Research Article
Volume 1 Issue 2 - 2017
Cardiovascular Disease Associated Risk Factors: A Possible Link Between Nutrition and Environmental Contamination
EuroEspes Biotechnology, Department of Health Biotechnology, Bergondo, Corunna, Spain
*Corresponding Author: Dr. Valter R.M. Lombardi, EuroEspes Biotechnology, Dept. of Health Biotechnology. Polígono Industrial de
Bergondo; C/. Parroquia de Guísamo s/n, Parcela A6, NaveF, 15166 - Bergondo, La Corunna, Spain.
Received: May 25, 2017; Published: June 05, 2017
Abstract
Human and animal health, safety, and environmental concerns are and will remain to be a top priority. However, there is often a gap between public perceptions of risk and realities of risk as evaluated by scientific methods. Living organisms are exposed daily to various concentrations of chemicals present in the environment that reach sensitive tissues. On the basis of their biologic action these chemicals can be classified as: irritants, asphyxiants, narcotics, systemic poisons, carcinogens, mutagens, teratogens and sensitizers and they can have damaging effects on health even when they are below the “safe-level” for individual chemicals. Human exposure to environmental chemicals is inevitable, but there is evidence that several pollutants, such as polychlorinated biphenyls (PCBs), accumulating in the fatty tissue of living organisms including humans, are directly involved in the development of chronic diseases, such as neurodegenerative and metabolic disorders, cardiovascular disease (CVD), type 2 diabetes, obesity, and some types of cancer. Knowledge of the effects of environmental toxicants on health is an essential background component for establishing effective programs to provide adequate protection to the biological community of interacting organisms and their physical environment. New data now implicate the importance of an individual’s nutritional status and the use of protective bioactive food components to decrease the overall toxicity of environmental pollutants to biological systems. It has been proven that fresh fish, vegetables, mushrooms, medicinal herbs, herbal teas, omega 3 fat acids (n-3 FAs), complex carbohydrates, yogurt, kefir and seaweed, by stimulating T-cells and other immune cells, are important modulators of inflammatory and antioxidant pathways, especially with regard to environmental insults. Interestingly, novel research now shows that bioactive food components that are environmentally friendly can also be integrated into remediation technologies, which in turn allow for more sustainable, inexpensive, and effective pollutant removal and detoxification. The aim of the present work was to review the current knowledge in this subject at the interface of nutritional and environmental sciences. It is to be hoped that implementing bioactive molecules in both biomedical and environmental science settings, together, will allow for decreased overall body burden and human toxicity of a multitude of pollutants.
Keywords: Cardiovascular Disease; PCBs; Nutrition
PCBs: Chemistry, Properties and Uses [1]
Exposure to environmental chemicals plays a significant role in the pathophysiology of many human and animal diseases, particularly dietary intake. PCBs are a family of 209 homologue or isomeric chemically related compounds and the individual substances are called PCB congeners. Th estructure of PCBs consist of two connected benzene rings (biphenyl), in which one or more of the 10 hydrogen atoms are substituted by chlorine (Figure 1). These compounds were used in an extreme variety of industrial and consumer applications: coolants, pesticide extenders, caulking sealants, carbonless copy paper, wood floor finishes and many other products. Although PCBs were banned in the United States in the 1970s, and most other industrialized countries since then, they are extremely stable chemicals and still pose toxicological risks due to their resistence to environmental degradation [2].
Exposure to environmental chemicals plays a significant role in the pathophysiology of many human and animal diseases, particularly dietary intake. PCBs are a family of 209 homologue or isomeric chemically related compounds and the individual substances are called PCB congeners. Th estructure of PCBs consist of two connected benzene rings (biphenyl), in which one or more of the 10 hydrogen atoms are substituted by chlorine (Figure 1). These compounds were used in an extreme variety of industrial and consumer applications: coolants, pesticide extenders, caulking sealants, carbonless copy paper, wood floor finishes and many other products. Although PCBs were banned in the United States in the 1970s, and most other industrialized countries since then, they are extremely stable chemicals and still pose toxicological risks due to their resistence to environmental degradation [2].
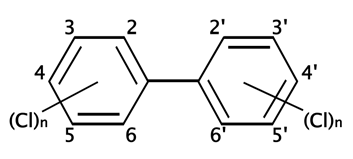
Figure 1: Basic PCB chemical structure. PCBs consist of a biphenyl (two benzene rings with a carbon to carbon bond between carbon 1 on one ring and carbon 1' on the second ring) with a varying number of chlorines. The possible positions of chlorine atoms on the benzene rings are indicated by numbers assigned to the carbon atoms. By D.328 - Own work, CC BY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=1048994.
Once released into the environment, PCBs can be transported via environmental media and migratory species far from the site of production and use. PCBs are ubiquitous in the environment and are found in biota, air, soil, sediment, and water worldwide, including in polar regions and deep oceans. PCB concentrations vary by several orders of magnitude. Furthermore, congener patterns differ to varying degrees in air, water, sediments and soils as a consequence of transport, and transformation processes such as dechlorination. In the environment, PCBs volatilize easily, or are ingested by fish and other animals and transferred to the food chain, where their concentration may increase. The general population is exposed primarily through ingestion of contaminated food [3]. Food can become contaminated with PCBs by: (i) uptake from the environment by fish, birds, livestock; (ii) contamination of the foodstuffs through usual practice or industrial processing; and (iii) accidental contamination. In contrast to vegetables and crops, fatty foods typically contain high concentrations of PCBs. Most foodstuffs will have a shift in the congener profile in favour of less volatile, more highly chlorinated congeners. Although it has been decades since their extensive use, PCBs are still found at sometimes extremely high concentrations in animals and humans around the world. PCB circulates and accumulates in the body associated with lipids and lipoproteins. The highest concentrations of PCB occur in fat-rich tissues like adipose tissue and human milk. At “steady-state” where the rate of uptake and rate of elimination of PCB are the same, the PCB concentration will approximately be the same in blood, muscle, adipose and milk, if the levels are reported on lipid basis. Though the levels of PCB in fats are about the same, adipose tissue has much higher fat % (about 80 %) and can contain 300-fold more PCB than the blood serum/plasma (lipid content about 0.3 %) and in that way regulate and dilute the blood-PCB. Mature human milk has a fat % of about 3.5 % while colostrum only contains 1-2 % lipids. Since PCBs tend to accumulate in fatty tissues, they are a common contaminant in human breast milk [4].
Animal studies dating from as far back as the 1970’s have demonstrated that exposure to dioxin and PCBs can result in a variety of health effects. From controlled studies of rats and non-human primates there is significant evidence that exposure to both mixtures of PCBs (e.g., Arochlor 1254) as well as single PCB congeners (e.g., PCB 126) results in hepatomegaly, enzyme induction, and elevation in the levels of serum lipids (cholesterol and triglycerides). PCBs have been shown to induce the liver to make more of the enzymes that synthesize lipids, and to cause an increase in plasma triglycerides and a decrease in high density lipoproteins. A major source of human exposure to PCBs is through dietary intake of contaminated foods and through inhalation of airborne pollutants [5].
There is a number of reports indicating that exposure to persistent, fat-soluble chlorinated organics, such as dioxins, furans, PCBs and chlorinated pesticides, including dichloro-diphenyl-trichloroethane (DDT) and its metabolite, dichloro-diphenyl-dichloro-ethylene (DDE), is associated with an elevation of serum lipids. In humans, gastrointestinal absorption of PCBs was estimated to vary from 50% of the ingested amount to close to 100%, the absorption decreasing as the number of chlorine atoms of the congener increased. Because most halogenated POPs, including PCBs, are lipid soluble, they easily accumulate in human tissues, leading to a perpetually increasing disease risk throughout a life span, especially in overweight populations [6].
A hallmark of the pathology of vascular diseases, including atherosclerosis, includes a change in the cellular redox status and a resultant increase in oxidative stress, which favors chronic and low level inflammation [7]. PCBs induce production of reactive oxygen species, activationof NF-κB transcription factors, and suppression of plasma membrane proteins, constituents of gap, adherens, and tight junctions, all of which may play a significant role in tumour promotion and progression [8]. PCBs can increase cellular oxidative stress and induce inflammatory parameters such as inflammatory cytokines, chemokines, and adhesion molecules in the vascular endothelium, which are metabolic events that foster an inflammatory response and atherosclerosis [9]. Through these pro-inflammatory mechanisms, PCBs and related environmental toxicants have been correlated with increased risk of multiple human chronic disease phenotypes including myocardial infarction, diabetes, stroke, and hypertension [10-12].
PCBs and CVD Risk Factors
Hypertension, Type 2 diabetes, Obesity and Dyslipidemia
Hypertension, which affects one billion people worldwide, is the most important and well-known risk factor for CVD, the leading cause of death in the world. It is estimated that in 2013 hypertension was responsible for > 45% of mortality caused by heart disease, 51% of mortality caused by stroke, and it contributed to 12.8% of deaths worldwide. Hypertension is often considered a lifestyle disease, and its prevention has been focused entirely on the change in behavioral factors, such as diet, high alcohol consumption, smoking, stress, and physical inactivity.
Hypertension, Type 2 diabetes, Obesity and Dyslipidemia
Hypertension, which affects one billion people worldwide, is the most important and well-known risk factor for CVD, the leading cause of death in the world. It is estimated that in 2013 hypertension was responsible for > 45% of mortality caused by heart disease, 51% of mortality caused by stroke, and it contributed to 12.8% of deaths worldwide. Hypertension is often considered a lifestyle disease, and its prevention has been focused entirely on the change in behavioral factors, such as diet, high alcohol consumption, smoking, stress, and physical inactivity.
CVD is a disease that encompasses a group of disorders of the heart and blood vessels including coronary heart disease, cerebrovascular disease, peripheral arterial disease, rheumatic heart disease, congenital heart disease, deep vein thrombosis, and pulmonary embolism [13]. The World Health Organization estimates that 17.3 million people died from cardiovascular diseases (CVD) in 2008 alone, accounting for 30% of global deaths and serving as the number one cause of death globally [14]. Several risk factors and associated diseases including type 2 diabetes, hypertension, obesity, sedentary lifestyle, and over nutrition can contribute to the pathology of CVD. However, these lifestyle changes alone fail to completely justify the current magnitude and disease burden of hypertension in most countries. Consequently, more attention has been paid to the growing body of evidence linking the risk of CVD and hypertension with additional exogenous factors, such as PCBs. Considering the significant burden that CVDs have on global mortality, it is imperative to explore and assess factors that may promote or exacerbate the pathogenesis of these diseases [15].
Many factors are suspected in the global surge in type 2 diabetes, among them exposures to toxic chemicals including PCBs [16]. These studies involve a variety of cohorts and subject ages, suggesting that PCB exposure increases type 2 diabetes risk regardless of age or cohort. In addition to type 2 diabetes, several studies showed that women with high PCB levels in early pregnancy had a higher risk for gestational diabetes. Serum concentrations of PCB 138 and PCB 180 were associated with increased 2-hour glucose levels and PCB 180 with increased immunoreactive insulin levels, suggesting that PCBs contribute to insulin resistance in these mothers [17]. Gestational diabetes, characterized by the development of any degree of glucose intolerance in mothers during pregnancy, occurs during approximately 7% of pregnancies, or 200,000 cases annually. Women who develop gestational diabetes are at an increased risk for the development of type 2 diabetes after pregnancy [18].
While the molecular mechanisms that regulate the development of type 2 diabetes from PCB exposure remain elusive, several recent studies have shed light on this subject. Some, like the gestational diabetes paper, have proposed the development of insulin resistance as the mechanism by which PCBs cause type 2 diabetes [19]. One recent study suggests that PCBs exhibit toxic effects by adversely affecting insulin producing β-cells in the pancreas as opposed to simply decreasing insulin sensitivity [20]. Additional studies have reported no association with exposure and insulin resistance [21], suggesting a PCBs effect through toxicity β-cell rather tan through decreased insulin sensitivity. Cross-sectional studies among adults have reported a positive association of fasting glucose with PCB exposure, suggesting a long-term toxic effect on β-cells [22]. It has also been suggested that PCBs may modulate adiponectin levels, leading to increased rates of type 2 diabetes development [23]. Adiponectin is a hormone produced by adipocytes that is significantly reduced in patients with insulin resistance, and is suggested as a strong predictor of type 2 diabetes. A negative correlation between levels of adiponectin and PCB 153 concentrations in human plasma, which may explain the role of PCB exposure in the development of type 2 diabetes, has been described [23]. Additionally, PCB exposure has been associated with a decreased production of insulin growth factor I, providing another potential link between exposure to environmental pollutants such as PCBs and increased risk for the development of type 2 diabetes [24].
Overweight and obesity around the world are becoming epidemic and contribute to other madly co-morbidity. Around 3.4 million adults die each year as a result of being overweight or obese. In USA, based on the report from May 2014 by CDC, 69.0% of adults age 20 years and over are overweight, including obesity, and 18.4% children (12–19 years) are obese, and even higher than Japan and South Korea (4% each). Population studies revealed that high-caloric diet and lifestyle changes set in motion this towards epidemic. The behavioral and genetic factors does not suffice enough to make this an epidemic, therefore this genetic susceptibility have to represent heritable variation upon which environmental factors exert their influences. There is growing evidence that perturbations of central endocrine regulatory systems by PCBs exposure during early gestation may contribute to the development of obesity in later life. The field of PCBs was declared a high research priority by the WHO in 2010. The far reaching effects of such PCBs in developing endocrine-related diseases among children have now been observed worldwide, as well as in the United States.
While atherosclerosis and hypertension are the most common risk factors for the development of cardiovascular disease, obesity serves as a primary modulator of these diseases and has been implicated heavily in CVD development [25]. Limited information has implicated PCB exposure in the etiology of obesity development, with primary correlations drawn from laboratory studies [26], as well as from multiple epidemiological studies that have shown an association that warrants further examination [27].
Epidemiological studies into the association of PCB exposure and the development of obesity primarily have relied upon the comparison of serum PCB levels and body mass index (BMI) or birth weight, although studies have yielded mixed results. An interesting prospective study of 12,313 non-obese participants in the Seguimiento Universidad de Navarra (SUN) cohort showed that after a median 8.1 years there were 621 new incidences of obesity among these participants, with a direct correlation seen between increased dietary PCB intake (estimated from an earlier study of dioxin-like PCB concentrations in food) and incidences of obesity. Additional studies of adolescent and adult patients have reported total PCB56b, [28] and congener-specific [29] obesity modulation, while others have found no correlation [30] or even an inverse association, [31] indicating the complexity of this question. These discrepancies, and especially data indicating an inverse relationship between obesity rates and level of PCB exposure, are likely due to bioaccumulation of highly lipophilic PCBs in human and animal fat tissue [32], leaving low and/or inconsistent levels of PCB remaining in serum samples for analysis. This suggests that serum PCB concentration measurement may not provide an accurate estimate of a subject’s PCB exposure or body burden that contributes to obesity risk factors. Recent weight loss or gain may also significantly alter the measured concentration of PCBs, which would also impact findings. While there are still many questions about the role of PCBs in modulating obesity risk factors, growing epidemiological evidence along with in vitro and in vivo findings have implicated PCBs as a part of a much larger group of environmental pollutants that act as obesogens, or chemicals capable of inappropriately activating molecular pathways that induce adipocyte differentiation and lead to a predisposition to obesity through dysfunctional weight-control [33].
Dyslipidemia is recognized as a prominent risk factor for CVD. Efforts aimed at primary prevention offer the greatest opportunity for reducing the onset and burden of CVD. While lowering LDL-C with a statin remains the cornerstone in the management of dyslipidemia, this approach may be insufficient in patients with other lipid abnormalities including low HDL-C and elevated triglycerides (TG), both of which are independently associated with CVD risk [34]. Lifestyle interventions that include reduction in total calories as well as intake of saturated and trans fats that are coupled to increased physical activity with associated weight loss continue to play an important role in controlling mixed dyslipidemia [35]. In addition, a combination lipid-modifying therapy is a potentially useful strategy for achieving lipid targets in patients with mixed dyslipidemia.
Similar to findings on the association between obesity and PCB exposure, the most compelling and complete evidence comes from laboratory studies [36]. For instance, ApoE (-/-) mice injected with PCB 77 exhibited increased serum cholesterol and atherosclerosis. Although the small volume of epidemiological literature directly examining a potential causal relationship between dyslipidemia and PCB exposure is certainly a limiting factor, serum lipid measurements are widely published within much larger analyses that implicate environmental stressors more broadly in metabolic dysfunction. Despite these limitations, though, there is epidemiological evidence suggesting that PCB exposure may contribute to dyslipidemia [37].
Among the contributors to dyslipidemia, elevated TG levels has been most consistently associated with PCB exposure [38]. Increases in both LDL-C [39] and total colesterol [40] have also been reported, although there are findings of no correlation [38]. An inverse relationship between PCB exposure and HDL-C has also been shown [39] which is significant considering that increasing levels of HDL-C are associated with a decrease in CVD risk [41].
3. Nutrition and Industrial Chemical-Induced Toxicity [42]
Harmful diet-related effects to human health resulting from exposure to environmental pollutants
There is a large collection of evidence pointing to the role that a person’s dietary makeup and eating habits can play in the promotion of chronic inflammation, and it is becoming increasingly clear that chronic inflammation is the root cause of many serious illnesses including heart disease, many cancers, Alzheimer’s disease, metabolic disorders and vascular diseases [43]. There is a growing body of knowledge that PCBs can initiate inflammatory responses in vivo, and this inflammation can be either exacerbated or ameliorated by nutrition [44]. Data indicate that diets high in certain dietary lipids such as omega-6 fatty acids can worsen PCB-induced vascular toxicity while diets enriched with bioactive food components such as polyphenols and n-3 FAs can improve the toxicant-induced inflammation. Multiple research groups have shown negative interactions between unhealthy or refined diets and environmental pollutants ranging from heavy metals to PCBs.
Harmful diet-related effects to human health resulting from exposure to environmental pollutants
There is a large collection of evidence pointing to the role that a person’s dietary makeup and eating habits can play in the promotion of chronic inflammation, and it is becoming increasingly clear that chronic inflammation is the root cause of many serious illnesses including heart disease, many cancers, Alzheimer’s disease, metabolic disorders and vascular diseases [43]. There is a growing body of knowledge that PCBs can initiate inflammatory responses in vivo, and this inflammation can be either exacerbated or ameliorated by nutrition [44]. Data indicate that diets high in certain dietary lipids such as omega-6 fatty acids can worsen PCB-induced vascular toxicity while diets enriched with bioactive food components such as polyphenols and n-3 FAs can improve the toxicant-induced inflammation. Multiple research groups have shown negative interactions between unhealthy or refined diets and environmental pollutants ranging from heavy metals to PCBs.
There is clear evidence that bacteria-dependent metabolism of pollutants modulates the toxicity for the host. Conversely, environmental contaminants from various chemical families have been shown to alter the composition and/or the metabolic activity of the gastrointestinal bacteria, which may be an important factor contributing to shape an individual’s microbiotype. The physiological consequences of these alterations have not been studied in detail but PBCs-induced alterations of the gut bacteria are likely to contribute to their toxicity. However, it is unclear how the gastrointestinal tract (GIT) microbiota and environmental chemicals interact and whether these interactions are relevant for human health. In a recent review, it was suggested that GI microbes might affect obesity and diabetes by altering the absorption, disposition, metabolism and excretion of environmental chemicals [45].
Animal fats such as those found in beef and chicken are significant sources of proinflammatory n-6 FAs as well as PCBs and other related POPs [46]. Mechanistically, synergism between unhealthy diets (e.g., highfat/high caloric diets) and toxicants makes logical sense because both factors act upon many of the same receptors and cell signaling pathways. For example, n-6 FAs and PCBs have both been implicated in inflammatory initiation through AhR, cytochrome P450’s, and toll-like receptors (TLRs) [47]. With the growing rates of obesity and chronic oxidative and inflammatory stress, it is important to elucidate interactions between proinflammatory diets and toxicants. Although it does appear that unhealthy nutrition can work in concert with PCBs and related toxicants to create an increasingly proinflammatory phenotype, a new paradigm has emerged implicating certain bioactive food components in protection against persistent organic pollutant-induced vascular toxicity.
Nutrition and pro-inflammatory PCBs
PCBs induce chronic oxidative stress and disregulated inflammatory responses through immunosuppression and immune stimulation/inflammation. Epidemiological studies show that PCBs are associated with modification of both innate and adaptive immunity, including having effects on immune cells and signaling molecules, with implications for both immune response and initiation. Such effects are manifested as an increased incidence of infections; insufficient antibody response to vaccination; and changes in immune organs, lymphocyte subsets, and lymphocyte function, as well as in an increase in the incidence of ulcerative colitis (UC) and Crohn’s disease (CD) [48].
PCBs induce chronic oxidative stress and disregulated inflammatory responses through immunosuppression and immune stimulation/inflammation. Epidemiological studies show that PCBs are associated with modification of both innate and adaptive immunity, including having effects on immune cells and signaling molecules, with implications for both immune response and initiation. Such effects are manifested as an increased incidence of infections; insufficient antibody response to vaccination; and changes in immune organs, lymphocyte subsets, and lymphocyte function, as well as in an increase in the incidence of ulcerative colitis (UC) and Crohn’s disease (CD) [48].
Diet and nutrition have played an important role in maintaining physiological homeostasis. Recent literature emphasizes potential therapeutic effects of micronutrients found in natural products, indicating positive applications for controlling the pathogenesis of chronic diseases driven by a chronic inflammatory status [49]. Nutritional compounds which display anti-inflammatory and antioxidant effects have specific applications in preventing oxidative stress-induced injury which characterizes their pathogenesis. Patient control over diet and disease has been demonstrated in diabetes mellitus, CVD, rheumatology, carcinogenesis and other diseases [50]. Polyphenolic compounds are ubiquitous dietary components, mainly flavonoids and tannins that have been shown to decrease toxicant-induced diseases including liver diseases, tumor formation and growth, and endothelial cell activation [51-53]. By using different in vitro and in vivo models we were able to show that natural extracts can have a potential therapeutic effect [54,55].
n-3 FAs such as docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA) are the main components of fish oil and have been shown to decrease inflammation, reduce vascular diseases, and protect against dioxin and PCB-mediated toxicity [56]. Unfortunately, most Western diets lack adequate levels of n-3 FAs, and the ratio of unhealthy n-6 FAs to n-3 FAs is very high [57]. Experimentally, it was determined that diets predominantly made up of oils rich in linoleic acid (n-6 FA) increased PCB-induced cellular dysfunction, but this negative effect was blunted as the ratio favored protective omega-3’s [58]. Interestingly, it has been shown that mice fed a DHA-supplemented diet and subsequently exposed to coplanar PCB exhibited a more profound antioxidant response as observed by higher expression levels of protective heme-oxygenase 1 (HO-1) and NAD(P) H:quinoneoxidoreductase 1 (NQO1) [59]. This work illustrates that n-3 FAs may help to protect against PCB-induced vascular toxicity by allowing for a more efficient and intense endogenous protective response that utilizes multiple physiological cell signaling pathways.
Nutrition, GIT and Environmental Health
The GIT is the site with the highest presence of microorganisms (also referred to as “the microbiota”) that work in a symbiotic fashion with the host and one of the most important interface organs between the environment and interior milieu, contributing to metabolism, immune response, detoxification and intestinal architecture. Gut epithelial cells are thus the first cells to be exposed to nutrients and the microbiota, with complementary functions between the small intestine aiming at digestion and nutrient absorption and the large intestine specialized in the fermentation of undigested materials. The gut epithelium is also the first line of defense and protection. Its action is complementary to that of the associated mucosal immune system whose development and maintenance are induced by the microbiota [60]. Thus gut epithelial cells - enterocytes and colonocytes - are polarized key players influenced by food components, pathogens, environmental toxicants and body metabolism and functions. The gut epithelium has developed over time various mechanisms for sensing not only nutrients but also microbial structural components, metabolites or secreted molecules, and all these mechanisms make the molecular basis of the crosstalk between the host and the gut microbiota at the epithelial level.
The GIT is the site with the highest presence of microorganisms (also referred to as “the microbiota”) that work in a symbiotic fashion with the host and one of the most important interface organs between the environment and interior milieu, contributing to metabolism, immune response, detoxification and intestinal architecture. Gut epithelial cells are thus the first cells to be exposed to nutrients and the microbiota, with complementary functions between the small intestine aiming at digestion and nutrient absorption and the large intestine specialized in the fermentation of undigested materials. The gut epithelium is also the first line of defense and protection. Its action is complementary to that of the associated mucosal immune system whose development and maintenance are induced by the microbiota [60]. Thus gut epithelial cells - enterocytes and colonocytes - are polarized key players influenced by food components, pathogens, environmental toxicants and body metabolism and functions. The gut epithelium has developed over time various mechanisms for sensing not only nutrients but also microbial structural components, metabolites or secreted molecules, and all these mechanisms make the molecular basis of the crosstalk between the host and the gut microbiota at the epithelial level.
An emerging paradigm implicates healthful nutrition as an effective protective modulator of environmental toxicant-induced inflammation and human disease. Several studies have shown the decreased toxicities of environmental pollutants due to bioactive nutrients such as n-3 FAs. The n-3 FAs EPA and DHA, found in fatty fish and fish oil supplements, suppress the production of proinflammatory eicosanoids and stimulate the synthesis of anti-inflammatory eicosanoids (lipoxins) from arachadonic acid. n-3 FAs also reduce the generation of the proinflammatory cytokines TNF-alpha, IL-1 beta, IL-6, and IL-8. In addition, EPA and DHA can be converted to compounds known as resolvins, which inhibit proinflammatory signaling. Unfortunatelly, many of these studies rely on in vitro assays that lack the complexity of a whole body organismal approach. Importantly, emerging classes of bioactive food components such as polyphenols also have been shown to modulate the pro-inflammatory effects of environmental toxicants.
Numerous lines of evidence suggest that dietary polyphenols such as resveratrol, (-)-epigallocatechin-3-gallate (EGCG), curcumin and quercetin have the capacity to mitigate age-associated celular damage, can decrease toxicant-induced oxidative stress and inflammation in multiple cell types, tissues and animal species [61].
The toxic effects of PCBs have been primarily associated with the activation of the Aryl Hydrocarbon Receptor (AHR) and subsequent induction of responsive genes, such as cytochrome P450 CYP1A1 [62]. In endothelial cells, EGCG and quercetin reduced PCB-mediated increase in AhR-DNA binding activity and expression of CYP1A1 [63], whereas in the liver of mice fed with green tea and subsequently exposed to PCB-126 an upregulation of AhR was observed [64]. In a work using rats treated with the potent AhR agonist PCB-169, feeding caffeic acid derivatives (chlorogenic acid, ferulic acid and rosmarinic acid) increased the level of hepatic glutathione and antioxidant enzyme activities, and protective effects were observed in reducing CYP1A1 activity and oxidative damage markers [65].The anti-cancer potential of dietary flaxseed (linseed) [66] has been underlined and secoisolariciresinol, a phytoestrogen flax lignan, was recently reported to suppress morphological abnormalities in zebra fish embryos exposed to PCB-126 [67].
Diet can be a source of nutrients essential or auxiliary to our physiological detoxification processes, but food can simultaneously be a dangerous vehicle of toxic pollutants. Despite that nutritional or pharmacological therapies may give (some) protection against toxic chemicals, we cannot fail to point out that prevention through the use of adequate agricultural practices, hygienic practices in food handling and the definition and implementation of environmental, food and health policies are crucial to minimize the risk of developing diseases. Preventing and treating the sources of toxicants in food production, water supplies, indoor and ambient air, industrial and other workplaces, must always be the first line of action in environmental health. However, potentially toxic chemicals will never be completely avoided and the possibility that nutritional factors modify the toxic outcomes of pollutants should be considered in the complex interaction of environment (including nutrition) and health (Figure 2).
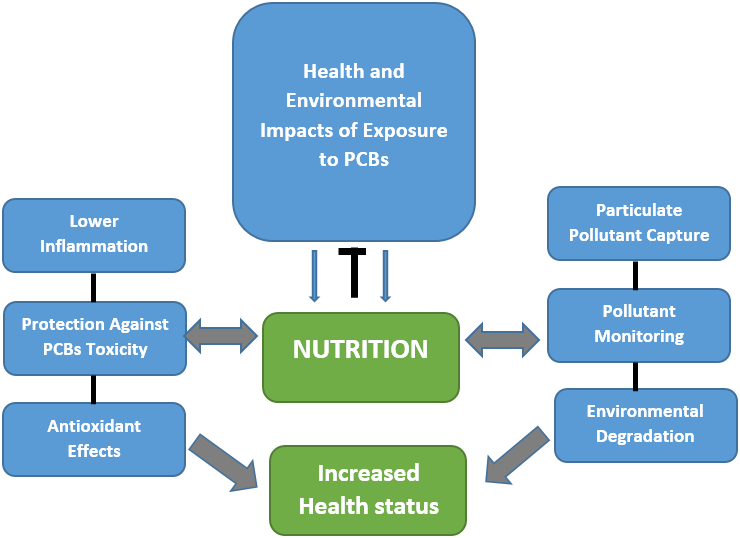
Figure 2: Illustration of nutrition as a modulator in the interplay of health risks associated with environmental exposure and toxicity to PCBs. A possible protective role of diet-derived bioactive compounds through their antioxidant and anti-inflammatory properties is suggested considering that the use of natural and inherent traits of plants and associated microbes can also participate in a remediation of environmental contaminant including their role in PCBs capture and sensing technologies, thus allowing to contain, remove, and/or destroy contaminants of concern from the environment and finally to protect human health.
Current Status and Research Needs
One critically important area in need of further investigation involves the metabolism of protective nutrients, and to our knowledge, there are no reported clinical trials at the moment on the safety and efficacy of bioactive natural compounds in protection against specific environmental toxicants. With the advent of more precise and high-resolution analytical techniques, researchers have finally begun to elucidate truly causative bioactive food components. In a physiological system, both nutrients and xenobiotics interact with similar cell signaling molecules and pathways. Both nutrients and toxicants are impacted by all aspects of absorption, distribution, metabolism and excretion, and understanding how bioactive compounds are altered or influenced at each step will allow for more efficient biomodulation. Specifically, the detection and identification of bioactive metabolites is of utmost importance due to the fact that many parent compounds are altered and modified in vivo.
One critically important area in need of further investigation involves the metabolism of protective nutrients, and to our knowledge, there are no reported clinical trials at the moment on the safety and efficacy of bioactive natural compounds in protection against specific environmental toxicants. With the advent of more precise and high-resolution analytical techniques, researchers have finally begun to elucidate truly causative bioactive food components. In a physiological system, both nutrients and xenobiotics interact with similar cell signaling molecules and pathways. Both nutrients and toxicants are impacted by all aspects of absorption, distribution, metabolism and excretion, and understanding how bioactive compounds are altered or influenced at each step will allow for more efficient biomodulation. Specifically, the detection and identification of bioactive metabolites is of utmost importance due to the fact that many parent compounds are altered and modified in vivo.
In a recent investigation on a population with elevated concentrations of PCBs, fruit and vegetable intake was significantly associated with a reduced risk of developing type 2 diabetes [68]. In different toxicity intervention trials, dietary supplementation with silibinin [69] and green tea polyphenol extract [70] returned positive results, as did decaffeinated green tea in smokers [71]. Several studies demonstrate polyphenols can alleviate acute effects, following short-term toxic exposures, and there is increasing data suggesting a significant role in modulation of chronic or delayed effects associated to long-term (sub-clinical) exposures or to toxic events at critical time windows, including trans generational effects [72].
The potential to modify the chronic effects of toxic exposures is strengthened in the case of some cancers, cardiovascular and neurodegenerative complications [73-75] for which environmental chemicals give a contribution and, by other side, a decreased risk is associated to green food consumption, according to distinct epidemiological studies. Uncertainties still remain whether a single component from the diet or a combination of nutrients and dietary habits are more effective for a protective outcome. Although the safety and tolerability of bioactive compounds present in green foods in humans is generally very good, specific trials that can translate the preclinical data with environmental toxicants into new nutritional and therapeutic interventions are needed.
References
- Petriello MC., et al. “Modulation of persistent organic pollutant toxicity through nutritional intervention: emerging opportunities in biomedicine and environmental remediation”. The Science of the total environment 491-492 (2014): 11-6.
- Dziubanek G., et al.“Preliminary study of possible relationships between exposure to PCDD/Fs and dl-PCBs in ambient air and the length of life of people”. Science of the Total Environment 598 (2017): 129-34.
- Dervilly-Pinel G., et al. “Micropollutants and chemical residues in organic and conventional meat”.Food Chemistry 232 (2017): 218-28.
- Lundqvist C., et al. “The effects of PCBs and dioxins on child health”. Acta paediatrica (Oslo, Norway : 1992)Supplement 95.453 (2006): 55-64.
- Rawn DFK., et al. “Dioxins/furans and PCBs in Canadian human milk: 2008-2011”. Science of the Total Environment 595 (2017): 269-278.
- Kim MJ., et al. “Fate and complex pathogenic effects of dioxins and polychlorinated biphenyls in obese subjects before and after drastic weight loss”. Environmental health perspectives 119.3 (2011): 377-83.
- Fort-Gallifa I.,et al. “Galectin-3 in Peripheral Artery Disease. Relationships with Markers of Oxidative Stress and Inflammation”. International Journal of Molecular Science 18.5 (2017) pii: E973.
- Tang L., et al.“PCB 118-induced endothelial cell apoptosis is partially mediated by excessive ROS production”. Toxicol Mechanisms and Methods 27.5 (2017): 394-399.
- Turunen AW., et al.“Fish consumption, omega-3 fatty acids, and environmental contaminants in relation to low-grade inflammation and early atherosclerosis”. Environmental Research120 (2013): 43-54.
- Kataria A., et al. “The effects of environmental chemicals on renal function”. Nature Reviews Nephrolology 11.10 (2015): 610-25.
- Perkins JT., et al. “Polychlorinated biphenyls and links to cardiovascular disease” Environmental Science and Pollution Research International 23.3 (2016): 2160-2172.
- Kim SA., et al.“Associations of organochlorine pesticides and polychlorinated biphenyls with total, cardiovascular, and cancer mortality in elders with differing fat mass”. Environmental Research 138 (2015): 1-7.
- Wang J., et al. “Novel biomarkers for cardiovascular risk prediction”. Journal of Geriatric Cardiology 14.2 (2017): 135-50.
- Organization WH. “Global status report on noncommunicable diseases 2010”. WHO (2011).
- Gholami F., et al. “The effect of dairy consumption on the prevention of cardiovascular diseases: A meta-analysis of prospective studies”. Journal of Cardiovascular Thoracic Research9.1 (2017): 1-11.
- Airaksinen R., et al. “Association between type 2 diabetes and exposure to persistent organic pollutants”. Diabetes care34.9 (2011): 1972-1979.
- Arrebola JP., et al. “Relationship between serum concentrations of persistent organic pollutants and markers of insulin resistance in a cohort of women with a history of gestational diabetes mellitus”. Environmental research 136. (2015): 435-440.
- American Diabetes Association. “Gestational diabetes mellitus”. Diabetes care 27 Suppl 1 (2004): S88-90.
- Hofe CR., et al. “Fruit and vegetable intake, as reflected by serum carotenoid concentrations, predicts reduced probability of PCB-associated risk for type 2 diabetes: NHANES 2003–2004”.Nutrition Research 34.4 (2014): 285–293.
- Jensen TK., et al. “Polychlorinated biphenyl exposure and glucose metabolism in 9-year-old danish children”. The Journal of clinical endocrinology and metabolism99.12 (2014): E2643-51.
- Jorgensen ME., et al. “A cross-sectional study of the association between persistent organic pollutants and glucose intolerance among Greenland Inuit”. Diabetologia51.8 (2008): 1416-1422.
- Gadupudi G., et al. “PCB126 Inhibits Adipogenesis of Human Preadipocytes”. Toxicol In Vitro29.1 (2015): 132–141.
- Park SH., et al.“Serum Levels of Persistent Organic Pollutants and Insulin Secretion among Children Age 7–9 Years: A Prospective Cohort Study”. Environmental Health Perspectectives 124.12 (2016): 1924–1930.
- Octavio P., et al.“The Relationship between Dioxin-Like Polychlorobiphenyls and IGF-I Serum Levels in Healthy Adults: Evidence from a Cross-Sectional Study”. PLoS One 7.5 (2012): e38213.
- Vincent G.,et al.“The pathophysiology of hypertension in patients with obesity”. Nature Reviews Endocrinology 10.6 (2014): 364–376.
- Banrida Wahlang, K., et al. “Polychlorinated Biphenyl 153 Is a Diet-dependent Obesogen Which Worsens Nonalcoholic Fatty Liver Disease In Male C57BL6/J Mice”. Journal of Nutritional Biochemistry 24.9 (2013): 1587-1595.
- Lee DH., et al. “Chlorinated Persistent Organic Pollutants, Obesity, and Type 2 Diabetes”. Endocrinology Reviews 35.4 (2014): 557-601.
- Lignell S., et al. “Prenatal exposure to polychlorinated biphenyls (PCBs) and polybrominated diphenyl ethers (PBDEs) may influence birth weight among infants in a Swedish cohort with background exposure: a cross-sectional study”. Environmental Health-Global 12 (2013): 44.
- Vainio H. and Weiderpass E. “Organochlorines in Swedish women: Determinants of serum concentrations”. Environmental health perspectives 111.3 (2003): 349-355.
- Glynn A., et al. “Determinants of serum concentrations of organochlorine compounds in Swedish pregnant women: a cross-sectional study.”Environmental Health Feb 1 (2007) 6:2.
- Ben Hassine S., et al. “Concentrations of organochlorine pesticides and polychlorinated biphenyls in human serum and their relation with age, gender, and BMI for the general population of Bizerte, Tunisia”. Environmental science and pollution research international21.10 (2014): 6303-6313.
- Givens ML., et al. “Maternal Exposure to Polybrominated and Polychlorinated Biphenyls: Infant Birth Weight and Gestational Age”. Chemosphere 69.8 (2007): 1295-1304.
- Grimm FA., et al. “Metabolism and metabolites of polychlorinated biphenyls (PCBs)”. Critical Reviews in Toxicology 45.3 (2015): 245-272.
- Baillie-Hamilton PF. “Chemical toxins: a hypothesis to explain the global obesity epidemic”. Journal of alternative and complementary medicine 8.2 (2002): 185-192.
- Huffman MD., et al. “Quantifying options for reducing coronary heart disease mortality by 2020”. Circulatio. 127.25 (2013): 2477-2484.
- Bell FP., et al. “Long-term effects of Aroclor 1254 (PCBs) on plasma lipid and carnitine concentrations in rhesus monkey”. Toxicology 89.2 (1994): 139-153.
- Lee DH., et al. “Low Dose Organochlorine Pesticides and Polychlorinated Biphenyls Predict Obesity, Dyslipidemia, and Insulin Resistance among People Free of Diabetes”. PLoS One 6.1 (2011): e15977.
- Baker EL., Jr., et al. “Metabolic consequences of exposure to polychlorinated biphenyls (PCB) in sewage sludge”. American Journal of Epidemiology 112.4 (1980): 553-563.
- Aminov, Z., et al. “Environmental Health Research, C., Analysis of the effects of exposure to polychlorinated biphenyls and chlorinated pesticides on serum lipid levels in residents of Anniston, Alabama”. Environmental health: a global access science source12 (2013): 108.
- Tokunaga S and Kataoka K. “A longitudinal analysis on the association of serum lipids and lipoproteins concentrations with blood polychlorinated biphenyls level in 135 chronic "Yusho" patients”. Hukuoka acta medica 94.5 (2003): 110-117.
- Linsel-Nitschke P and Tall AR. “HDL as a target in the treatment of atherosclerotic cardiovascular disease”. Nature reviews. Drug discovery4.3 (2005): 193-205.
- Tehrani R and Van Aken B. “Hydroxylated Polychlorinated Biphenyls in the Environment: Sources, Fate, and Toxicities”. Environmental and Science Pollution Research International 21.10 (2014): 6334-6345.
- Ganguly R and Pierce GN. “Trans fat involvement in cardiovascular disease”. Molecular nutrition & food research 56 .7 (2012): 1090-1096.
- Hennig B., et al. “Linoleic acid amplifies polychlorinated biphenyl-mediated dysfunction of endothelial cells”. Journal of biochemical and molecular toxicology13.2 (1999): 83-91.
- Lin L and Zhang J. “Role of intestinal microbiota and metabolites on gut homeostasis and human diseases”. BMC Immunology 18.1 (2017).
- Fresno M., et al. “Toll-like receptors, inflammation, metabolism and obesity”. Archives of physiology and biochemistry117.3 (2011): 151-164.
- Nebert DW and Karp CL. “Endogenous functions of the aryl hydrocarbon receptor (AHR): intersection of cytochrome P450 1 (CYP1)-metabolized eicosanoids and AHR Biology”. The Journal of biological chemistry283.52 (2008): 36061-36065.
- Heilmann C., et al. “Reduced Antibody Responses to Vaccinations in Children Exposed to Polychlorinated Biphenyls”. PLoS Medicine 3.8 (2006): e311.
- Brook E.,et al. “Nutrient composition and anti-inflammatory potential of a prescribed macrobiotic diet”.Nutrition and Cancer 67.6 (2015): 933-940.
- Minihane AM., et al.“Low-grade inflammation, diet composition and health: current research evidence and its translation”. British Journal of Nutrition 114.7 (2015): 999-1012.
- Park KII., et al. “Polyphenolic compounds from Korean Lonicera japonica Thunb. induces apoptosis via AKT and caspase cascade activation in A549 cells”. Oncology Letters13.4 (2017): 2521-2530.
- Petrick JL., et al. “Dietary intake of flavonoids and oesophageal and gastric cancer: incidence and survival in the United States of America (USA)”.British Journal of Cancer112.7 (2015): 1291-1300.
- Pasinetti GM., et al.“Roles of Resveratrol and Other Grape-Derived Polyphenols in Alzheimer’s Disease Prevention and Treatment”. Biochimica et Biophysica Acta 1852.6 (2015): 1202-1208.
- Lombardi VRM.,et al. “Effects of fish-derived lipoprotein extracts on activation markers, Fas expresión and apoptosis in peripheral blood lymphocytes”. International Immunopharmacology5.2 (2005): 253-262.
- Lombardi VRM., et al. “Prevention of Chronic Experimental Colitis Induced by Dextran Sulphate Sodium (DSS) in Mice Treated with FR91”. Journal of Biomedicine and Biotechnology(2012): 826178. doi: 10.1155/2012/826178.
- Turkez H., et al. “Ameliorative effect of docosahexaenoic acid on 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced histological changes, oxidative stress, and DNA damage in rat liver”. Toxicology and industrial health28.8 (2012): 687-696.
- Watkins BA., et al. “Dietary ratio of n-6/n-3 PUFAs and docosahexaenoic acid: actions on bone mineral and serum biomarkers in ovariectomized rats”. The Journal of nutritional biochemistry 17.4 (2006): 282-289.
- Wang L., et al. “Changing ratios of omega-6 to omega-3 fatty acids can differentially modulate polychlorinated biphenyl toxicity in endothelial cells”. Chemico-biological interactions 172.1 (2008): 27-38.
- Hofe CR., et al. “Fruit and vegetable intake, as reflected by serum carotenoid concentrations, predicts reduced probability of PCB-associated risk for type 2 diabetes: NHANES 2003–2004”.Nutrition Research 34.4 (2014): 285-293.
- Wang M. et al. “Impact of early gut microbiota on immune and metabolic development and function”. Seminars in Fetal Neonatal Medicine 21.6 (2016): 380-387.
- Choi YJ., et al.“Quercetin blocks caveolae-dependent pro-inflammatory responses induced by coplanar PCBs”. Environment international 36 .8 (2010): 931-934.
- Tian J., et al.“The Aryl Hydrocarbon Receptor: A Key Bridging Molecule of External and Internal Chemical Signals”. Environmental Science and Technology 49.16 (2015): 9518-9531.
- Vorrink SU., et al. “Hypoxia perturbs aryl hydrocarbon receptor signaling and CYP1A1 expression induced by PCB 126 in human skin and liver-derived cell lines”. Toxicology and Applied Pharmacology274.3 (2014): 408-416.
- Zhang W., et al. “PCB 126 and Other Dioxin-Like PCBs Specifically Suppress Hepatic PEPCK Expression via the Aryl Hydrocarbon Receptor”. PLoS One7.5 (2012): e37103.
- Bunaciu RP., et al. “The Effect of Dietary Glycine on the Hepatic Tumor Promoting Activity of Polychlorinated Biphenyls (PCBs) in Rats”. Toxicology239.3 (2007): 147-155.
- Lombardi VRM., et al. “In Vitro Screening for Cytotoxic Activity of Herbal Extracts”. Evidence-Based Complementary and Alternative Medicine (2017): 2675631.
- Glazer L., et al.“Delayed effects of developmental exposure to low levels of the aryl hydrocarbon receptor agonist 3,3′,4,4′,5-pentachlorobiphenyl (PCB126) on adult zebrafish behavior”. Neurotoxicology 52 (2016): 134-143.
- Donat-Vargas C., et al. “Association between dietary intakes of PCBs and the risk of obesity: the SUN Project”. Journal of Epidemiology and Community Health68.9 (2014): 834-841.
- Loguercio C and Festi D. “Silybin and the liver: From basic research to clinical practice” World Journal of Gastroenterology 17.18 (2011): 2288-2301.
- Kanwar J., et al. “Recent advances on tea polyphenols”. Frontiers in Bioscience (Elite Ed) 4 (2012): 111-131.
- Yuan JM. “Green tea and prevention of esophageal and lung cancers”. Molecular Nutrition & Food Research 55.6 (2011): 886-904.
- Li SH., et al. “The Acute Effects of Grape Polyphenols Supplementation on Endothelial Function in Adults: Meta-Analyses of Controlled Trials”. PLoS One8.7 (2013): e69818.
- Chung S., et al. “Cancer Prevention by Tocopherols and Tea Polyphenols”. Cancer Letters 334.1 (2013): 79-85.
- Khan N., et al. “Cocoa Polyphenols and Inflammatory Markers of Cardiovascular Disease”. Nutrients 6.2 (2014): 844-880.
- Khushwant S.,et al. “Polyphenols: Multipotent Therapeutic Agents in Neurodegenerative Diseases”. Oxidative Medicine and Cellular Longevity (2013).
Citation:
Valter RM Lombardi. et al. “Cardiovascular Disease Associated Risk Factors: A Possible Link Between Nutrition and Environmental
Contamination”. Nutrition and Food Toxicology 1.2 (2017): 70-81.
Copyright: © 2017 Valter RM Lombardi. et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.



































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.