Research Article
Volume 2 Issue 3 - 2017
Quantification of Ethanol Content in Beverages Alcoholic Using Direct and Fast Method
Laboratory of Spectro-Applied Chemometric and Environment (LSCAE), Faculty of Sciences and Techniques, University Sultan Moulay Slimane, Beni-Mellal, Morocco
*Corresponding Author: A Hirri, Laboratory of Spectro-Applied Chemometric and Environment (LSCAE), Faculty of Sciences and Techniques, University Sultan Moulay Slimane, Beni-Mellal, Morocco.
Received: December 04, 2017; Published: December 11, 2017
Abstract
The aim of this paper is to introduce an innovative method for measuring the concentration of ethanol in alcoholic beverages. The novel approach consists in using MIR spectroscopic data for ethanol content quantification. To quantify ethanol a rapid Fourier transformed infrared (FT-IR) attenuated total reflectance (ATR) spectroscopic is used. 54 samples are prepared for developing a suitable calibration model. Spectra are recorded for all samples in wavelength region 4000-600 cm-1 using a vector 22 Bruker FTIR Spectrophotometer in ATR mode. Calibration equation is obtained using partial least squares (PLS) method. Correlation coefficient and root mean square error values for ethanol are R2 cal: 0.99, R2 val: 0.99 and RMSEC: 0.17, RMSECV: 1.35 Spectral measurement is done for 14 samples and their ethanol concentration is predicted using the developed calibration model. The reliability and repeatability of developed method may be appreciated by the agreement between ATR-PLS predicted results to those of actual values.
Keywords: Alcoholic beverages; Ethanol; MIR ATR and PLS
Introduction
Alcoholic beverages are popular in many countries and often consumed. Due to its effects on the human organism the popularity is linked to the content of ethanol [1]. An alcoholic beverage is a complex mixture of components presenting volatile compounds, responsible for aroma and flavor, and fixed compounds, consisting of a large variety of substances with different characteristics [2]. Some of the most important parameters used for the characterization and differentiation of several types of beverages are alcoholic content, ash content, dry extract, pH, fixed acidity, volatile acidity, etc.
The main components of most alcoholic beverages are ethanol and water [3]. Percentage by volume (% vol) is used to indicate the ethanol content of beverages, which is also called the French or Gay-Lussac system. Alcohol content differs according to the main beverage type and may also vary by country. Commonly, 4-5% vol are contained in beer, about 12% vol in wine and about 40% vol in distilled spirits [4].
Currently, ethanol determination in alcoholic beverages can be performed in several ways: boiling point depression of the ethanol solution relative to water [5], dens metric analysis [6], refractive index method [5,6], oxidation of the distillate [7], dichromate oxidation spectrophotometry [8], enzymatic method [9,10], biosensor [11], potentiometry [12], gas chromatography(GC) [13] and high performance liquid chromatography (HPLC[14]. Most of this analyses used for characterization of ethanol content in beverages alcoholic are time-consuming, expensive and involve a considerable amount of manual work. Very often, complex chemical treatment of the sample and the use of sophisticated instruments are required [15]. Recently, Fourier transform infrared (FT-IR) spectroscopy has become an emerging well-accepted analytical technique, due to its simplicity with advantages in terms of cost per sample [16].
It achieves high analysis speed and requires little or no sample preparation. FT-IR spectroscopy has been widely used as an analytical tool in many laboratories and industrial sectors such as food agricultural [17], petrochemical [18], textile [19] and pharmaceutical [20]. FT-IR data have been often combined with chemo metric techniques to develop methods of classification and characterization. This approach has been found to be very useful in many applications, due to the ability of these methods in achieving the spectral resolution of the FTIR signals. Up to now, a lot of studies have been published on the utilization of near and mid FT-IR for authentication, identification or classification of many agro-foods, by multivariate statistical analysis of spectral data [21].
Ideally, an analytical method used to verify the quality and authenticity of alcoholic beverages should to perform an analysis without sample pre-treatment. In addition, it should to accomplish a fast data acquisition and carry out the data treatment accurately with relatively low costs. The combination of chemo metric methods with MIR spectroscopy is a good way to reach these premises.
The objective of this study is to develop, by FT-MIR spectroscopy coupled to chemo metric treatment, a direct and rapid test method that quantified ethanol content in the real beverages alcoholic.
Materials and Methods
Sampling
Highly Pure ethanol 99,9% and distilled water were used for the set of mixtures. Fifty five mixtures were prepared in concentration range 1% to 55% solution of ethanol in the distilled water. The concentrations of ethanol in the majority of spirits are in the prepared range of concentration of ethanol in distilled water. Mixtures were prepared in glass vials and analyzed both directly by FT-IR. Advantage of vials consists in preventing evaporation of the ethanol, which is relatively fast.
Highly Pure ethanol 99,9% and distilled water were used for the set of mixtures. Fifty five mixtures were prepared in concentration range 1% to 55% solution of ethanol in the distilled water. The concentrations of ethanol in the majority of spirits are in the prepared range of concentration of ethanol in distilled water. Mixtures were prepared in glass vials and analyzed both directly by FT-IR. Advantage of vials consists in preventing evaporation of the ethanol, which is relatively fast.
FT-IR analysis
FTIR spectra are obtained using a Vector 22 Bruker FTIR Spectrophotometer equipped with an attenuated total reflectance accessory (ATR single-reflexion, Diamond, incident angle 45°), DTGS detector, Globar (MIR) source and KBr Germanium separator, with a resolution of 4 cm-1 at 98 scans. Spectra are scanned in the absorbance mode from 4000 to 600 cm-1 and the data are handled with OPUS logiciel. Analyses are carried out at room temperature. The background is collected before each sample was measured.
FTIR spectra are obtained using a Vector 22 Bruker FTIR Spectrophotometer equipped with an attenuated total reflectance accessory (ATR single-reflexion, Diamond, incident angle 45°), DTGS detector, Globar (MIR) source and KBr Germanium separator, with a resolution of 4 cm-1 at 98 scans. Spectra are scanned in the absorbance mode from 4000 to 600 cm-1 and the data are handled with OPUS logiciel. Analyses are carried out at room temperature. The background is collected before each sample was measured.
Partial least square regression (PLS)
PLS is a supervised analysis which is based on the relation between the signal intensity and the characteristics of the sample [22]. Interference and overlapping of the spectral information may be overcome by using powerful multicomponent analysis such as PLS regression. La PLS [23] allows a sophisticated statistical approach using spectral region rather than unique and isolated analytical bands. The first step is to perform a calibration model.
PLS is a supervised analysis which is based on the relation between the signal intensity and the characteristics of the sample [22]. Interference and overlapping of the spectral information may be overcome by using powerful multicomponent analysis such as PLS regression. La PLS [23] allows a sophisticated statistical approach using spectral region rather than unique and isolated analytical bands. The first step is to perform a calibration model.
This involves collecting a set of reference calibration samples, which should contain all chemical and physical variations to be expected in the unknown samples, which will be predicted later. The model was built by full cross-validation methods during the calibration development. The optimal number of PLS Latentes variables (LVs) were found according to the full cross-validation procedure. The second step is to test the model using a prediction set (different to the calibration one), i.e. to compare the values obtained by the model to the values obtained by the reference method. The evaluation of the errors in the calibration is estimated by computing the standard error of calibration (SEC) after comparing the real concentration with the computed one for each component. The formula for the standard error of calibration is:
Where Ci is the known value, Ci is the calculated value, N the number of samples and p is the number of independent variables in the regression optimized by cross validation. The standard error of prediction (SEP) gives an estimation of the prediction performance during the step of validation of the calibration equation
Where Ci is the known value, Ci is the value calculated by the calibration equation, and M is the number of samples in the prediction set. Another useful parameter is the relative error of prediction (REP %) that shows the predictive ability of the model. This is calculated from the equation
Quality assessment of the obtained results is discussed by comparison of predicted values versus measured values, both for calibration and for validation data sets. All chemo metrics calculations were performed using the Unscramble x software version 10.2 from CAMO (Computer Aided Modeling, Trondheim, Norway) was used for chemo metric treatments of FTIR-ATR data spectra.
The predictive ability of the model should also be expressed by the bias and the square of correlation coefficient (R2) also called determination coefficient, usually called Q2 in prediction. The regression coefficients are the numerical coefficients which express the link between the predictor variations and the response variations. The bias is systematic difference between predicted and measured values.
The bias is computed as average value of the residuals. The residual is the measure of the variation which is not taken into account by the model. The residual for a given sample and a given variable is computed as the difference between observed value and fitted (projected or predicted) value of the variable on the sample.
Results and Discussion
Spectra of ethanol
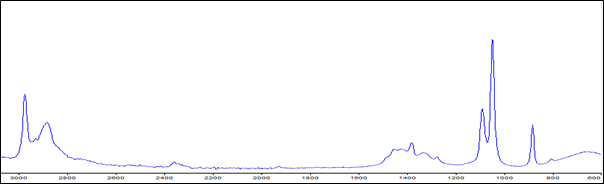
In the region 4000–600 cm−1, ethanol has an absorbance at 3005–2960, 1200–950 and 900–850 cm−1. The absorption is due to C–H stretching, C–O stretching and O–H bending vibration, respectively. Each of them differs by sensitivity and interference effect. However, the spectra at 1200–950 and 900–850 cm−1 were the most sensitive and exclusive absorbance region for ethanol, respectively. Thus, the range 1200–850 cm−1 was selected as a spectral region, because it satisfied both. Therefore, for quantifying ethanol using PLS at optimal spectral region, ethanol spectra in the presence of sugars were developed (Figure 1).
In the region 4000–600 cm−1, ethanol has an absorbance at 3005–2960, 1200–950 and 900–850 cm−1. The absorption is due to C–H stretching, C–O stretching and O–H bending vibration, respectively. Each of them differs by sensitivity and interference effect. However, the spectra at 1200–950 and 900–850 cm−1 were the most sensitive and exclusive absorbance region for ethanol, respectively. Thus, the range 1200–850 cm−1 was selected as a spectral region, because it satisfied both. Therefore, for quantifying ethanol using PLS at optimal spectral region, ethanol spectra in the presence of sugars were developed (Figure 1).
PLS modeling
The PLS model is built by considering the two frequency intervals 3005-2960 cm-1 and 1200–850 cm-1 as X variables and the Y variables is associated to the content of ethanol. The PLSR models are evaluated using coefficient of determination (R2) in calibration, root-mean-square error of calibration (RMSEC) and cross validation (RMSECV). The performance of the PLSR models on the independent validation set is assessed using R2 and RMSEP and the residual prediction deviation (RPD).
The PLS model is built by considering the two frequency intervals 3005-2960 cm-1 and 1200–850 cm-1 as X variables and the Y variables is associated to the content of ethanol. The PLSR models are evaluated using coefficient of determination (R2) in calibration, root-mean-square error of calibration (RMSEC) and cross validation (RMSECV). The performance of the PLSR models on the independent validation set is assessed using R2 and RMSEP and the residual prediction deviation (RPD).
Here, the criteria of classifying RPD values [24] is adopted as follows: an RPD value below 1.5 indicates that the calibration is not usable; an RPD value between 1.5 and 2.0 indicates the possibility of differentiating between high and low values; an RPD value between 2.0 and 2.5 makes possible approximate quantitative predictions. For RPD value between 2.5 and 3.0 and beyond 3.0, the prediction is classified as good and excellent, respectively. Generally, a good model should have high values of R2 and RPD, and low values of RMSEC, RMSECV and RMSEP.
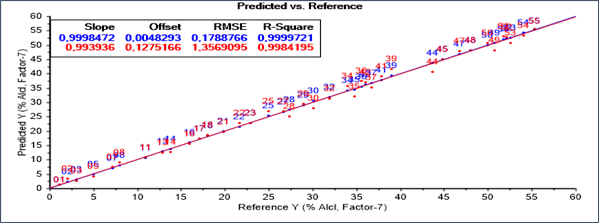
The resulting model seems to be able to determine the ethanol content for 40 samples of calibration. As it can be seen from Figure 2. The PLS model is validated by full cross validation. The obtained statistical parameters RMSEC, RMSECV and R2 are summarized in figure 2. The coefficient of determination (R2) of 0.99, RMSEC of 0.17 and RMSECV of 1.35, could be considered satisfactory. Seven VLs are necessary to have a good PLS performance.
Determination of ethanol content in the new samples
The validity of the resulting preliminary and the developed calibration model based using the set of 40 samples, were tested using a validation set of the unknown ethanol content samples (table 2). The prediction equation obtained from the final calibration model (n = 40; Figure 2) was applied to the validation set in order to calculate the statistical parameters related to the estimation of ethanol content in unknown beverages alcoholic samples. The obtained results are given in tables 1 and 2.
The validity of the resulting preliminary and the developed calibration model based using the set of 40 samples, were tested using a validation set of the unknown ethanol content samples (table 2). The prediction equation obtained from the final calibration model (n = 40; Figure 2) was applied to the validation set in order to calculate the statistical parameters related to the estimation of ethanol content in unknown beverages alcoholic samples. The obtained results are given in tables 1 and 2.
| External validation | LVs | Rp2 | RMSEP | Bias | RPD | REP % |
| 7 | 0.994 | 0.982 | 0.2 | 12.9 | 1.3 |
Table 1: Statistical parameters carried out by external validation on PLS.
| Samples | Predicted ethanol content |
Reference ethanol content |
| 04 | 4.17 | 4.08 |
| 06 | 5.96 | 6.00 |
| 09 | 8.90 | 8.91 |
| 12 | 12.49 | 12.14 |
| 15 | 15.28 | 14.87 |
| 20 | 19.21 | 19.84 |
| 24 | 23.63 | 24.00 |
| 26 | 25.39 | 25.86 |
| 31 | 31.17 | 31.00 |
| 33 | 33.10 | 32.92 |
| 38 | 38.13 | 37.97 |
| 40 | 34.88 | 35.53 |
| 43 | 42.80 | 42.69 |
| 46 | 44.87 | 44.37 |
Table 2: Prediction of arbequine EVOO percentage.
Table 2 shows clearly that the FTIR-PLS method is an effective method for the determination of ethanol content in beverages alcoholic. The results indicate that there is no significant difference between the reference values and the proposed one. Therefore, the PLS model for the FT-IR data treatment appears to be an appropriate approach to predict the ethanol content in beverages alcoholic.
Conclusion
PLS-R calibration models developed in 1 and 55% concentration calibration range w/w, gave less than 2 % relative error for external validation samples. Therefore, we can conclude that the FT-IR spectra of alcoholic beverages can be properly modeled by PLSR using first the derivation of the absorbance spectra for the baseline removal using the Savitzoky-Golay method, with a first order polynomial and 20 smoothing points as initial data pretreatments. This study had also underlined that the spectral regions 3005–2960 cm-1 and 1200–850 cm-1 were useful for good predictions of ethanol content.
FT-IR spectroscopy coupled to chemo metrics techniques is reported as an adequate method for the determination of ethanol content in alcoholic beverages, without any previous sample pretreatment and any destructive sampling manipulation. Therefore, the proposed spectroscopic method provides a convenient alternative in terms of time and solvent saving for routine analysis of large number of alcoholic beverages samples. This approach can be considered fast, clean and affordable methodology.
References
- H Vaskova. “Spectroscopic Determination of Methanol Content in Alcoholic Drinks”. International Journal of Biology and Biomedical Engineering 8 (2014): 27-34.
- Arvanitoyannis IS., et al. “Application of quality control methods for assessing wine authenticity: Use of multivariate analysis (chemometrics)”. Trends in Food Science & Technology 10.10 (1999): 321-336.
- (O’Neil MJ, editor. Te Merck Index, 13th ed. Whitehouse Station, NJ, Merck & Co., Inc., (2001): 1818.
- IARC. Alcohol consumption and ethyl carbamate. IARC Monogr Eval Carcinog Risks Hum, 96 (2010): 3-1383.
- Anonymous. Chapter 4-Alcohols. In Wine Analysis. Research Institute for Wines, Taiwan Tobacco & Wine Monopoly Bureau (1992): 1-7.
- AOAC Official Method of Analysis. Wines. “In Official Methods of Analysis of AOAC International”. 15th edition, (1990): 739-750.
- Caputi A Jr and Wright D. Collaborative study of the determination of ethanol in wines by chemical oxidation. Journal - Association of Official Analytical Chemists 52 (1969): 85-88.
- Caputi A., et al. “Spectrophotometric determination of ethanol in wines”. Am. J. Enol. Vitic. 19 (1968): 160-165.
- Jones, A W. “Measuring ethanol in saliva with the QED enzymatic test device: comparison of results with blood and breath alcohol concentration”. Journal of Analytical Toxicology 19.3 (1995): 169-174.
- McCloskey LP and Replogle LL. “Evaluation of an enzymatic method for estimating ethanol in wines using an enzyme kit”. American Journal of Enology and Viticulture 25 (1974): 194-197.
- Mason M. “Ethanol determination in wine with an immobilized enzyme electrode”. American Journal of Enology and Viticulture 34 (1983): 173-175.
- Kokovkin V V and Smolyakov B S. “Ethanol determination in aqueous solutions and wine stocks by potentiometry with ion selective electrodes”. Journal of Analytical Chemistry 50 (1995): 569-573.
- Naviglio D., et al. “Rapid determination of ethanol content in spirits and in beer by high resolution gas chromatography”. Italian Food & Beverage Technology 24 (2001): 19-21.
- Martin E., et al. “Ethanol determination by HPLC in alcoholic beverages”. Mitteilungen aus dem Gebiete der Lebensmitteluntersuchung und Hygiene. 77 (1986): 528-534.
- Bassbasi M., et al. “Determination of Solid Non Fat (SNF) in raw milk using PLS and SVM chemometric methods”. Food Chemistry 146 (2014): 250-254.
- Hirri A., et al. “Prediction of polyphenol fraction in virgin olive oil using Mid-infrared Attenuated Total Reflectance ATR-MIR coupled with partial least squares regression”. International Journal of Food Properties 19.7 (2016): 1504-1512.
- Aouidi F., et al. “Discrimination of five Tunisian cultivars by Mid InfraRed spectroscopy combined with chemometric analyses of olive”. Food Chemistry 131.1 (2012): 360-366.
- Roman MB and Ravilya ZS. “Gasoline classification by source and type based on near infrared (NIR) spectroscopy data”. Fuel 87.7 (2008): 1096-1101.
- Langeron Y., et al. “Classifying NIR spectra of textile products with kernel methods”. Engineering Applications of Artificial Intelligence 20.3 (2007): 415-427.
- Wu YW., et al. “Fourier transforms Mid Infrared (MIR) and Near Infrared (NIR) spectroscopy for rapid quality assessment of Chinese medicine preparation Honghua Oil”. Journal of Pharmaceutical and Biomedical Analysis 46.3 (2008): 498-504.
- Hirri A., et al. “FTIR spectroscopy and PLS-DA classification and prediction of four commercial grade virgin olive oils from Morocco”. Food Analytical Methods (2015).
- Beebe KR and Kowalski BR. “An introduction to multivariate calibration and analysis”. Analytical Chemestry 59.17 (1987): 1007-1017.
- Fuller MP and Griffiths PR. “Diffuse reflectance measurements by infrared fourier transform spectrometry”. Analytical Chemistry 50.13 (1978): 1906-1910.
- Mouazen AM., et al. “Effect of wavelength range on the measurement accuracy of some selected soil constituents using visual-near infrared spectroscopy”. Journal of Near Infrared Spectroscopy 14.3 (2006): 189-199.
Citation:
A Hirri., et al. “Quantification of Ethanol Content in Beverages Alcoholic Using Direct and Fast Method”. Nutrition and Food
Toxicology 2.3 (2017): 339-345.
Copyright: © 2017 A Hirri., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.



































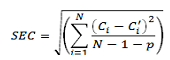
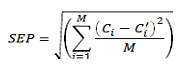
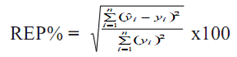
 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.