Research Article
Volume 1 Issue 3 - 2017
Effects of Various Environmental Conditions on the Rancidity Levels of Some Edible Oil Sold In Lokoja Metropolis of Kogi State
Department of Biosciences, College of Natural and Applied Sciences. Salem University, Lokoja- Kogi State, Nigeria
*Corresponding Author: Ekpa Emmanuel, Department of Biosciences, College of Natural and Applied Sciences.Salem University, Lokoja- Kogi State, Nigeria.
Received: August 29, 2016; Published: July 14, 2017
Abstract
Acid value (A.V) and free fatty acid (FFA) were determined over a period of five days in seven samples of sun exposed and shade exposed edible oils sold within lokoja metropolis of Kogi state using the normal potassium hydroxide titrimetric method as indicators of rancidity. Edo palm oil displayed a higher rancidity value of 251.2 mg KOH/g and 207.5 for sun and shade exposure respectively on day 5. While Macco vegetable oil gave the lowest acid value of 8.19 mg KOH/g of all the sun exposed oils with time and the highest acid value of 223.9 mg KOH/g of shade exposed oils after five days. Igala and Edo palm oil tend to be more susceptible to rancidity with time. On the whole this work shows that oils exposed in the sun are prone to rancidity faster than shade exposed oils. And photo oxidation is faster/more damaging than microbial oxidation of lipids.
Introduction
Lipids are one of the major constituents of foods, and are important in our diet for a number of reasons. They are a major source of energy and essential nutrients (Abdallah., et al. 2006). Nevertheless, over-consumption of certain lipid components can be detrimental to our health, e.g. cholesterol and saturated fats. In many foods the lipid component plays a major role in determining the overall physical characteristics, such as flavor, texture, mouth feel and appearance. For this reason, it is difficult to develop low-fat alternatives of many foods, because once the fat is removed some of the most important physical characteristics are lost (Abrashev., et al. 2002). Finally, many fats are prone to lipid oxidation, which leads to the formation of off-flavors and potentially harmful products (Williams, 2000). Each type of fat has a different profile of lipids present which determines the precise nature of its nutritional and physico-chemical properties. The terms fat, oil and lipid are often used interchangeably by food scientists. Although sometimes the term fat is used to describe those lipids that are solid at the specified temperature, whereas the term oil is used to describe those lipids that are liquid at the specified temperature (Bahruddin, 2008).
Acid value (AV) is a common parameter in the specification of fats and oils. It is defined as the weight of potassium hydroxide (KOH) in milligram (mg) needed to neutralize the organic acids present in 1g of fat and it is a measure of the free fatty acids (FFA) present in the fat or oil. An increment in the amount of FFA in a sample of oil or fat indicates hydrolysis of triglycerides. Such reaction occurs by the action of lipase enzyme and it is an indicator of inadequate processing and storage conditions (i.e., high temperature, relative humidity, and tissue damage). The source of the enzyme can be the tissue from which the oil or fat was extracted or it can be a contaminant from other cells including microorganisms. Besides FFA, hydrolysis of triglycerides produces glycerol (Brimberg., et al. 2009). FFA are a source of flavors and aromas. On one side, we have short chain FFA which tend to be water soluble and volatile with characteristic smell. On the other side, we have long chain saturated and unsaturated fatty acids. The latter are more prone to oxidation in their free form and their breakdown products (aldehydes, ketones, alcohols, and organic acids) provide characteristic flavors and aromas. In most cases these flavors and aromas are considered a defect in oils, fats, and foods that contain them. However, there are instances where hydrolysis of triglycerides and oxidation of FFA are key in the development of desirable flavor and aroma in foods. This is the case of aged cheeses and some processed meat (Gertz., et al. 2007).
Rancidity is the chemical or microbial decomposition of fats, oils and other lipids. When these processes occur in food, undesirable odors and flavors can result. In some cases, however, the flavors can be desirable (as in aged cheeses).In processed meats, these flavors are collectively known as warmed over flavor. Rancidification can also affect the nutritional value of food. There are two basic types of rancidity that cause and/or contribute to the degradation of stored edible oils: oxidative and hydrolytic. Oxidative rancidity, known as auto oxidation, occurs when oxygen is absorbed from the environment. In the presence of oxygen and/or ultraviolet (UV) radiation, most lipids will break down and degrade, forming several other compounds. Oxygen is eight times more soluble in fats than it is in water; it is this exposure that is the main cause of the autoxidation process, increasing the saturation of the oil (Gordon., et al. 1995). Another degradation process is microbial rancidity, in which micro-organisms such as bacteria, molds and yeast use their enzymes to break down chemical structures in the oil, producing unwanted odors and flavors. Water needs to be present for microbial growth to occur.
Edible oil sold in the market comes from various sources and the length of time they stay before being sold varies. Also the condition of storage at home before usage affects their palatability and edibility. Due to the many application of these vegetable oil in both home and industrial processes, the need to study factors that enhance their quality becomes imperative. Also the parameters to be investigated in this work will help us in addressing both oxidative and microbial degradation of our common local oils. This is with a view to proffering better ways of preserving these oil samples for their intended use.
Materials and Method
Collection of sample
Different oil samples were purchased fresh from different markets within Lokoja metropolis of kogi state-Nigeria, and they include; Macco vegetable oil, Ify-ochi vegetable oil, Avon vegetable oil, Olive oil, Igala palm-oil, and Edo palm-oil.
Different oil samples were purchased fresh from different markets within Lokoja metropolis of kogi state-Nigeria, and they include; Macco vegetable oil, Ify-ochi vegetable oil, Avon vegetable oil, Olive oil, Igala palm-oil, and Edo palm-oil.
Test for Acid value and Free Fatty acid (Nedyalka., et al. 2001)
Two grams of each of the fresh oil samples were weighed into a standard conical flask and dissolved in 20 ml of Fat solvent (Ethanol: Ether 1:1), three drops of phenolphthalein was added and then titrated with standard KOH (0.975N) until the first pink color persists. Acid values and FFA were gotten from the calculation below:
Two grams of each of the fresh oil samples were weighed into a standard conical flask and dissolved in 20 ml of Fat solvent (Ethanol: Ether 1:1), three drops of phenolphthalein was added and then titrated with standard KOH (0.975N) until the first pink color persists. Acid values and FFA were gotten from the calculation below:
Principle behind the titration for Acid value and free fatty acid in edible oil
Titration technique is a laboratory method used to determine the unknown concentration of a reactant. Fat may become rancid after long storage, Fat or oil is hydrolyzed by different microorganisms with the formation of free fatty acids. The amount of free fatty acid present in the oil indicates the age and the quality of that oil. Thus, the high acid number will indicate that the oil is old and rancid.
Titration technique is a laboratory method used to determine the unknown concentration of a reactant. Fat may become rancid after long storage, Fat or oil is hydrolyzed by different microorganisms with the formation of free fatty acids. The amount of free fatty acid present in the oil indicates the age and the quality of that oil. Thus, the high acid number will indicate that the oil is old and rancid.
Results and Discussion
Discussion
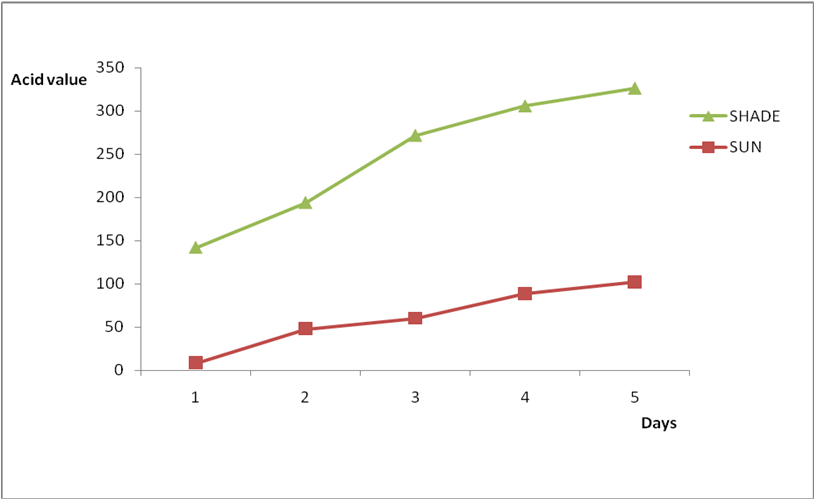
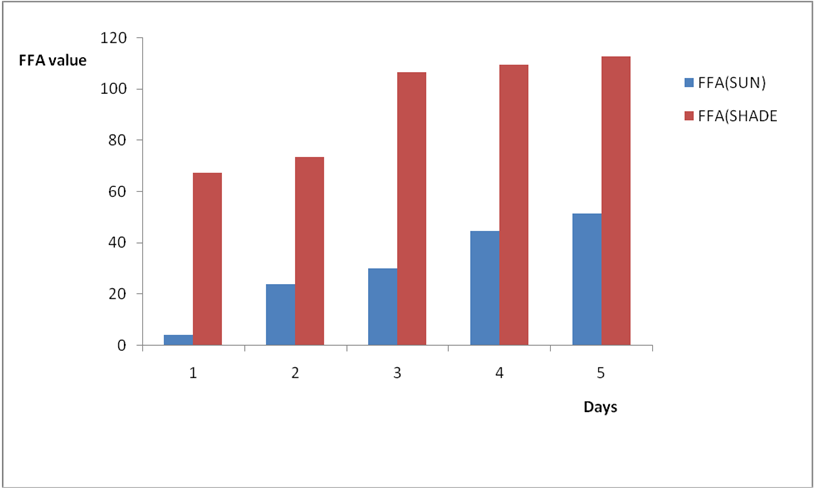
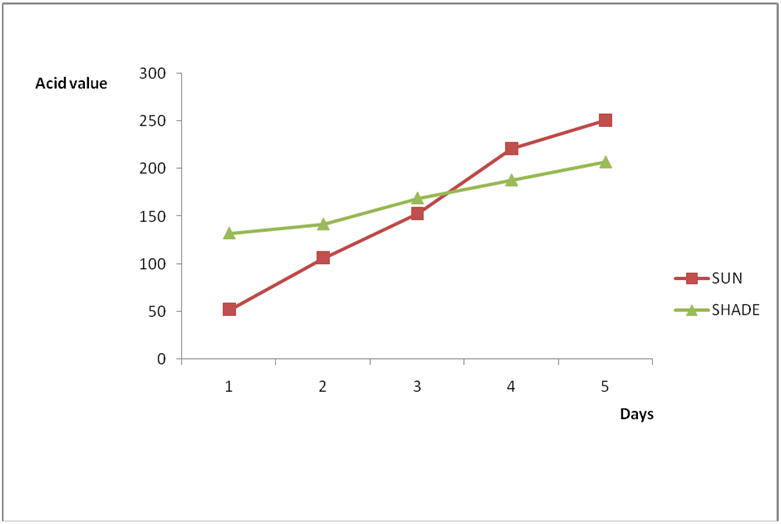
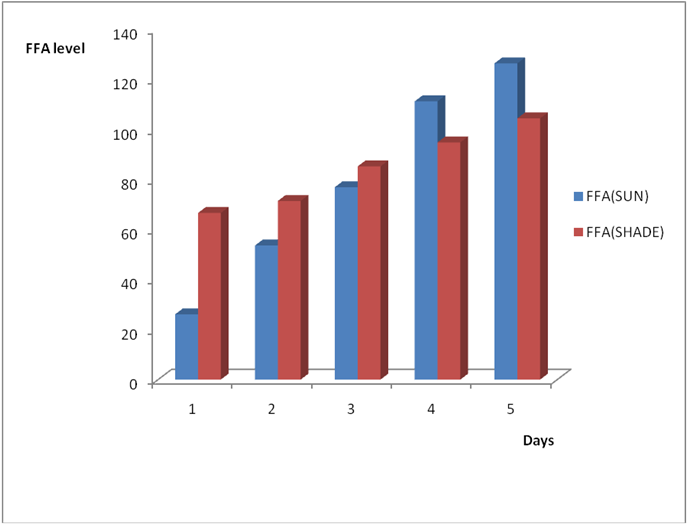
Effect of various environmental conditions (exposure in the sun and in shade) on the acid value of some edible oil sold within Lokoja metropolis was determined over a period of 5 days for each oil as shown in Figures 1a to 8c. Macco vegetable oil (Figure 1a and b) showed an acid value (A.V) of 8.19 and free fatty acid (FFA) of 4.1 for exposure to sun while A.V of 133.8 and FFA of 67.3 was under shade exposure. This trend was observed to be increasing from day 2 to day 5.The fresh sample that was not exposed has a low acid value of 2.73 and a low free fatty acid of 1.4.
Effect of various environmental conditions (exposure in the sun and in shade) on the acid value of some edible oil sold within Lokoja metropolis was determined over a period of 5 days for each oil as shown in Figures 1a to 8c. Macco vegetable oil (Figure 1a and b) showed an acid value (A.V) of 8.19 and free fatty acid (FFA) of 4.1 for exposure to sun while A.V of 133.8 and FFA of 67.3 was under shade exposure. This trend was observed to be increasing from day 2 to day 5.The fresh sample that was not exposed has a low acid value of 2.73 and a low free fatty acid of 1.4.
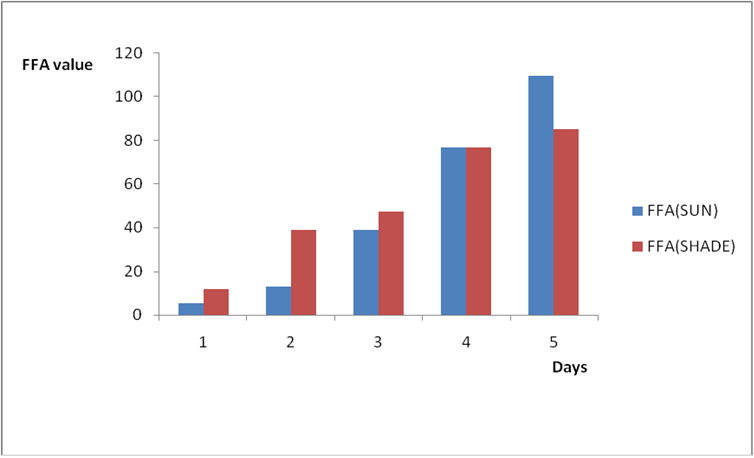
Figure 1b: Effect of time and environmental conditions on the free fatty acid level of Macco vegetable oil.
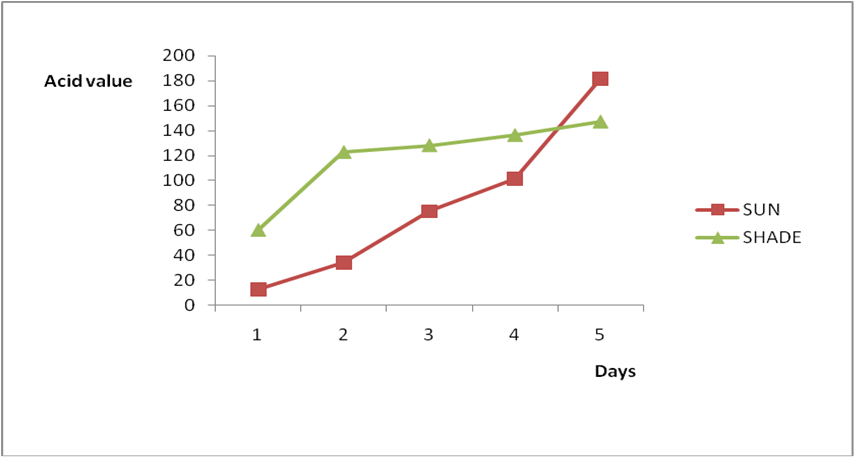
Results from all the other vegetable oils used also showed the same pattern in terms of their A.V’s and FFA’s on exposure with time in Figures 2a to 8c. This is because the rate of photo oxidation of oils generally is affected by factors like oxygen, intensity of radiation, degree of unsaturation and temperature (PORIM, 1991). When oils are exposed to sunlight, pi bonds within the lipid molecules become photo oxidized (Ruger., et al. 2002). In the process, oxygen reacts with the double bonds of fatty acids to form peroxide and/or free radicals in the presence of light. Therefore for all the observed value in this work the increases could be due to higher level of unsaturation, possible pigmentation of the sources of these oils and their decomposition products which can act as photo sensitizers to generate singlet oxygen radicals. As observed by PORIM (1991), oxygen being a strong electrophile reacts with pi bond of unsaturated fatty acids to form hydro peroxides which can subsequently breakdown to free radicals thereby initiating autoxidation.
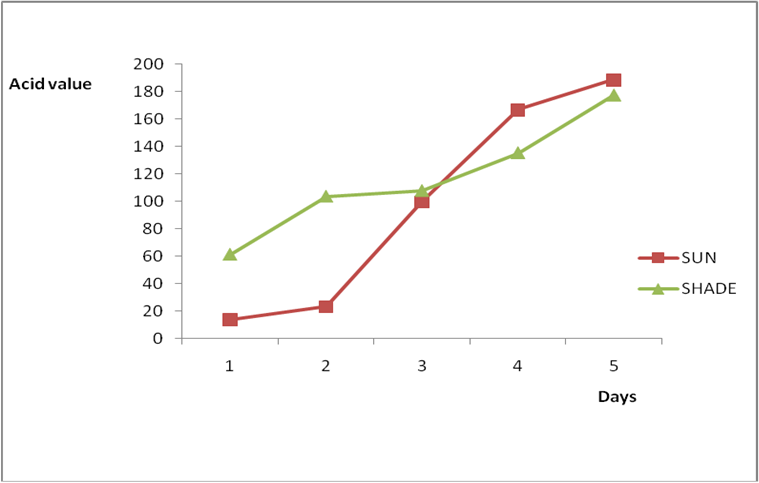
Figure 2a: Effect of time and environmental conditions on rancidity level of Ify
Ochi vegetable oil.
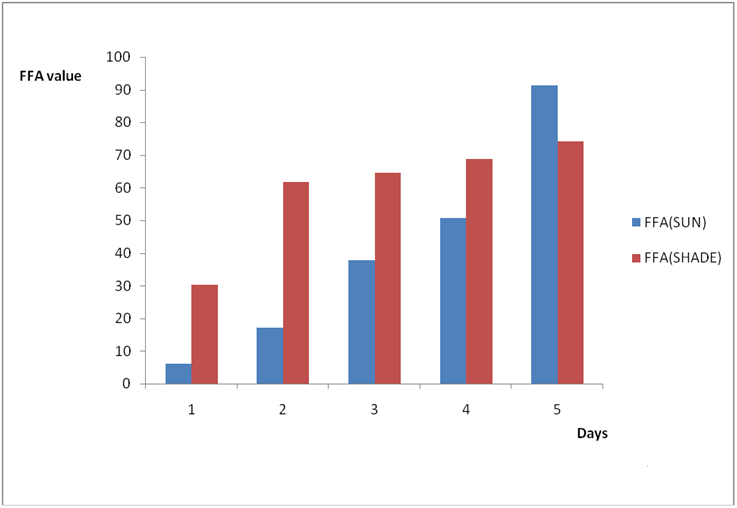
Figure 2b: Effect of time and environmental conditions on free fatty acid level
of Ify Ochi vegetable oil.
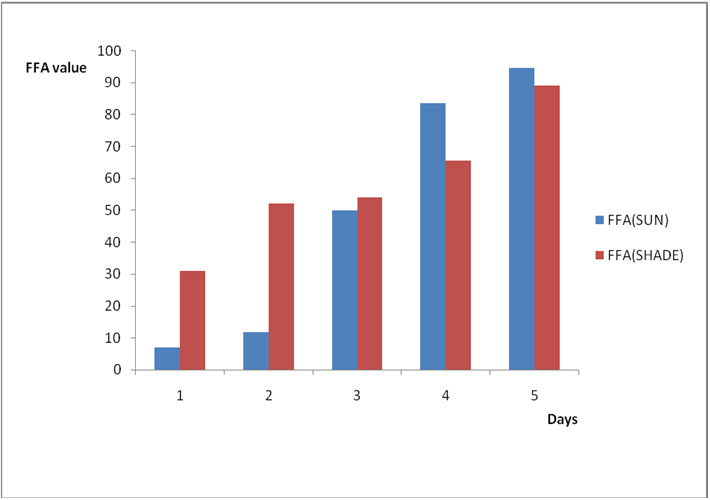
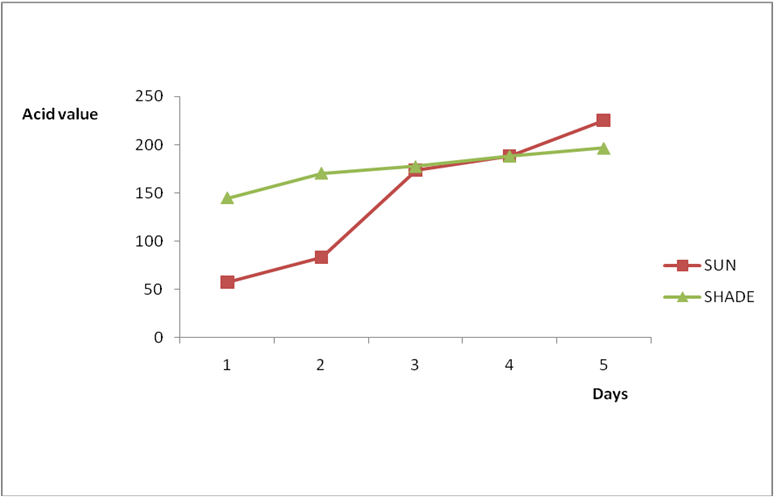
Figure 3b: Effect of time and environmental conditions on the free fatty acid level
of Avon vegetable oil.
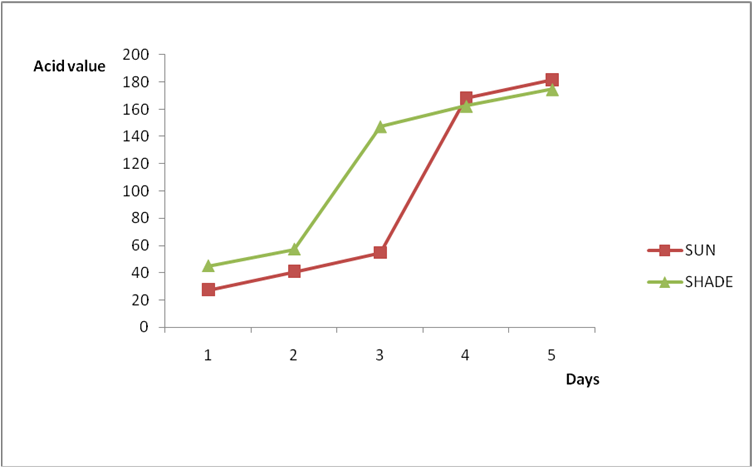
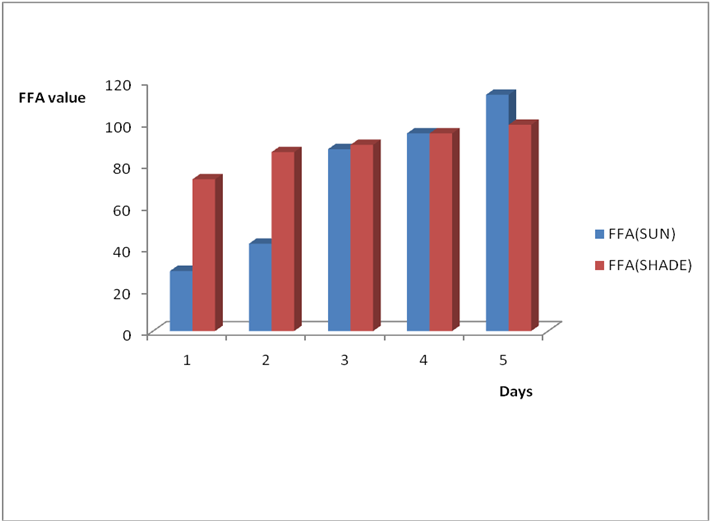
Figure 4a: Effect of time and environmental conditions on rancidity level of Goya
(bottle) vegetable oil.
Figure 4b: Effect of time and environmental conditions on the free fatty acid level
of Goya (bottle) vegetable oil.
Figure 5a: Effect of time and environmental conditions on rancidity level of Goya
(plastic) vegetable oil.
Figure 5b: Effect of time and environmental conditions on the free fatty acid level
of Goya (plastic) vegetable oil.
Figure 6b: Effect of time and environmental condition on the free fatty acid level
of Igala palm oil.
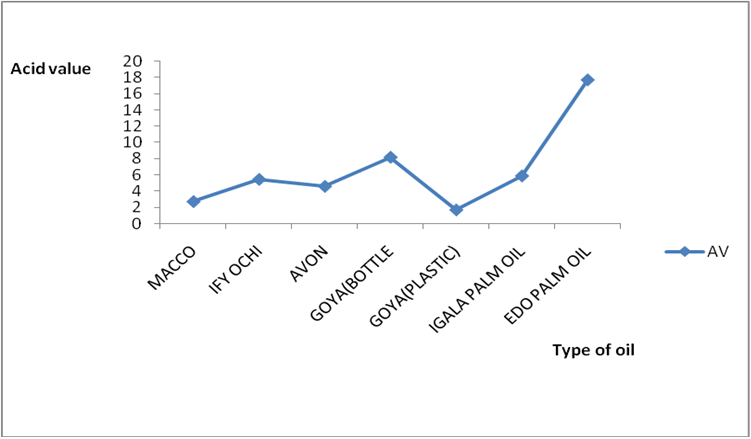
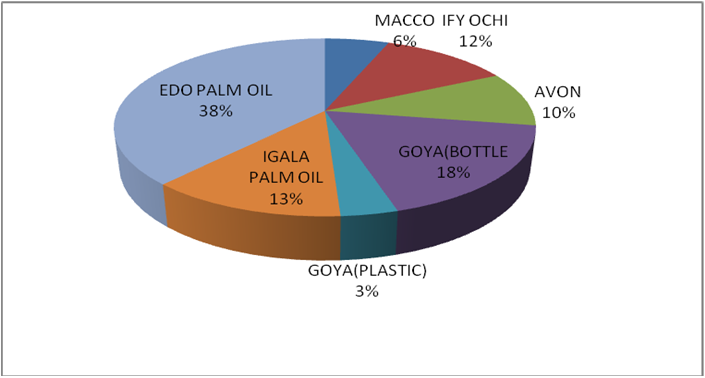
Figure 8c: % distribution of rancidity level in different raw oil samples sold
in Lokoja markets of Kogi state.
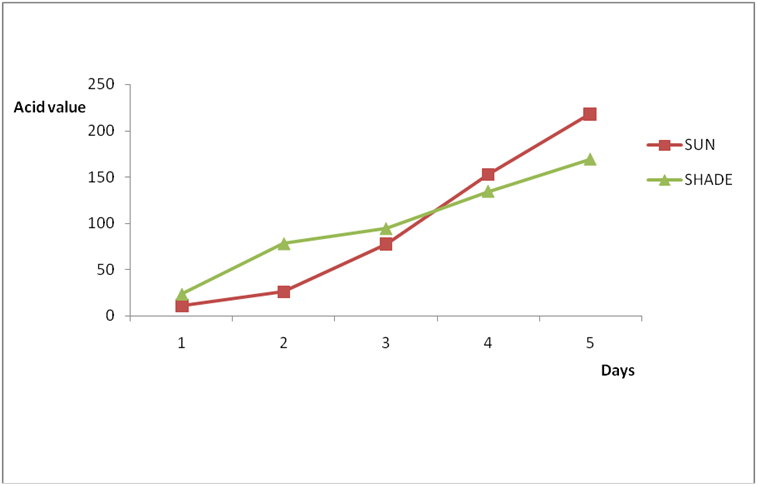
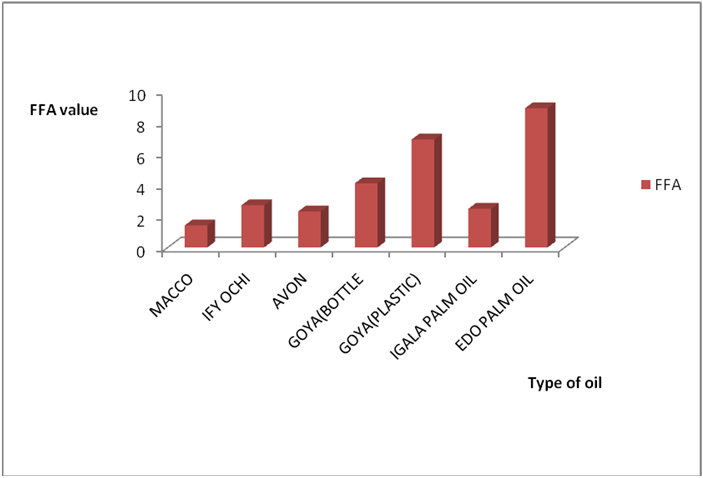
Results for Igala and Edo palm oil are shown in Figure 6a-7b. The higher value observed in these palm oils could be due to the formation of sludge’s at the bottom of this oils leading to an increase in thickness over the period of exposure which might be due to photolysis in addition to photo oxidation. The level of pigment present (e.g. chlorophyll and carotenoids) has been observed to be highly oxidizable due to their pi bond contents also (PORIM, 1991). In both palm oils and vegetable oils, exposure to sunlight causes a substantial loss of antioxidants, especially tocopherols leading to increase in rancidity as compared to oils stored in dark. However some of the oils exposed under the shade (dark) still displayed varying levels of rancidity majorly due to other deteriorating factors like air, moisture, etc. In addition, oils which have low total polyphenols and high levels of polyunsaturated fatty acids are susceptible to oxidation no matter the environment they are kept (Malik, 1991). Another factor that could have enhanced rancidity in shade exposed oils could be due to the atmospheric temperature at which they are stored since temperature is a factor in a chemical reaction. Microbial degradation of the known sunlight exposed oils may have contributed to the high level of rancidity of all the oils since conditions at which they were stored are suitable for microbial growth. As such the combined effects of both oxidative and microbial degradation of the shade oils may have given high value of rancidity in some of the oils. The results of A.V and FFA for all the oil in this work is in conformity with Poku (2002), Nwobi., et al. (2006) and PORIM (1991).
Storage of edible oils under poor conditions will result in the loss of quality and therefore value of the oil which have grave health implications. This can take place throughout the lifecycle of a given oil. Every batch of oil is different, its raw-material source and the condition at the point of purchase in the market must be taken into cognizance in order to ascertain its quality. This current work has shown that storage conditions affects quality of oil if not properly handled. It also shows that long term storage and environmental conditions of storage point have an influence on the oxidative stability of the oil. This is important for those involved in the production, storage and sell of these oils.
References
- Abdalla HS and Patel S. “The performance and oxidation stability of sustainable metalworking fluid derived from vegetable extracts”. Proceedings of the Institution of Mechanical Engineers, Part B: J. Engine. Manufacture (2006).
- Abrashev I., et al. “A Biodegradation Activity of Microbial Associations”. Journal of Culture Collections 3.1(2002): 43-47.
- Bahruddin SW., et al. “Comparative Study on Oxidative Decomposition Behavior of Vegetable Oils and Its Correlation with Iodine Value Using Thermo-gravimetric Analysis”. Journal of Oleo Science 57.4 (2008): 257-266.
- Brimberg UI and Afaf KE. “On the kinetics of the autoxidation of fats: influence of pro-oxidants, antioxidants and synergists”. European Journal Lipid Science and Technology 105.2 (1994): 83-91.
- Brown Ellie., et al. “Cruel Oil: How Palm Oil Harms Health, Rainforest & Wildlife. Washington, D.C.: Center for Science in the Public Interest. pp. iv,3–5. (2005): OCLC 224985333.
- Demirba A. “Biodegradability of Biodiesel and Petrodiesel Fuels.” Energy Sources, Part A: Recovery, Util. Environ Effects 31.2 (2009): 169-174.
- Gertz C., et al. “Testing and Comparing Oxidative Stability of Vegetable Oils and Fats at Frying Temperature. Europe”. Journal Lipid Science Technology 102.8-9 (2000): 543-541.
- Gordon MH and Lenka K. “The effects of antioxidants on changes in oils during heating and deep frying Journal”. Science Food Agricultural 68.3 (1995): 347-353.
- Nedyalka VY and Emma M M. “Stabilisation of edible oils with natural antioxidants”. European Journal of Lipid Science and Technology 103.1 (2001): 1752-1767.
- Nwobi BE Ofoegbu O and Adesina OB. “Extraction and Qualitative Assessment of African Sweet Orange Seed Oil”. African Journal of Food, Agriculture, Nutrition and Development 6.2 (2006).
- Petlyuk A and Adams M Richard J. “Oxidation Stability and Tribological Behavior of Vegetable Oil Hydraulic Fluids”. Tribology Transactions 47.2 (2004): 182-187.
- Poku Kwasi. "Small-Scale Palm Oil Processing in Africa”. FAO Agricultural Services Bulletin 148. Food and Agriculture Organization (2002): ISBN 92-5-104859-2.
- PORIM (Palm Oil Research institute Malaysia). “Palm oil and human nutrition”. A Report by Director General of the Palm Oil Research Institute of Malaysia (1991): pp 4-5.
- Ruger CW., et al. “Abilities of some antioxidants to stabilize soybean oil in industrial use conditions”. Journal of the American Oil Chemists' Society 79 (2002).
Citation:
Ekpa Emmauel., et al. “Effects of Various Environmental Conditions on the Rancidity Levels of Some Edible Oil Sold In Lokoja
Metropolis of Kogi State”. Nutrition and Food Toxicology 1.3 (2017): 118-129.
Copyright: © 2017 Ekpa Emmauel., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.



































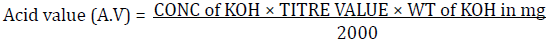

 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.