Research Article
Volume 2 Issue 4 - 2018
Deer Oil Extract Suppresses LPS-Induced Inflammatory Signaling in Skin Immune Cells
1Laboratory of Cosmetic pharmacology, BIOSTANDARD, Seoul 04631, Republic of Korea
2Department of Biotechnology, Yon-Sei University, Seoul 03722, Republic of Korea
2Department of Biotechnology, Yon-Sei University, Seoul 03722, Republic of Korea
*Corresponding Author: Young Wook Jo, Department of Biotechnology, Yon-Sei University, Seoul 03722, Republic of Korea.
Received: January 24, 2018; Published: February 08, 2018
Abstract
The effect of Deer Oil extracted with deer antler (DADO) on lipopolysaccharide (LPS)-induced inflammatory responses in RAW264.7 cells. Deer oil was fractionated by liquid–liquid extraction to obtain two fractions: methanol fraction (DAE-M) and hexane fraction (DAE-H). TLC showed that DAE-M had relatively more hydrophilic lipid complexes, including unsaturated fatty acids, than DADO and DAE-H. The relative compositions of tetradecenoyl carnitine, α-linoleic acid, and palmitoleic acid increased in the DAE-M fraction by 61, 38, and 32%, respectively, compared with DADO. The concentration of sugar moieties was 3-fold higher in the DAE-M fraction than DAE-H. DAE-M significantly decreased LPS-induced nitric oxide (NO) production in RAW264.7 cells in a dose-dependent manner. This DAE-M-mediated decrease in NO production was due to downregulation of mRNA and protein levels of inducible nitric oxide synthase (iNOS). In addition, mRNA expression of pro-inflammatory mediators, such as cyclooxygenase (COX-2), interleukin (IL)-1β, and IL-12β, was suppressed by DAE-M. Our data showed that DAE-M, which has relatively higher sugar content than DADO and DAE-H, could play an important role in suppressing inflammatory responses by controlling pro-inflammatory cytokines and mediators.
Introduction
Inflammation is a normal physiological process that heals injury and fights pathogens. Although early responses of the body against harmful stimuli are beneficial, [1] prolonged or chronic inflammation can be detrimental to the body, destroying healthy cells in the absence of any foreign pathogen and causing a wide range of disease conditions. [1,2] Chronic inflammation has been linked with diverse diseases, such as cancer, diabetes, obesity, and heart disease. [2-5] Chronic inflammatory responses are characterized by excessive or prolonged pro- duction of pro-inflammatory cytokines and mediators, such as nitric oxide (NO), inducible nitric oxide synthase (iNOS), cyclooxygenase-2 (COX-2), and inflammatory interleukins. Uncontrolled release or production of pro-inflammatory cytokines has been found in various diseases, including cancer and arthritis. [6] All stages of cancer development have been associated with chronic and excessive production of pro-inflammatory cytokines from immune cells, such as macrophage and neutrophils, which stimulate cell proliferation, reduce apoptosis, and enhance angiogenesis. [6] Autoimmune diseases, such as rheumatoid arthritis, have also been implicated in the dysregulation of pro-inflammatory cytokines. [7] Controlling unregulated cytokines has been a target for the prevention and alleviation of chronic inflammatory responses. Generally, non-steroidal anti-inflammatory drugs (NSAIDs) have been used to suppress the action of inflammatory mediators. [8] However, NSAIDs have side effects, including gastric bleeding, kidney damage, and exacerbation of asthma. Therefore, the potential use of natural anti-inflammatory agents without any side effects has emerged as an alternative solution. [9,10].
In East Asia, deer antler and deer oil from subcutaneous fat has been widely used to relieve the symptoms of various diseases. [11] Water extracts of deer horn have various biological effects, including anti-aging, anti-inflammatory, anti-oxidant, and anti-amnesic activities. [11-14] However, studies on the biological effects of these deer antler extracted deer subcutaneous fat-derived lipids have not yet been investigated. Components of deer antler extracted deer subcutaneous fat include uronic acids and sialic acid as well as components related to protection of cartilage, such as glucosamine, calcium, chondroitin sulfate, and collagen hydrolysate. [13,15] Deer antler is also rich in a large number of lipid components, including neutral lipids, glycolipids, ganglioside, and phospholipids. [15–18] In particular, pantocrine, an alcoholic extract of the lipid fraction from deer antler, has been used as a supplementary tonic agent for the enhancement of testosterone and growth factors in the body. [19] In this study, we analyzed the lipid components of deer antler extracted deer oil (DADO) and examined the effect of the methanol fraction of DADO (DAE-M) on inflammatory responses in RAW264.7 cells. The DAE-M fraction reduced inflammatory responses induced by lipopolysaccharide (LPS) by suppressing pro-inflammatory cytokines and mediators. Our study suggests the potential use of deer oil as an anti-inflammatory agent.
Materials and Methods
Reagents
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetra-zolium bromide (MTT), dimethyl methylene blue (DMMB), and phenol were purchased from Junsei (Tokyo, Japan), and sulfuric acid was from Showa (Tokyo, Japan). Solvents, including hexane, methanol, acetone, chloroform, and ether were purchased from Daejung Chemical & Materials (Gyonggi-do, Korea). Phosphatidyl choline, cholesterol, fatty acids mixture, and triglyceride were purchased from Avanti Polar Lipids, Inc. (Alabaster, AL, U.S.A.). Fetal bovine serum (FBS), penicillin, streptomycin, and Dulbecco’s modified Eagle’s medium (DMEM) with high glucose were purchased from Welgene (Fresh Media™, Daegu, Korea). iNOS and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibodies and second- ary antibody were obtained from Cell Signaling Technology (Boston, MA, U.S.A.). Griess reagent and LPS (Escherichia coli, serotype 0111:04) were obtained from Sigma Aldrich (St. Louis, MO, U.S.A.). All other reagents were of the highest commercial grade available.
3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetra-zolium bromide (MTT), dimethyl methylene blue (DMMB), and phenol were purchased from Junsei (Tokyo, Japan), and sulfuric acid was from Showa (Tokyo, Japan). Solvents, including hexane, methanol, acetone, chloroform, and ether were purchased from Daejung Chemical & Materials (Gyonggi-do, Korea). Phosphatidyl choline, cholesterol, fatty acids mixture, and triglyceride were purchased from Avanti Polar Lipids, Inc. (Alabaster, AL, U.S.A.). Fetal bovine serum (FBS), penicillin, streptomycin, and Dulbecco’s modified Eagle’s medium (DMEM) with high glucose were purchased from Welgene (Fresh Media™, Daegu, Korea). iNOS and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) antibodies and second- ary antibody were obtained from Cell Signaling Technology (Boston, MA, U.S.A.). Griess reagent and LPS (Escherichia coli, serotype 0111:04) were obtained from Sigma Aldrich (St. Louis, MO, U.S.A.). All other reagents were of the highest commercial grade available.
Preparation and Analysis of Deer Oil Extract
Deer antler (Cervus elaphus) from adult male New Zealand elks (2 to 4 years old) was kindly provided from Biostandard inc. (Seoul, Korea). The extraction procedure was modified from described by Lee., et al. [13] In brief, the deer antler was extracted two times with 6-fold volume deer subcutaneous fat oil at 65–70°C for 4h, and then cooled. The pure lipid fraction of the DADO was obtained with an oil/water separator. The impurities filtered with filter paper.
Deer antler (Cervus elaphus) from adult male New Zealand elks (2 to 4 years old) was kindly provided from Biostandard inc. (Seoul, Korea). The extraction procedure was modified from described by Lee., et al. [13] In brief, the deer antler was extracted two times with 6-fold volume deer subcutaneous fat oil at 65–70°C for 4h, and then cooled. The pure lipid fraction of the DADO was obtained with an oil/water separator. The impurities filtered with filter paper.
Solvent Fractionation by Liquid–Liquid Extraction
Deer oil was fractionated by liquid–liquid extraction to yield relatively hydrophilic and hydrophobic parts. Two immiscible solvents, hexane and methanol with a ratio of 2:1 (v/v), were used to separate each fraction. Deer oil was first dissolved in hexane to 10% (w/v) and transferred to separating funnel. Then, the half volume of methanol was added to the solution, vigorously shaken, and stilled until the two separate layers appeared. Two solvent layers were separately collected, DAE-M and hexane fraction of Deer oil (DAE-H), and solvents were eliminated by high vacuum rotary evaporator. Two fractions were resuspended with the proper solvents for experimental use.
Deer oil was fractionated by liquid–liquid extraction to yield relatively hydrophilic and hydrophobic parts. Two immiscible solvents, hexane and methanol with a ratio of 2:1 (v/v), were used to separate each fraction. Deer oil was first dissolved in hexane to 10% (w/v) and transferred to separating funnel. Then, the half volume of methanol was added to the solution, vigorously shaken, and stilled until the two separate layers appeared. Two solvent layers were separately collected, DAE-M and hexane fraction of Deer oil (DAE-H), and solvents were eliminated by high vacuum rotary evaporator. Two fractions were resuspended with the proper solvents for experimental use.
Lipid Analysis of Each Fraction by TLC
Eight microliters of each sample (fractions) was dissolved in chloroform and applied on a 250 µm analytical grade silica gel 60F254 TLC plate (Merck, KGaA, Darmstadt, Germany). The samples were developed using the solvent system of hexane–diethylether–formic acid (40:10:1, v/v/v) for approximately 1h. The spots were visualized by spraying the TLC plate with a 2,7-di-chlorofluoroscein solution (0.2% in 95% methanol). Separated lipids spots from samples were compared to those of standard lipids (phosphatidyl choline, cholesterol, fatty acids mixture, and triglyceride).
Eight microliters of each sample (fractions) was dissolved in chloroform and applied on a 250 µm analytical grade silica gel 60F254 TLC plate (Merck, KGaA, Darmstadt, Germany). The samples were developed using the solvent system of hexane–diethylether–formic acid (40:10:1, v/v/v) for approximately 1h. The spots were visualized by spraying the TLC plate with a 2,7-di-chlorofluoroscein solution (0.2% in 95% methanol). Separated lipids spots from samples were compared to those of standard lipids (phosphatidyl choline, cholesterol, fatty acids mixture, and triglyceride).
Identification of Fatty Acid Composition by Gas Chromatography (GC)
Fatty acid composition of whole lipid from deer antler oil and its two solvent fractions (DAE-M and DAE-H) were determined by GC. Samples were acid hydrolyzed and methylated with boron trifluoride (BF3) in methanol before GC analysis. The fatty acid methyl ester was subsequently analyzed by a Varian 3800 GC (Walnut Creek, CA, U.S.A.) equipped with a supelcowax 10 fused-silica capillary column (30m × 0.32 mm i.d.; Supelco, Bellefonte, PA, U.S.A.) and flame-ionization detector. The column was held at 180°C for 1 min and then heated to 230°C for 34.33 min at a rate or 1.5°C/min. Helium was used as the carrier gas, with a total gas flow rate of 1.0 mL/min. The injector and detector temperatures were set at 240 and 250°C, respectively. The fatty acids were identified by comparison with the retention times of the standards. Heptadecanoic acid was used as an internal standard (IS).
Fatty acid composition of whole lipid from deer antler oil and its two solvent fractions (DAE-M and DAE-H) were determined by GC. Samples were acid hydrolyzed and methylated with boron trifluoride (BF3) in methanol before GC analysis. The fatty acid methyl ester was subsequently analyzed by a Varian 3800 GC (Walnut Creek, CA, U.S.A.) equipped with a supelcowax 10 fused-silica capillary column (30m × 0.32 mm i.d.; Supelco, Bellefonte, PA, U.S.A.) and flame-ionization detector. The column was held at 180°C for 1 min and then heated to 230°C for 34.33 min at a rate or 1.5°C/min. Helium was used as the carrier gas, with a total gas flow rate of 1.0 mL/min. The injector and detector temperatures were set at 240 and 250°C, respectively. The fatty acids were identified by comparison with the retention times of the standards. Heptadecanoic acid was used as an internal standard (IS).
Identification of Total Sugar Content of Each Fraction
Total sugar content in whole lipid and its solvent fractions of deer antler oil was analyzed by the phenol–sulfuric acid method [20] with external calibration curve of glucose. The absorbance was measured at 490 nm by microplate reader.
Total sugar content in whole lipid and its solvent fractions of deer antler oil was analyzed by the phenol–sulfuric acid method [20] with external calibration curve of glucose. The absorbance was measured at 490 nm by microplate reader.
Cell Culture and Treatment in RAW264.7 Cells
RAW264.7 cells witch is major skin immunogenic cells were purchased from Korean Cell Line Bank (KCLB, Seoul, Korea). Cells were maintained with 10% FBS and 1% penicillin streptomycin in DMEM at 37°C in a humidified chamber with a 5% CO2 atmosphere. Cells were sub-cultured before they reached confluence, approximately 70% confluence, by gently scraping the attached cells. Cells were incubated with various concentrations of samples and then stimulated with LPS at 1 µg/mL for the indicated times.
RAW264.7 cells witch is major skin immunogenic cells were purchased from Korean Cell Line Bank (KCLB, Seoul, Korea). Cells were maintained with 10% FBS and 1% penicillin streptomycin in DMEM at 37°C in a humidified chamber with a 5% CO2 atmosphere. Cells were sub-cultured before they reached confluence, approximately 70% confluence, by gently scraping the attached cells. Cells were incubated with various concentrations of samples and then stimulated with LPS at 1 µg/mL for the indicated times.
Cell Viability Assay
RAW264.7 cells were seeded on 96-well plates (2.5 × 105 cells/mL) and preincubated for 24h. Then, they were treated with DAE-M at various concentrations, followed by treatment with or without LPS 1h later. After 20h of DAE-M treatment, the media were removed, and cells were treated with 1 mg/mL of MTT reagent. Four hours later, cells were treated with 20% sodium dodecyl sulfate (SDS) in acidic isopropyl alcohol. After overnight incubation, the absorbance at 540 nm was determined using an enzyme-linked immuno- sorbent assay (ELISA) plate reader (Molecular Devices, LLC, Sunnyvale, CA, U.S.A.).
RAW264.7 cells were seeded on 96-well plates (2.5 × 105 cells/mL) and preincubated for 24h. Then, they were treated with DAE-M at various concentrations, followed by treatment with or without LPS 1h later. After 20h of DAE-M treatment, the media were removed, and cells were treated with 1 mg/mL of MTT reagent. Four hours later, cells were treated with 20% sodium dodecyl sulfate (SDS) in acidic isopropyl alcohol. After overnight incubation, the absorbance at 540 nm was determined using an enzyme-linked immuno- sorbent assay (ELISA) plate reader (Molecular Devices, LLC, Sunnyvale, CA, U.S.A.).
NO Assay
Briefly, RAW264.7 cells were seeded on 96-well plates (2.5 × 105 cells/mL) and incubated for 20h. Then, they were treated with various concentrations of DAE-M and then incubated with or without 1 µg/mL of LPS for 20h. The level of nitrite, a stable NO product, in the culture media was determined using Griess reagent. One hundred microliters of media was mixed with same volume of Griess reagent for 10 min. Absorbance was measured at 540 nm using an ELISA reader. Nitrite concentration was determined by a calibration curve using sodium nitrite.
Briefly, RAW264.7 cells were seeded on 96-well plates (2.5 × 105 cells/mL) and incubated for 20h. Then, they were treated with various concentrations of DAE-M and then incubated with or without 1 µg/mL of LPS for 20h. The level of nitrite, a stable NO product, in the culture media was determined using Griess reagent. One hundred microliters of media was mixed with same volume of Griess reagent for 10 min. Absorbance was measured at 540 nm using an ELISA reader. Nitrite concentration was determined by a calibration curve using sodium nitrite.
Quantitative Real-Time Polymerase Chain Reaction (qPCR)
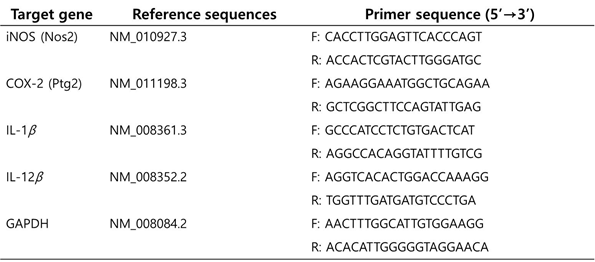
Total RNA was extracted from cell cultures using TRIzol LS reagent (Invitrogen Corporation, Carlsbad, CA, U.S.A.) based on the manufacturer’s instructions. The amount of RNA was calculated as the ratio of absorbance at 260 nm/280 nm, using a microplate reader with a NanoQuant™ plate (Tecan, Männedorf, Switzerland). cDNA was synthesized from 1 µg of isolated RNA using oligo-dT and Superscript II reverse transcriptase (Invitrogen) according to the manufacturer’s protocol. Genomic DNA (gDNA) was eliminated by using gDNA purification kit from Promega (Madison, WI, U.S.A.). SYBR Green Real-Time Master Mix (Applied Bio- system, Life Technologies™, Waltham, MA, U.S.A.) was used for qPCR. The primers of target genes were obtained from Bioneer Inc. and are listed in Table 1. Using StepOnePlus™ Real-Time PCR System (Applied Biosystem, Life Technologies™), relative values of individual genes to that of the endogenous control gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene, were calculated to determine the mRNA expression level of each gene. The values are expressed in 2-Ct. The PCR cycling condition was 2 min at 50°C, 10 min at 95°C, and 50 cycles of 15 s at 95°C and 1 min at 57.5°C.
Total RNA was extracted from cell cultures using TRIzol LS reagent (Invitrogen Corporation, Carlsbad, CA, U.S.A.) based on the manufacturer’s instructions. The amount of RNA was calculated as the ratio of absorbance at 260 nm/280 nm, using a microplate reader with a NanoQuant™ plate (Tecan, Männedorf, Switzerland). cDNA was synthesized from 1 µg of isolated RNA using oligo-dT and Superscript II reverse transcriptase (Invitrogen) according to the manufacturer’s protocol. Genomic DNA (gDNA) was eliminated by using gDNA purification kit from Promega (Madison, WI, U.S.A.). SYBR Green Real-Time Master Mix (Applied Bio- system, Life Technologies™, Waltham, MA, U.S.A.) was used for qPCR. The primers of target genes were obtained from Bioneer Inc. and are listed in Table 1. Using StepOnePlus™ Real-Time PCR System (Applied Biosystem, Life Technologies™), relative values of individual genes to that of the endogenous control gene, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) gene, were calculated to determine the mRNA expression level of each gene. The values are expressed in 2-Ct. The PCR cycling condition was 2 min at 50°C, 10 min at 95°C, and 50 cycles of 15 s at 95°C and 1 min at 57.5°C.
Western Blot
Cells were lysed by lysis buffer (pH 7.4) including phenylmethanesulfonylfluoride (PMSF), benzamidine, aprotinin, leupeptin, pepstatin, sodium orthovanadate, sodium fluoride, sodium pyrophosphate, and β-glycerophosphate. The cell lysate was centrifuged at 14000 rpm for 10 min at 4°C, and the supernatant was collected. The protein content was determined using a bicinchoninic acid (BCA) protein assay kit (Thermo Fisher Scientific, Rockford, IL, U.S.A.). Thirty micrograms of protein were resolved on 10-12% SDS poly- acrylamide gel electrophoresis (PAGE) and transferred to a polyvinylidene fluoride (PVDF) membrane. Membranes were blocked with 3% skim milk in Tris buffered saline (TBS)/Tween 20 buffer (50 mM Tris–HCl (pH 7.5), 150 mM NaCl, and 0.1% Tween [20] for 2h at 4°C. Membranes were then incubated overnight at 4°C with primary antibodies against iNOS or GAPDH, followed by incubation with horseradish peroxidase-conjugated secondary antibody in 3% skim milk in TBS buffer for 2h at room temperature. The membranes were washed with TBS/Tween 20 buffer and treated with a chemi- luminescence reagent (Pierce® ECL Plus detection kit, Thermo Fisher Scientific) according to the manufacturer’s instructions. The bands were visualized by Gel Doc™ and analyzed by Image Lab™ software (Bio-Rad, Hercules, CA, U.S.A.). The relative protein expression of iNOS was expressed as the ratio to that of the endogenous control protein, GAPDH.
Cells were lysed by lysis buffer (pH 7.4) including phenylmethanesulfonylfluoride (PMSF), benzamidine, aprotinin, leupeptin, pepstatin, sodium orthovanadate, sodium fluoride, sodium pyrophosphate, and β-glycerophosphate. The cell lysate was centrifuged at 14000 rpm for 10 min at 4°C, and the supernatant was collected. The protein content was determined using a bicinchoninic acid (BCA) protein assay kit (Thermo Fisher Scientific, Rockford, IL, U.S.A.). Thirty micrograms of protein were resolved on 10-12% SDS poly- acrylamide gel electrophoresis (PAGE) and transferred to a polyvinylidene fluoride (PVDF) membrane. Membranes were blocked with 3% skim milk in Tris buffered saline (TBS)/Tween 20 buffer (50 mM Tris–HCl (pH 7.5), 150 mM NaCl, and 0.1% Tween [20] for 2h at 4°C. Membranes were then incubated overnight at 4°C with primary antibodies against iNOS or GAPDH, followed by incubation with horseradish peroxidase-conjugated secondary antibody in 3% skim milk in TBS buffer for 2h at room temperature. The membranes were washed with TBS/Tween 20 buffer and treated with a chemi- luminescence reagent (Pierce® ECL Plus detection kit, Thermo Fisher Scientific) according to the manufacturer’s instructions. The bands were visualized by Gel Doc™ and analyzed by Image Lab™ software (Bio-Rad, Hercules, CA, U.S.A.). The relative protein expression of iNOS was expressed as the ratio to that of the endogenous control protein, GAPDH.
Statistical Analysis
The results are presented as the mean standard deviation (S.D.) or standard error (S.E.). Differences among the groups were analyzed using one-way ANOVA with Tukey’s multiple range tests. Statistic values of p < 0.05 were considered significantly significant. All the statistical analyses were performed using the Statistical Package for Social Sciences (SPSS, IBM® SPSS® Statistics 21, Armonk, NY, U.S.A.).
The results are presented as the mean standard deviation (S.D.) or standard error (S.E.). Differences among the groups were analyzed using one-way ANOVA with Tukey’s multiple range tests. Statistic values of p < 0.05 were considered significantly significant. All the statistical analyses were performed using the Statistical Package for Social Sciences (SPSS, IBM® SPSS® Statistics 21, Armonk, NY, U.S.A.).
Results
Solvent Fractionation by Liquid–Liquid Extraction
Deer oil has poor solubility in dimethyl sulfoxide (DMSO), a common solvent for dissolving hydrophobic materials in cell culture experiments. The low solubility of deer oil hampered investigation of the anti-inflammatory effects of deer oil in this study. Accordingly, DADO was fractionated using solvents with differential hydrophobicity. We fractionated DADO into two fractions by liquid–liquid extraction using methanol and hexane. DAE-M and DAE-H were obtained and used to deter- mine cell viability and NO production in RAW264.7 cells. The yield of DAE-M was approximately 3-4%. Fractionation of DAE-M is shown in Figure 1. DAE-M fraction was solubilized in DMSO, a vehicle solvent in this study, with a yellowish liquid state, unlike DAE-H fraction, which is beige solid state in room temperature (25°C) (Figure 1S).
Deer oil has poor solubility in dimethyl sulfoxide (DMSO), a common solvent for dissolving hydrophobic materials in cell culture experiments. The low solubility of deer oil hampered investigation of the anti-inflammatory effects of deer oil in this study. Accordingly, DADO was fractionated using solvents with differential hydrophobicity. We fractionated DADO into two fractions by liquid–liquid extraction using methanol and hexane. DAE-M and DAE-H were obtained and used to deter- mine cell viability and NO production in RAW264.7 cells. The yield of DAE-M was approximately 3-4%. Fractionation of DAE-M is shown in Figure 1. DAE-M fraction was solubilized in DMSO, a vehicle solvent in this study, with a yellowish liquid state, unlike DAE-H fraction, which is beige solid state in room temperature (25°C) (Figure 1S).
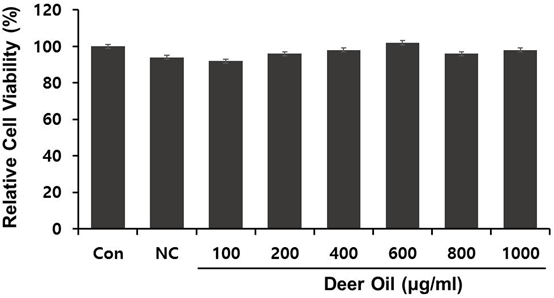
Figure 1a: RAW264.7 cells were treated with various concentrations of deer oil (μg/mL)
with or without lipopolysaccharide (LPS) (1 μg/mL). Values are the mean ± S.D. (n = 4).
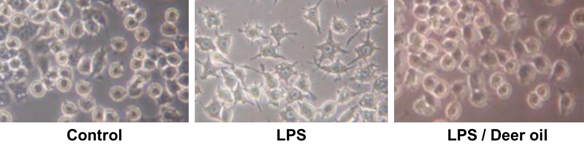
Figure 1b: RAW 264.7 cells were treated with DMSO or Deer oil (μg/mL) with or without LPS (1 μg/mL). Morphology of the cells were observed and photographed under microscope after 24h incubation. CON: DMSO only treated; LPS: LPS only treated. Deer oil/LPS: deer oil is treated followed by LPS treatment.
Figure 1: Effect of Methanol Extract of deer oil on Cell Viability in RAW264.7 Cells.
Effects of DAE-M on RAW264.7 Cell Viability
The effect of DAE-M on cell viability was determined. DAE-M was nontoxic to the RAW264.7 cells at the indicated range of concentrations (0–800 µg/mL) (Figure 2). Cell viability tended to increase at concentrations greater than 400 µg/mL. In addition, DAE-M showed no morphological alterations in the cells, while LPS (only)-treated cells were shown to be activated with morphological changes. However, such morphological difference in LPS(only)-treated group was not observed in DAE-M-pretreated group (Figure 2). These results indicated that DAE-M was not cytotoxic at any given concentration, and suppressed the LPS-induced activation of macrophage.
The effect of DAE-M on cell viability was determined. DAE-M was nontoxic to the RAW264.7 cells at the indicated range of concentrations (0–800 µg/mL) (Figure 2). Cell viability tended to increase at concentrations greater than 400 µg/mL. In addition, DAE-M showed no morphological alterations in the cells, while LPS (only)-treated cells were shown to be activated with morphological changes. However, such morphological difference in LPS(only)-treated group was not observed in DAE-M-pretreated group (Figure 2). These results indicated that DAE-M was not cytotoxic at any given concentration, and suppressed the LPS-induced activation of macrophage.
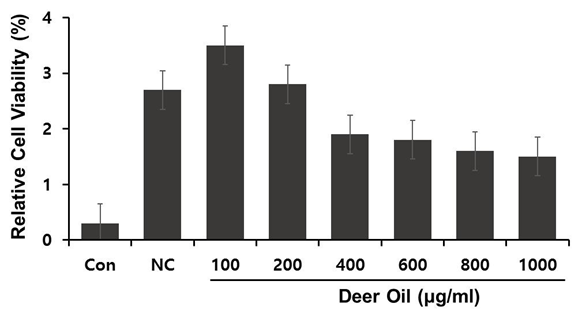
Figure 2: Effects of deer oil on Nitric Oxide (NO) Production in RAW264.7 Cells RAW264.7 cells were treated with various concentrations of deer oil (μg/mL), which had no adverse effect on the cell viability, with or without LPS (1 μg/mL). The level of nitrite in the culture media was determined using Griess reagent. Values are the mean ± S.D. (n = 4).
Effects of DAE-M on NO Production in LPS-Activated RAW264.7 Cells
We examined the effect of DAE-M on LPS-induced NO production, a mediator of the inflammatory response. DAE-M alone (without LPS) did not induce significant NO production up to 800 µg/mL, the concentration shown to be safe for cell viability. DAE-M inhibited LPS-induced NO production in RAW264.7 cells from concentrations of 250 µg/mL and higher (Figure 3). A high concentration of DAE-M (800 µg/mL) decreased NO production by approximately 60% (Figure 3). Thus, DAE-M effectively suppressed LPS-induced NO production, suggesting an anti-inflammatory effect of DAE-M. The effect of DAE-H was also tested on LPS-induced NO production. Due to the poor solubility of DAE-H, a suspension of DAE-H in DMSO was used. DAE-H did not suppress NO production (Figure 3S).
We examined the effect of DAE-M on LPS-induced NO production, a mediator of the inflammatory response. DAE-M alone (without LPS) did not induce significant NO production up to 800 µg/mL, the concentration shown to be safe for cell viability. DAE-M inhibited LPS-induced NO production in RAW264.7 cells from concentrations of 250 µg/mL and higher (Figure 3). A high concentration of DAE-M (800 µg/mL) decreased NO production by approximately 60% (Figure 3). Thus, DAE-M effectively suppressed LPS-induced NO production, suggesting an anti-inflammatory effect of DAE-M. The effect of DAE-H was also tested on LPS-induced NO production. Due to the poor solubility of DAE-H, a suspension of DAE-H in DMSO was used. DAE-H did not suppress NO production (Figure 3S).
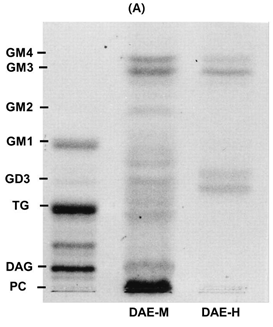
Figure 3a: The spots were visualized by spraying the TLC plate with a 2,7-dichlorofluoroscein solution (0.2% in 95% methanol). Separated lipids spots from samples were compared to standard lipids (PC; phosphatidyl choline, DAG; diacylglycerol, TG; triglyceride, FFA (s); free fatty acids, C; cholesterol).
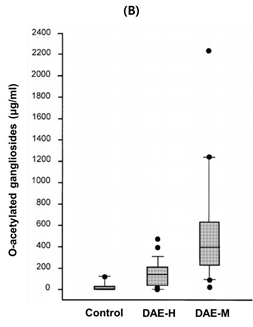
Figure 3b: Total sugar content in solvent fractions of DBOE was analyzed by phenol–sulfuric acid method. methanol fraction of deer oil extract. Means with different superscript letters indicate significant differences with p < 0.05 by ANOVA of Tukey’s multiple range tests.
Figure 3: TLC Analysis and Total Sugar Content of Fractions from DAE-M and DAE-H The fractions from liquid–liquid extraction of DBOE were developed using the solvent system of hexane–diethyl ether–formic acid (40:10:1, v/v/v)
Lipid Analysis of Each Fraction by TLC
To estimate lipid components of DADO, fractions from DADO were analyzed by TLC. Spots on TLC revealed that DADO and DAE-H contained more triglyceride (TG) but less free fatty acid, cholesterol, and phospholipids than DAE-M. DAE-M had relatively more hydrophilic lipid complexes, which were located between free fatty acid and phospholipids, in comparison to DADO and DAE-H (Figure 4A). These results suggested that the relatively hydrophilic components in DAE-M afforded its increased solubility in DMSO.
To estimate lipid components of DADO, fractions from DADO were analyzed by TLC. Spots on TLC revealed that DADO and DAE-H contained more triglyceride (TG) but less free fatty acid, cholesterol, and phospholipids than DAE-M. DAE-M had relatively more hydrophilic lipid complexes, which were located between free fatty acid and phospholipids, in comparison to DADO and DAE-H (Figure 4A). These results suggested that the relatively hydrophilic components in DAE-M afforded its increased solubility in DMSO.
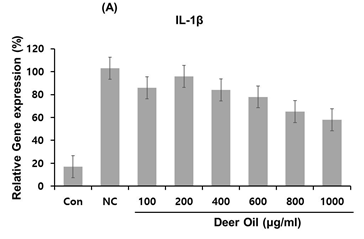
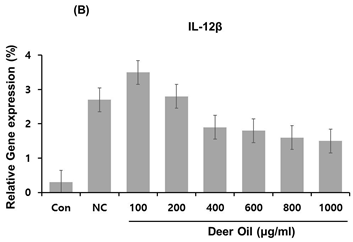
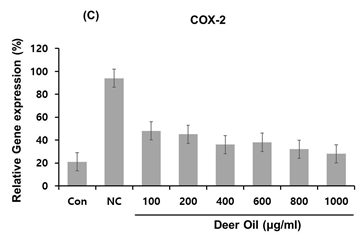
Figure 4: Effects of deer oil on mRNA Expression of IL-1β, IL-12β, and COX-2 in RAW264.7 Cells RAW264.7 cells were treated with deer oil at 100 to 1000 μg/mL dissolved in DMSO, which had no adverse effect on cell viability, with LPS (1 μg/mL). mRNA levels of interleukin (IL)-1β (A), I L-12β (B), and cyclooxygenase 2 (COX-2) (C) were determined by quantitative real time polymerase chain reaction (qRT-PCR). Control group (CON; without LPS) and negative control group (NC; with LPS) were treated with dimethyl sulfoxide (DMSO) of the amount equivalent to the sample.
Fatty Acid Composition of Each Fraction
Fatty acid composition of each fraction was analyzed by GC (Table 1). DAE-M exhibited a higher compositional level of unsaturated fatty acid than DADO and DAE-H, while the composition of saturated fatty acid, such as stearic acid, was significantly reduced in DAE-M.
Fatty acid composition of each fraction was analyzed by GC (Table 1). DAE-M exhibited a higher compositional level of unsaturated fatty acid than DADO and DAE-H, while the composition of saturated fatty acid, such as stearic acid, was significantly reduced in DAE-M.
Compositions of tetradecenoyl carnitine, α-linoleic acid, and palmitoleic acid were increased 61, 38, and 32%, respectively, in DAE-M compared with DADO (Table 1). Stearic acid level of DAE-M was reduced by 23% compared to DADO. In addition, short chain fatty acids, such as myristic acid, were shown to be increased (20%) in the DAE-M fraction relative to DADO. This result indicated that the relative increase in unsaturated fatty acid and short chain fatty acid contributed to the higher solubility and lower melting point of DAE-M.
Total Sugar Content of Each Fraction
To investigate sugar levels of DADO and its two fractions, total sugar content was measured by the phenol–sulfuric acid method. The concentration of sugar moieties in DAE-M was significantly higher than DAE-H (Figure 4B). The DAE-M fraction contained 3-fold higher levels of sugar than DADO. These results sug- gest that the higher level of sugar content in DAE-M com- pared to the DADO could contribute to the enhanced solubility of DAE-M in DMSO and that DAE-M is composed of higher amounts of mixed lipids containing sugar, such as glycolipids, than DADO. Inhibitory Effects of DAE-M on Gene Expressions of Interleukin (IL)-1β, IL-12β, and COX-2 in LPS-Activated RAW264.7 Cells
To investigate sugar levels of DADO and its two fractions, total sugar content was measured by the phenol–sulfuric acid method. The concentration of sugar moieties in DAE-M was significantly higher than DAE-H (Figure 4B). The DAE-M fraction contained 3-fold higher levels of sugar than DADO. These results sug- gest that the higher level of sugar content in DAE-M com- pared to the DADO could contribute to the enhanced solubility of DAE-M in DMSO and that DAE-M is composed of higher amounts of mixed lipids containing sugar, such as glycolipids, than DADO. Inhibitory Effects of DAE-M on Gene Expressions of Interleukin (IL)-1β, IL-12β, and COX-2 in LPS-Activated RAW264.7 Cells
To investigate the effects of DAE-M on regulation of several important pro-inflammatory cytokines in RAW264.7 cells, mRNA expression levels of IL-1β and IL-12β were analyzed with real-time PCR in DAE-M treated cells. The expression of IL-1β was greatly increased by LPS (Figure 5A). The LPS-induced increase in IL-1β was significantly de- creased by DAE-M treatment. However, the effect of DAE-M was not dose-dependent. High dose of DAE-M (800 µg/mL) reduced IL-1β mRNA expression by 65% compared with the control group (Figure 5A). Transcriptional expression of IL-12β, another proinflammatory cytokine, was effectively reduced by DAE-M treatment. mRNA level of IL-12β was decreased by over 70% with DAE-M (800 µg/mL) treatment compared with control (Figure 5B). DAE-M also inhibited LPS-induced expression of COX-2, an important enzyme responsible for inflammation and pain. DAE-M suppressed LPS-induced expression of COX-2 by over 60% (Figure 5C). These results showed that DAE-M effectively suppresses LPS-induced expression of pro-inflammatory cytokines and mediators. Our data suggest that DAE-M can be used to control inflammatory responses.
Inhibitory Effect of DAE-M on iNOS Expression in LPS-Activated RAW264.7 Cells
DAE-M treatment effectively reduced LPS-induced increase in iNOS mRNA at all given concentrations, although the effect was not dose dependent (Figure 6A). iNOS expression was reduced approximately 55% by DAE-M (800 µg/mL). This DAE-M-mediated downregulation of iNOS was also observed at the protein level. DAE-M treatment decreased iNOS protein levels in a dose-dependent manner. A high dose (800 µg/mL) of DAE-M caused a 90% reduction in iNOS protein expression (Figured 6B, C). This result showed that DAE-M-mediated reduction of iNOS protein level was due to the downregulation of DAE-M mRNA expression. These data on the iNOS expression cor- related with data on NO production, which was decreased by DAE-M treatment as well (Figures 3). These results indicated that LPS-induced NO production was due to the upregulation of iNOS and that DAE-M-mediated downregulation of iNOS was responsible for the reduction of NO production. Therefore, our data shows that DAE-M is an effective suppressor of inflammatory responses caused by upregulation of inflammatory cytokines or mediators
DAE-M treatment effectively reduced LPS-induced increase in iNOS mRNA at all given concentrations, although the effect was not dose dependent (Figure 6A). iNOS expression was reduced approximately 55% by DAE-M (800 µg/mL). This DAE-M-mediated downregulation of iNOS was also observed at the protein level. DAE-M treatment decreased iNOS protein levels in a dose-dependent manner. A high dose (800 µg/mL) of DAE-M caused a 90% reduction in iNOS protein expression (Figured 6B, C). This result showed that DAE-M-mediated reduction of iNOS protein level was due to the downregulation of DAE-M mRNA expression. These data on the iNOS expression cor- related with data on NO production, which was decreased by DAE-M treatment as well (Figures 3). These results indicated that LPS-induced NO production was due to the upregulation of iNOS and that DAE-M-mediated downregulation of iNOS was responsible for the reduction of NO production. Therefore, our data shows that DAE-M is an effective suppressor of inflammatory responses caused by upregulation of inflammatory cytokines or mediators
Discussion
In this study, we investigated the anti-inflammatory effect of DADO with fractional analysis. Numerous studies have been performed on the pharmacological effects of deer antler and antler, [11-14] but most studies were executed using water extraction of deer antler, which produces a large amount of oil remnants that are not well studied. Here, we examined the biological effects of oil fractions from deer antler extraction and performed compositional analysis of the lipids. Deer oil is insoluble in DMSO because of its hydrophobicity, making it difficult to perform experiments. Therefore, liquid–liquid fractionation of the sample was performed, yielding a fraction (DAE-M) that was more hydrophilic and soluble in DMSO.
DAE-M was in liquid form at room temperature, while DADO needs heating process (50–60°C) to be liquid state. The content of unsaturated fatty acids and sugars in DAE-M was higher than the other fractions (DAE-H and DADO) (Table 2, Figure 4), which contributed to it increased solubility and lower melting point.
Although deer antler-derived active substances for therapeutic effects have not been identified, several studies have re- ported the presence of complex lipids, such as glycolipids and phospholipids, as the active substances in deer antler. [16,17,21] TLC analysis in current study revealed that the DAE-M fraction possessed complex lipids containing a much more hydrophilic moiety than the DAE-H fraction, which had no anti- inflammatory effect (Figure 4). Previously, another study using the same solvent system as ours showed the same positions of phospholipids and glycolipids in TLC analysis, suggesting that those complex lipids in our study likely include phospholipids and glycolipids. [22]
Several studies have suggested a relationship between ganglioside, a biological molecule that is com- posed of glycosphingolipids and sialic acid, and the pharmacological effect of deer antler. [12,17,21] Specifically, gangliosides are regulators of local and systemic inflammation and have anti-inflammatory effects. [23,24] Therefore, DADO-derived anti-inflammatory substances are considered a kind of glycolipid such as ganglioside, but further detailed analysis is needed to identify the active substances.
Our data also showed that DAE-M fraction significantly decreased the production of NO (Figure 3), a mediator of inflammatory responses, in RAW264.7 cells. In contrast, DAE-H, a fraction with higher hydrophobicity than DAE-M, did not inhibit NO production (Figure 3). These data indicated that molecules containing a relatively hydrophilic moiety of deer antler oil are inhibitory in inflammatory responses. These data were correlated with the higher sugar content in DAE-M (Figure 4). Indeed, the DAE-H fraction was difficult to dissolve in DMSO, a vehicle solvent in cell treatment, and a suspension of DAE-H was applied to the cells for the NO assay. However, DAE-H in suspension becomes clear in the cell culture medium, suggesting DAE-H is dissolved in the medium when it is treated to the cells. DAE-M inhibited NO production, implying that DAE-M is a regulator of inflammatory responses. Normal levels of NO are involved in vasodilation, but excessive production of NO in abnormal conditions leads to inflammation. [25,26] In osteoarthritis patients, levels of NO are high in their cartilage even in the absence of inflammatory stimuli, such as IL-1β and LPS. [25,26] In addition, the synthesis of proteoglycan and collagen, components of healthy and elastic skin, are known to be inhibited by NO. [26] In our study, the de- crease of NO synthesis with DAE-M treatment was due to the downregulation of iNOS, a pro-inflammatory enzyme responsible for NO production. DAE-M effectively suppressed LPS- induced upregulation of iNOS at both the mRNA and protein level (Figure 6). Increase of NO production has been shown to stimulate the expression of COX-2, another pro-inflammatory biomarker. [27] COX-2, which is associated with the conversion of arachidonic acid to prostaglandin and eicosanoids, is clinically important as a major target of NSAIDs, like aspirin. [28,29] However, these synthetic drugs are linked with undesirable side effects, such as intestinal bleeding, swelling of tissue, and allergies. [9,10] DAE-M effectively suppressed COX-2 expression at the mRNA level (Figure 5), suggesting that deer antler oil can be used as a natural source to suppress the COX-2-associated inflammatory responses.
Expression of iNOS and COX-2, which are representative inflammatory markers, are upregulated by pro-inflammatory cytokines, such as IL-1β and IL-12β, in various immune cell types. [25,30] IL-1β is usually produced by macrophages, and particularly, IL-1β-induced COX-2 induction in the central nervous system is known to be responsible for inflammatory pain hypersensitivity. [31] IL-12β is linked to autoimmune dis- ease with induction of the T helper cell immune response. [32] In addition, IL-12β seemed to be an important mediator for perpetuating inflammation and successive destruction in the diseased area. [33] Our data showed that DAE-M significantly inhibited mRNA expressions of these inflammatory cytokines, suggesting that DAE-M regulates the expression of cytokines to suppress the inflammatory response. These data are supported by other studies showing improvement of inflammation conditions with suppression of inflammatory cytokines. [13,34,35]
In addition, a recent study reported that deer antler extract activated macrophages to alleviate neutropenia, which is characterized by a low neutrophil number and an increased level of cytokines (IL-6 and tumor necrosis factor alpha (TNF-α) in mouse neutropenia model system). [36] Collectively, deer antler-derived samples seem to have beneficial effects on inflammation, as shown in our study. However, this study did not address signaling pathways involved in DAE-M-mediated inhibition of inflammatory responses. Inflammatory cytokines and mediators, including iNOS and COX-2, have been shown to be controlled by nuclear factor kappa B (NF-κB), a transcription factor responsible for the synthesis of inflammatory substances. [37] Translocation of NF-κB into the nucleus increases its binding to the genes of inflammatory cytokines, thereby promoting their expression. [37] Activation of NF-κB is controlled by upstream signaling pathways like the Toll like receptor (TLR) system. [38] Chronic stimulation of TLR leads to the activation of inflammatory genes via NF-κB activation. [39] Li., et al. showed that chloroform extract of deer antler inhibited osteoclast differentiation by receptor activator of NF-κB ligand (RANKL)-stimualted mouse antler marrow. [40] Based on these data, DAE-M may mediate its anti-inflammatory effects by inhibiting NF-κB signaling. Future efforts should focus on determining the detailed mechanistic action of deer oil in anti-inflammation, including the role of the TLR and NF-κB pathways.
In conclusion, DAE-M fraction of deer antler oil is enriched with unsaturated fatty acid and sugars, which can contribute to the solubility and the formation of active components in DADO. DAE-M effectively inhibited inflammatory responses by suppressing inflammatory cytokines and mediators. Our study suggests that deer antler oil can be used as a natural anti-inflammatory agent.
References
- Serhan CN., et al. “Fundamentals of inflammation”. Cambridge University Press (2010).
- Coussens LM and Werb Z. “Inflammation and cancer”. Nature 420.6917 (2002): 860–867.
- Libby P. “Inflammation and cardiovascular disease mechanisms”. The American Journal of Clinical Nutrition 83.2 (2006): 456S-460S.
- Wellen KE and Hotamisligil GS. “Inflammation, stress, and diabetes”. The Journal of Clinical Investigation 115.5 (2005): 1111–1119.
- Xu H., et al. “Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance”. The Journal of Clinical Investigation 112.12 (2003): 1821–1830.
- Landskron G., et al. “Chronic inflammation and cytokines in the tumor microenvi- ronment”. Journal of Immunology Research 2014 (2014): 149185.
- McInnes IB and Schett G. “Cytokines in the pathogenesis of rheumatoid arthritis”. Nature Reviews Immunology 7.6 (2007): 429–442.
- Rashad S., et al. “Effect of non-steroidal anti-inflammatory drugs on the course of osteoarthritis”. Lancet 334.8662 (1989): 519-522.
- Huang J., et al. “Therapeutic properties of quercetin on monosodium urate crystal-induced in- flammation in rat”. The Journal of Pharmacy and Pharmacology 64.8 (2012): 1119–1127.
- Jiang Y., et al. “Effects of extract from Man- gifera indica leaf on monosodium urate crystal-induced gouty arthritis in rats”. Evidence-Based Complementary and Alternative Medicine 2012 (2012): 967573.
- Zhao L., et al. “Antioxidant activity of protein hydrolysates from aqueous extract of velvet antler (Cervus elaphus) as influenced by molecular weight and enzymes”. Natural Product Communications 6.11 (2011): 1683–1688.
- Du CN., et al. “Deer antler extract prevents against scopolamine-induced memory impairment in mice”. Journal of Medicinal Food 18.2 (2015): 157-165.
- Lee H., et al. “Effects of deer antler extract on the expression of pro-inflammatory cytokine and cartilage-related genes in monosodium iodoacetate- induced osteoarthritic rats”. Bioscience, Biotechnology, and Biochemistry 78.10 (2014): 1703-1709.
- Wang B-X., et al. “Effects of repeated administration of deer antler extract on biochemical changes related to aging in senescence-accelerated mice”. Chemical and Pharmaceutical Bulletin 36.7 (1988): 2587–2592.
- Ha H and Yoon S. “Analytical studies of constituents of antlers”. Journal of the Korean Society of Food Science and Nutrition 25 (1996): 279-282.
- Ivankina NF., et al. “Prostaglan- din-like activity, fatty acid and phospholipid composition of sika deer (Cervus nippon) antlers at different growth stages”. Comparative Biochemistry and Physiology B 106.1 (1993): 159–162.
- Kim Y., et al. “Biochemical studies on antler (Cervus nip- pon taiouanus) (V) a study on glycolipids and phospholipids of ant- ler velvet layer and pantocrin”. Journal of Biochemistry and Molecular Biology 10 (1977): 153–159.
- Zhao SL. “Chemical constituents of hairy young horn of Capreolus capreolus-isolation and identification of fat-soluble constituents”. Zhong Yao Tong Bao 9.4 (1984): 174.
- Ge R and Pu H. “Effects of ginsenosides and pantocrine on the repro-ductive endocrine system in male rats”. Journal of Traditional Chinese Medicine 6.4 (1986): 301–304.
- DuBois M., et al. “Colorimet- ric method for determination of sugars and related substances”. Analytical Chemistry 28.3 (1956): 350-356.
- Jhon GJ., et al. “Studies of the chemical structure of gangliosides in deer antler, Cervus nippon”. Chemical and Pharmaceutical Bulletin 47.1 (1999): 123–127.
- Kupke IR and Zeugner S. “Quantitative high-performance thin-layer chromatography of lipids in plasma and liver homogenates after direct application of 0.5-µL samples to the silica-gel layer”. Journal of Chromatography B: Biomedical Sciences and Applications 146.2 (1978): 261–271.
- Ohmi Y., et al. “Gangliosides are essential in the protection of in- flammation and neurodegeneration via maintenance of lipid rafts: elucidation by a series of ganglioside-deficient mutant mice”. Journal of Neurochemistry 116.5 (2011): 926–935.
- Park EJ., et al. “Dietary ganglioside inhibits acute inflammatory signals in intestinal mucosa and blood induced by systemic inflam- mation of Escherichia coli lipopolysaccharide”. Shock 28.1 (2007): 112–117.
- Amin AR and Abramson SB. “The role of nitric oxide in articular cartilage breakdown in osteoarthritis”. Current Opinion in Rheumatology 10.3 (1998): 263–268.
- Sharma JN., et al. “Role of nitric oxide in in- flammatory diseases”. Inflammopharmacology 15.6 (2007): 252–259.
- Patel R., et al. “Regulation of cytosolic COX-2 and prostaglandin E2 production by nitric oxide in activated murine macrophages”. The Journal of Immunology 162.7 (1999): 4191–4197.
- Budenholzer BR. “Are selective COX 2 inhibitors superior to tradi- tional NSAIDs? Rofecoxib did not provide unequivocal benefit over traditional NSAIDs”. BMJ 325.7356 (2002): 161.
- Hawkey C., et al. “Comparison of the effect of rofecoxib (a cyclooxygenase 2 inhibitor), ibuprofen, and placebo on the gastroduodenal mucosa of patients with osteoarthritis: a randomized, double-blind, placebo-controlled trial. The Rofecoxib Osteoarthritis Endoscopy Multinational Study Group”. Arthritis & Rheumatology 43.2 (2000): 370–377.
- Kuwano T., et al. “Cyclooxygenase 2 is a key enzyme for inflam- matory cytokine-induced angiogenesis”. The FASEB Journal 18.2 (2004): 300–310.
- Shaftel SS., et al. “The role of interleukin-1 in neuroinflammation and Alzheimer’s disease: an evolving perspec- tive”. Journal of Neuroinflammation 5 (2008): 7.
- Zundler S and Neurath MF. “Interleukin-12: Functional activities and implications for disease”. Cytokine & Growth Factor Reviews 26.5 (2015): 559–568.
- Sakkas LI., et al. “Interleu- kin-12 is expressed by infiltrating macrophages and synovial lining cells in rheumatoid arthritis and osteoarthritis”. Cellular Immunology 188.2 (1998): 105–110.
- Kang S-K., et al. “Im- munosuppressive activity of deer antler extracts of Cervus korean Temminck var. mantchuricus Swinhoe, on type II collagen-induced arthritis”. In Vitro Cellular & Developmental Biology 42(3-4) (2006): 100–107.
- Kim KS., et al. “Protective and anti-arthritic effects of deer antler aqua-acupuncture (DAA), inhibiting dihydroorotate dehydrogenase, on phosphate ions-mediated chondrocyte apoptosis and rat collagen- induced arthritis”. International Immunopharmacology 4.7 (2004): 963–973.
- Choi HS., et al. “Water extract of deer antlers activates macrophages and alleviates neutropenia”. Evidence-Based Complementary and Alternative Medicine 2013 (2013): 617302.
- Brown KL., et al. “Complexities of targeting innate immunity to treat infection”. Trends in Immunology 28.6 (2007): 260–266.
- Rhee SH and Hwang D. “Murine TOLL-like receptor 4 confers lipopolysaccharide responsiveness as determined by activation of NF kappa B and expression of the inducible cyclooxygenase.” The Journal of Biological Chemistry 275.44 (2000): 34035-34040.
- Rossol M., et al. “LPS-induced cytokine production in human mono- cytes and macrophages”. Critical Reviews in Immunology 31.5 (2011): 379–446.
- Li Y-J., et al. “Chloro- form extract of deer antler inhibits osteoclast differentiation and antler resorption”. Journal of Ethnopharmacology 113.2 (2007): 191–198.
Citation:
Young Wook Jo., et al. “Deer Oil Extract Suppresses LPS-Induced Inflammatory Signaling in Skin Immune Cells”. Nutrition and
Food Toxicology 2.4 (2018): 407-418.
Copyright: © 2018 Young Wook Jo., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.



































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.