sickle cell disease is linked to their hemolytic crises which caused an increase in elimination of zinc through their urinary tract.
Key words: Pregnancy; Zinc; Anemia
Introduction
Zinc is an important micro-element for a human's organism. There is usually about 2500 mg. of zinc in our body which is to be found as it follows: 30% in our bones, 60% in our muscles, and the remaining quantity is to be found in our tissues. Our hair, eyes, prostaglandins, etc. are the tissues which contain most of the zinc. The following factors lower the zinc level in the serum:
- Mal absorption in the pipes (chronic enteropathie)
- Low zinc intake because of going on diets (i.e. vegetarian diets)
- Specific conditions of the organism which increase the need for more zinc such as: unique pregnancy, twins, or three embryos.
- Diseases which increase the hemolytic erythrocytes such as: sickle cell disease, spherocytosis, deficit of glucose, 6 phosphate dehydrogenises [16].
Zinc plays an important role in the synthesis of nucleic acids, in cell's division, in strengthening our immune system [3, 12, 13, 15].
There are factors who modify the absorption of zinc:
- Proteins and amino acids increase the absorption of zinc.
- Glucides do not affect in the absorption of zinc, but they lower its quantity through urine.
- Use of alcohol decreases zinc's absorption and stimulates its excretion through urine [15].
Unsaturated fatty acids and animal proteins increase the absorption of zinc. Excretion of zinc by our organism is done through sweat, faeces, biliary fluid and urine. Zinc is composed of about 300 metalo-enzims which are synthesized in liver and they take part in many metabolic processes of our organism [13-15].
Synthesis of metalo-enzims is increased under the influence of gluco-corticoid hormones (glucagon and epinephrine]. This explains the decrease of zinc in the serum in a state of stress (cold or low temperatures, heat or high temperature, or strong emotions). Acute infections through interleukin 2 increase the synthesis of metalo-enzims.
Zinc serves as a catalyst of several reactions of dehydrogenating and dehydration. It stimulates hormones by activating the co-factors of the hormones. Zinc stabilizes the tertiary structure of peptide hormones by making them active, stabilizes the insulin (by creating the zinc-insulin complex), it prohibits the sickle cell creation of erythrocytes, and it affects the stability of polypeptides chains of hemoglobin [16].
Zinc has an effect on ovulation, our cell's immunity, cutan integrity in the metabolism of nucleic acids by influencing the polymerase RNA, sintetase RNA, reverse transcriptase and thymidine kinase [3,12,14]. Some foods that are rich in zinc are: meat, fish, integrated bread, sea food, etc.
Material and Method
We surveyed 24 pregnant women suffering from anemia. 14 of them suffered from hemoglobinopathies (10 of them from homozygous sickle cell and trait sickle cell and 4 of them were carriers of thalasemia) and the other ones suffered from ferro-deficit anemia. In addition, we surveyed 20 pregnant women who did not suffer from anemia.
The collected data was analyzed according to Fisher Student Test.
| Congenital anemia | Ferro-deficit anemia | Total | ||
| Sickle cell trait cases | Sickle cell disease | The trait thalassemia | ||
| 6 | 4 | 4 | 10 | 24 |
Table 1: Pregnant women according to their type of anemia.
We measured the zinc level in the serum in the third trimester of pregnancy in 24 women who suffered from anemia (Hb. level was lower than 10 gr. % and HCT lower than 30%) and in 20 pregnant women who did not suffer from anemia.
| Normal pregnant women in third trimester ( No) | Zn seric (mcg %) | Hb in gr% | Pregnant women suffering from anemia (No) | Zn seric (mcg %) | Hb in gr% |
| 1 | 87 | 10.5 | 1 | 60 | 8 |
| 2 | 122 | 12,4 | 2 | 75 | 6.5 |
| 3 | 172 | 11,9 | 3 | 54 | 6.1 |
| 4 | 102 | 11 | 4 | 72 | 7.1 |
| 5 | 120 | 11,4 | 5 | 55 | 6.1 |
| 6 | 90 | 11 | 6 | 63 | 7.2 |
| 7 | 81 | 11 | 7 | 66 | 8.5 |
| 8 | 85 | 11 | 8 | 72 | 7.8 |
| 9 | 120 | 11 | 9 | 50 | 6.8 |
| 10 | 88 | 10.5 | 10 | 50 | 7,2 |
| 11 | 105 | 11 | 11 | 48 | 4 |
| 12 | 144 | 11.7 | 12 | 53 | 5,4 |
| 13 | 108 | 11 | 13 | 10 | 4.5 |
| 14 | 105 | 11 | 14 | 50 | 6,8 |
| 15 | 106 | 11,5 | 15 | 72 | 8,8 |
| 16 | 140 | 12 | 16 | 66 | 7.2 |
| 17 | 104 | 11 | 17 | 38 | 5,6 |
| 18 | 180 | 11 | 18 | 41 | 4. 8 |
| 19 | 182 | 11 | 19 | 75 | 9 |
| 20 | 100 | 12 | 20 | 67 | 7,2 |
| 21 | 60 | 6,8 | |||
| 22 | 57 | 5,8 | |||
| 23 | 73 | 7,4 | |||
| 24 | 66 | 6,1 | |||
Table 2: Data measurement for seric ZN in normal and anemic pregnant women.
The indicator that shows the difference between two groups is significant. Zinc level is lower in pregnant women suffering from anemia than in pregnant women who do not suffer from anemia i.e. P ˂ 0.001 T 6.66. The lowest zinc level in the serum is noticed in women who suffer from Sickle Cell Disease. When there is a hemolytic crisis in SCD, there is a higher zinc elimination through urine. [12,15, 16 ]. As a result, the zinc level in those women's blood decreases.
According to many authors, preeclampsia is higher in pregnant women who suffer from zinc deficit than in those pregnant women with a normal zinc level [3,4,5, 8,9,10,13,15,16 ].
| Congenital anemia | No. of pregnant women | Preeclampsia (nr or %) |
| Sickle cell trait | 6 | 3 |
| Sickle cell disease | 4 | 3 |
| The trait thalasemia | 4 | 1 |
| Ferro-deficit anemia | 10 | N/A |
| Total | 24 | 7 28% |
| Normal pregnant women | 20 | 1 5 % |
Table 3: Frequency of Preeclampsia in anemic and normal pregnant women.
Preeclampsia is more frequent in anemic pregnant women than in normal pregnant women. Zinc deficit during a pregnancy leads to: involuntary abortions, congenital anomalies, premature delivery, fetal hypotrophy, fetal sufferance, distacco placenta, serotonin pregnancy, and hemorrhage after delivery, and postpartum infections [1, 3].
Conclusion
With reference to our patients in this study, we came to the conclusion that the zinc level was low in those pregnant women who suffered from anemia. It was lower in pregnant women who suffered from hemogobinopathies (thalassemi and sickle cell disease). In addition, preeclampsia is more common in pregnant women who have a lower zinc level compared with the level of normal pregnant women.
References
- Laura E Caulfield., et al. “Potential contribution of Maternal Zinc supplementation during pregnancy to Maternal and child survival”. The American Journal of Clinical Nutrition 68 (1998): 4985-5085
- Rebeka L Willson., et al. “Association between Maternal Zinc Status, Dietary Zinc intake and Pregnancy Complications: A Systematic Review”. Nutrients 8.10 (2016): 641.
- Benjamin W Chaffee and Janet C King. “Effect of Zinc Supplementation on Pregnancy and infant Outcomes”. Paediatric and Perinatal Epidemiology 26.0-1 (2012): 118-137.
- Yue Ma., et al. “Relationship between serum zinc level and Preeclampsia: A Meta-Analysis”. Nutrients 7.9 (2015): 7806-7820.
- Ellen CG Grant. “Risk factors for preeclampsia at antenatal booking; systematic review of controlled studies”. BMJ (2005): 330-365.
- Janet C King. “Determinations of maternal zinc status during pregnancy”. The American Journal of Clinical Nutrition 71 (2000): 1334S-1343S.
- Jena D Hamadani, George J Fuchs, Saskia JM Osendarp, Syed N Huda and Sally M Grantham-Mc. Greor. The Lancet 360.9329 (2002) 290-294
- Lap Lambert. "Zinc Level in Pre-eclamptic Pregnant Women". Academic Publishing (2012).
- Kelly D Larrabee and Manju Monga. "Women with sickle cell trait are at increased risk for preeclampsia”. American Journal Obstetrics and Gynecology 177(1997): 425-428.
- Rebecca L. Wilson., et al. “Association between Maternal Zinc Status, Dietary Zinc Intake and Pregnancy complications: A Systematic Review”. Nutrients 8.10 (2016): 641.
- H James MD. “Sickle cell disease in pregnancy”. Obstetrics-Gynecology and Women's Health (2014).
- CBS Dangi., et al. “Copper and Zinc Quotient in Haemoglobinopathies”. Biomedical & Pharmacology Journal 1 (2011): 165-173.
- Alison D Germand., et al. “Micronutrient deficiencies in pregnancy worldwide: health effects and prevention” Nature Reviews Endocrinology 12.5 (2016): 274-289
- Maria J Salgueiro., et al. “The role of zinc in the growth and development of children”. Nutrition 18.6 (2002): 510-519.
- Jayant D Deshpande., et al. “Zinc: The trace element of major importance in human nutrition and health”. International Journal of Medical Science and Public Health (2013).
- Thomaj SH. “Hemoglobinopathies and Pregnancy” EUE (2017).
Citation:
Shpresa Thomaj and Bledar Benja. “The Role of Zinc in Pregnant Women Suffering from Anaemia”. Gynaecology and Perinatology 2.5 (2019): 366-370.
Copyright: © 2019 Shpresa Thomaj. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.