Research Article
Volume 2 Issue 6 - 2018
Pasting Properties and some Functional Properties of Starches from 8 Tropical Legumes Grown in Central Africa
1Faculty of Sciences, Department of Biological sciences University of Maroua P O Box 814 Maroua Cameroun
2National Advanced School of Enginering of Maroua, Department of Agriculture Livestock and By-products, P.O BOX 46, Maroua, Cameroon
3National School of Agro-Industrial sciences (ENSAI) University of Ngaoundéré, P O Box 455 Adamaoua, Cameroon
2National Advanced School of Enginering of Maroua, Department of Agriculture Livestock and By-products, P.O BOX 46, Maroua, Cameroon
3National School of Agro-Industrial sciences (ENSAI) University of Ngaoundéré, P O Box 455 Adamaoua, Cameroon
*Corresponding Author: Njintang Yanou Nicolas, Faculty of Sciences, Department of Biological sciences University of Maroua P O Box 814 Maroua Cameroun, Cameroon.
Received: April 09, 2018; Published: April 16, 2018
Abstract
Starches from five varieties of Phaseolus vulgaris L. (common bean), one variety of Vigna subterrenea V (bambara groundnut) and one variety of Vigna unguiculata (Cowpea) were isolated by wet milling process, centrifugation and freeze drying. The physicochemical and functional properties of the starches including swelling power and solubility pattern, water absorption capacity and pasting properties were examined. The result revealed significant differences amongst the properties of the starches. The swelling power of the legume starch isolates put them in the category of highly restricted-swelling starch. This characteristic is desirable for the manufacture of value-added products such as noodles and composite blends with cereals. The high BV was founded for Cowpea and Bambara groundnut and confirmed their low. Ability to resist heat and shear stress when compared to Common bean varieties studies. The factors which influence the pasting characteristics resulting to decrease in peak viscosity (PV), trough viscosity (TV) and final viscosity (FV) of starch are attributed to the interaction of starch with the protein, fat, etc. which depended to their variety.
Keywords: Common bean; Cowpea; Bambara groundnut; starch; Physico-chemical properties; Pasting properties; Functional properties
Introduction
Legumes seeds are important ingredients of a balanced human diet in many parts of the world due to their high protein (15-40%) and starch (35-60 %) contents. They are consumed traditionally as whole seeds or in the form of flour. Legume seeds are major sources of dietary proteins in the developing countries, as animal proteins are expensive. In addition to their protein contributions, legumes are also rich in other nutrients such as starch, dietary fiber, protective phytochemicals, oil, vitamins and mineral elements (Saikia., et al. 1999). Legumes contain about 60% carbohydrate including starch, reducing and non-reducing sugars, oligosaccharides of the raffinose family, etc. Legumes seeds protein isolates have been the interest of research for many years, as they may play a role as food ingredient and contribute in alleviating protein malnutrition in the world. In this respect, the physicochemical and nutritional properties of protein isolates of legumes seeds such as mung bean (Rahma., et al. 2000), chickpea soybean seeds and pea seeds (Withana-Gamage et al., 2010; Papalamprou., et al. 2009), faba bean seeds (Fernandez Quintela., et al. 1998) have been studied for their use as emulsifying agents and food ingredients. Hence several isolations techniques of proteins have been developed.
In some cases of the process of protein isolate production, the dry legume seeds are dehulled, ground into flour and solubilized in water. The mixture is filtered, and the residue resubmitted to extraction. The obtained extract which contains proteins and carbohydrates is keep overnight for particle to settle and the solution containing proteins directly of after precipitation. Studies by Pahane., et al. (2017) and Ngatchic., et al. (2013) reported the use of Bambara and mucuna seeds for legume seeds milk production and protein isolate. However, the production of legume seeds extracts or protein isolates can be of economic value only if their starch component is made profitable simultaneously. In this respect the physicochemical and functional properties of the legume starches need to be investigated.
Generally, starch is convertible to many useful materials by chemical and biochemical techniques, as well as by fermentation (Eliasson, 1996). It plays an important role in food industries because it affects the physical properties of many foods, and it mainly uses as thickener, water binding, emulsion stabilizer and gelling agent. Starches from various plant sources have their own unique properties that enable them to tolerate a wide range of processing techniques as well as various distributions, storage and final preparation conditions via either chemical or physical modified methods (Daniel and Weaver, 2000).
Starch characteristics such as swelling power and solubility pattern, physico-chemical and functional properties are important for improved quality of food products. Dry legume starches have been recognized as a potential food ingredient containing a relatively high proportion of amylose when compared to cereal starches (Yixiang Xu., et al. 2013). Legume starches have a higher resistance to swelling and rupture than do cereal starches. Among the commonly consumed food legumes, common bean (Phaseolus vulgaris L.) and cowpea (Vigna subterranea) are the most widely produced and consumed legumes in the world and occupies an important place in human nutrition in East and Great Lakes Regions of Africa (Shimelis and Rakshit, 2005; Doughty and Walker, 1982; De Godinez., et al. 1992). Greater attention is actually being paid to improve and diversify production of dry legume seeds common beans in Africa. In this respect, ongoing programs work towards development of high-yielding and disease resistant varieties through adaptation, selection and hybridization. Improving the use of dry seeds for protein and starch ingredients in food industries will go a long way open new avenue for new and value-added products developments. This study was undertaken to determine some physico-chemical and functional properties of native starches from common dry seeds legume cultivars.
Materials and Methods
Sources of Legume seeds and sample Preparation
Eight varieties of dry legume seeds belonging to 2 species (Vigna sp. and Phaseolus sp.) were used for this study. They were purchased from a local market in the Adamawa and west regions of Cameroon in the Central Africa. The dry seeds of each variety were separately cleaned, uniformed in size and color, and freed from foreign or abnormal seeds, odors and living or dead insects. For each variety, 2 kg portions of breeding seeds were placed in an aluminum box and transported to the Laboratory. For each lot, sufficient amount of legume seeds was taken as required, rinsed four times in deionized water to eliminate dust and the insecticide (used for protecting treatment), dried at room temperature and packed in plastic bags and stored at 4°C until used. All the chemicals used were analytical grade.
Eight varieties of dry legume seeds belonging to 2 species (Vigna sp. and Phaseolus sp.) were used for this study. They were purchased from a local market in the Adamawa and west regions of Cameroon in the Central Africa. The dry seeds of each variety were separately cleaned, uniformed in size and color, and freed from foreign or abnormal seeds, odors and living or dead insects. For each variety, 2 kg portions of breeding seeds were placed in an aluminum box and transported to the Laboratory. For each lot, sufficient amount of legume seeds was taken as required, rinsed four times in deionized water to eliminate dust and the insecticide (used for protecting treatment), dried at room temperature and packed in plastic bags and stored at 4°C until used. All the chemicals used were analytical grade.
Starch Extraction
Starches were extracted from legume seeds by the method adopted by Sathe., et al. (1982). In the procedure of starch extraction, 0.5 kg seeds was washed abundantly with tap water, soaked for 12h, manually dehulled and ground to obtain a paste. Water (5L) was then added to the paste and mixed manually. The mixture was kept for 12h during which the starch settled. The precipitated was then resuspended in 1L NaCl 1% and gently mixed. The mixture was kept for 12h, centrifuged (3800g, 20 min, 20°C). The precipitate was collected, washed twice with distilled water, suspended in 1L NaOH 0.03M, gently mixed and kept at 4°C for 12h. The starch solution was again centrifuged at 3800g for 20 min. the precipitate was washed with distilled water through a sieve mesh 125µm to eliminate fibers and the obtained solution was centrifuged (3800g, 20 min, 20°C). The resulting precipitate was dispersed on a tray and dried at 45°C for 45 min, grounded into powder in a mortar, packaged in polyethylene bag and stored at 4°C until used. Commercial rice starch (S-7260) purchased from Sigma Chemical Co (St. Louis, Mo., U.S.A) and was used as a control.
Starches were extracted from legume seeds by the method adopted by Sathe., et al. (1982). In the procedure of starch extraction, 0.5 kg seeds was washed abundantly with tap water, soaked for 12h, manually dehulled and ground to obtain a paste. Water (5L) was then added to the paste and mixed manually. The mixture was kept for 12h during which the starch settled. The precipitated was then resuspended in 1L NaCl 1% and gently mixed. The mixture was kept for 12h, centrifuged (3800g, 20 min, 20°C). The precipitate was collected, washed twice with distilled water, suspended in 1L NaOH 0.03M, gently mixed and kept at 4°C for 12h. The starch solution was again centrifuged at 3800g for 20 min. the precipitate was washed with distilled water through a sieve mesh 125µm to eliminate fibers and the obtained solution was centrifuged (3800g, 20 min, 20°C). The resulting precipitate was dispersed on a tray and dried at 45°C for 45 min, grounded into powder in a mortar, packaged in polyethylene bag and stored at 4°C until used. Commercial rice starch (S-7260) purchased from Sigma Chemical Co (St. Louis, Mo., U.S.A) and was used as a control.
Physico-chemical Properties of legume starch extracts
Chemical Composition Assessment
The chemical analysis of the isolated starch fractions included the determination of moisture using modified vacuum-oven method (AACC, 1983; Method 44-40), crude lipid content using Sox Tec service unit 1046 and Fibertec I and M systems (Foss Tecator, Sweden) according to method 923.05 and 962.09 respectively, of the Official Methods of AOAC (2000). Total ash content was also analyzed according to method 923.03 of the AOAC Official Methods (2000). Crude protein (N x 5.7 for starch) was performed using Kjeldhal block digestion and steam distillation (2200 Kjeltec Auto distillation, Foss Tecator, Sweden) method according to AOAC (2000) official methods 979.09.
Chemical Composition Assessment
The chemical analysis of the isolated starch fractions included the determination of moisture using modified vacuum-oven method (AACC, 1983; Method 44-40), crude lipid content using Sox Tec service unit 1046 and Fibertec I and M systems (Foss Tecator, Sweden) according to method 923.05 and 962.09 respectively, of the Official Methods of AOAC (2000). Total ash content was also analyzed according to method 923.03 of the AOAC Official Methods (2000). Crude protein (N x 5.7 for starch) was performed using Kjeldhal block digestion and steam distillation (2200 Kjeltec Auto distillation, Foss Tecator, Sweden) method according to AOAC (2000) official methods 979.09.
Water absorption capacity, Swelling Power and Solubility Pattern
Solubility index, water absorption and swelling power patterns at 60 and 90°C were determined using a modified version of Sathe and Salunkhe (1981) method. Briefly, 40 ml of a starch suspension (4, 6 and 8%, w/v) was prepared in a previously tared 50 ml centrifuge tube. A magnetic agitator was put in the tube, which was placed in a water bath for 30 min at a constant temperature (60 or 90°C). The suspension was then centrifuged at 2120 g for 15 min, the supernatant decanted and the swollen granules weighed. The supernatant was placed in a crucible and dried in an air convection oven (Imperial V) at 120°C for 4 h to constant weight. Solubility expressed in g particle/100g starch and swelling power expressed in g water/g starch were calculated using the formulas:
Solubility index, water absorption and swelling power patterns at 60 and 90°C were determined using a modified version of Sathe and Salunkhe (1981) method. Briefly, 40 ml of a starch suspension (4, 6 and 8%, w/v) was prepared in a previously tared 50 ml centrifuge tube. A magnetic agitator was put in the tube, which was placed in a water bath for 30 min at a constant temperature (60 or 90°C). The suspension was then centrifuged at 2120 g for 15 min, the supernatant decanted and the swollen granules weighed. The supernatant was placed in a crucible and dried in an air convection oven (Imperial V) at 120°C for 4 h to constant weight. Solubility expressed in g particle/100g starch and swelling power expressed in g water/g starch were calculated using the formulas:
Water absorption capacity expressed in g water/100g sample was measured using the same conditions as above, but expressed as weight of the gel formed per sample, divided by treated sample weight.
Pasting Profiles
A Rapid Visco Analyzer (Model RVA-4, Newport Scientific Pty. Ltd., Sydney, Australia, 1995) with Thermocline for windows software was used to evaluate the pasting properties of the dry legume starches. Test runs were conducted following standard profile 1 which include 1 min of mixing, stirring, and warming up to 50°C, 3 min and 42 sec of heating at 12°C/min up to 95°C, 2.5 min of holding at 95°C, 3 min and 48 sec of cooling down to 50°C, at the same rate as the heating (12°C/min) and 2 min holding at 50°C, where the process ends after 13 minutes (Deffenbaugh and Walker, 1989). Starch gelatinization (pasting) curves were recorded on RVA and viscosity was expressed in terms of centipoises (Cp).
A Rapid Visco Analyzer (Model RVA-4, Newport Scientific Pty. Ltd., Sydney, Australia, 1995) with Thermocline for windows software was used to evaluate the pasting properties of the dry legume starches. Test runs were conducted following standard profile 1 which include 1 min of mixing, stirring, and warming up to 50°C, 3 min and 42 sec of heating at 12°C/min up to 95°C, 2.5 min of holding at 95°C, 3 min and 48 sec of cooling down to 50°C, at the same rate as the heating (12°C/min) and 2 min holding at 50°C, where the process ends after 13 minutes (Deffenbaugh and Walker, 1989). Starch gelatinization (pasting) curves were recorded on RVA and viscosity was expressed in terms of centipoises (Cp).
Statistical Analysis
One-way analysis of variance (ANOVA) was conducted on each of the variables and the least significant difference (LSD) test at a significance level P < 0.05 was performed using SPSS/12 software for Windows to compare the difference between treatment means. Results were expressed as the means ± standard deviation of three separate determinations.
One-way analysis of variance (ANOVA) was conducted on each of the variables and the least significant difference (LSD) test at a significance level P < 0.05 was performed using SPSS/12 software for Windows to compare the difference between treatment means. Results were expressed as the means ± standard deviation of three separate determinations.
Results and Discussion
Physico-chemical Properties of legume Starches
The chemical composition of the dry legume starches studied is presented in table 1. Protein, fat, ash and moisture contents showed significant difference (P < 0.05) among the legume starches. Protein content ranged 0.30 to 2.71 g/100g, crude fat 0.40 to 0.96 g/100g, ash 0.17 to 0.35 g/100g and moisture 9.2 to 11.3 g/100g dry starch. These values are in the range reported for other legumes seeds (Barampama and Simard, 1993; Bishnoi and Khetarpaul, 1993; Tharanathan and Mahadevamma, 2003). The presence of components other than starch represents impurities, and in this case they were few. This is probably consequence of washing procedures used including NaCl and NaOH washings as also reported in earlier studies (Galvez and Ressuression, 1993).
The chemical composition of the dry legume starches studied is presented in table 1. Protein, fat, ash and moisture contents showed significant difference (P < 0.05) among the legume starches. Protein content ranged 0.30 to 2.71 g/100g, crude fat 0.40 to 0.96 g/100g, ash 0.17 to 0.35 g/100g and moisture 9.2 to 11.3 g/100g dry starch. These values are in the range reported for other legumes seeds (Barampama and Simard, 1993; Bishnoi and Khetarpaul, 1993; Tharanathan and Mahadevamma, 2003). The presence of components other than starch represents impurities, and in this case they were few. This is probably consequence of washing procedures used including NaCl and NaOH washings as also reported in earlier studies (Galvez and Ressuression, 1993).
Young., et al. (1996) reported in their study the range moisture content of potato starches higher (13.5-18.2%) than that of bean starches (10.8-11.0%). While the moisture contents of our starch samples were consistent with literature, they were lower compared to that of potato starch. According to Swinkels (1985), potato starch typically has higher moisture content than the other starches.
Ash content also showed no significant difference and the values are from 0.17 for red beans to 0.35g/100g dry starch for black beans. Similar contents were reported earlier (Lee., et al. 2006; Shimelis., et al. 2006; Sung and Stone 2004).
| Varieties | Moisture | Crude proteins | Crude fat | ash |
| cowpea | 10.22 ± 0.20a | 2.71 ± 0.05c | 0.54 ± 0.01a | 0.24 ± 0.01a |
| Bambara groundnut | 9.25 ± 0.18a | 0.30 ± 0.01a | 0.50 ± 0.01a | 0.28 ± 0.01a |
| Red beans | 11.25 ± 0.22a | 0.97 ± 0.02ab | 0.40 ± 0.01a | 0.17 ± 0.01a |
| White beans | 10.72 ± 0.21a | 1.44 ± 0.03b | 0.40 ± 0.01a | 0.26 ± 0.01a |
| Green beans | 9.23 ± 0.18a | 0.62 ± 0.01a | 0.6 ± 0.01a | 0.21 ± 0.01a |
| Black beans | 9.64 ± 0.19a | 1.11 ± 0.02b | 0.49 ± 0.01a | 0.35 ± 0.01a |
| Red spotted beans | 11.18 ± 0.22a | 0.95 ± 0.02ab | 0.96 ± 0.02b | 0.21 ± 0.01a |
| Yellow beans | 10.72 ± 0.21a | 0.60 ± 0.01a | 0.51 ± 0.01a | 0.31 ± 0.01a |
Table 1: Chemical composition (g/100 g) of starches from 8 dry legume seeds.
Water absorption capacity, Water Solubility Index and Swelling Power.
The swelling power and solubility of the dry legume starches at 60°C and 90°C are shown in Table 2. The swelling power (14.48%) and solubility index (17.0%) of Bambara starch at 90°C were the highest, followed by that of Cowpea starch with 12.7 % swelling power and 13.1 % solubility. The solubility index of the five varieties of common bean (range 10.0 to 11.1 g/100g) at 90°C did not significantly (p < 0.05) vary each other with values range. In other hand at 90°C, the swelling power of green bean, Yellow bean and Black bean did not significantly varied (range 6.72-7.8%), and White bean, Red bean and Red spotted bean had swelling power of 11.3, 10.2 and 10.3%, respectively. it can easily be seen from the table that WAC, SP and WSI increased with increase in temperature. Comparatively to properties of rice starch used as reference in these analysis with values at 60°C and 90°C of WAC (407% and 1395%), WSI (2 and 14% ) and SP (4% and 16%), the legume starches exhibited significantly lower values. Therefore, the resulting swelling power indicated that the starch extracts obtained were highly restricted type according to Schoch and Maywald (1968). The difference between the swelling powers of starch isolates might be due to the amylose content, the protein-amylose complex formation (Pomeranz, 1991). The observation reported by Lai and Varriano-Marston (1979) for black bean starch was 17.9% solubility at 95°C. Lii and Chang (1981) reported higher values of WSI (25%) and SP power (32%). Generally, we observed a significant and positive correlation (r = 0.67) between the solubility and swelling power. This is seen as mainly the result of granule swelling permitting the exudation of amylose (Dengate, 1984).
The swelling power and solubility of the dry legume starches at 60°C and 90°C are shown in Table 2. The swelling power (14.48%) and solubility index (17.0%) of Bambara starch at 90°C were the highest, followed by that of Cowpea starch with 12.7 % swelling power and 13.1 % solubility. The solubility index of the five varieties of common bean (range 10.0 to 11.1 g/100g) at 90°C did not significantly (p < 0.05) vary each other with values range. In other hand at 90°C, the swelling power of green bean, Yellow bean and Black bean did not significantly varied (range 6.72-7.8%), and White bean, Red bean and Red spotted bean had swelling power of 11.3, 10.2 and 10.3%, respectively. it can easily be seen from the table that WAC, SP and WSI increased with increase in temperature. Comparatively to properties of rice starch used as reference in these analysis with values at 60°C and 90°C of WAC (407% and 1395%), WSI (2 and 14% ) and SP (4% and 16%), the legume starches exhibited significantly lower values. Therefore, the resulting swelling power indicated that the starch extracts obtained were highly restricted type according to Schoch and Maywald (1968). The difference between the swelling powers of starch isolates might be due to the amylose content, the protein-amylose complex formation (Pomeranz, 1991). The observation reported by Lai and Varriano-Marston (1979) for black bean starch was 17.9% solubility at 95°C. Lii and Chang (1981) reported higher values of WSI (25%) and SP power (32%). Generally, we observed a significant and positive correlation (r = 0.67) between the solubility and swelling power. This is seen as mainly the result of granule swelling permitting the exudation of amylose (Dengate, 1984).
| Varieties | WAC (%) | WSI (g/100g) | SP (g/g) | |||
| 60°C | 90°C | 60°C | 90°C | 60°C | 90°C | |
| Cowpea | 335 ± 7e | 1102 ± 23e | 4.0 ± 0.1d | 13.1 ± .3b | 3.5 ± 0.5b | 12.7 ± 0.3d |
| Bambara groundnut | 390 ± 8f | 1203 ± 23f | 4.9 ± 0.2e | 17.1± 0.3c | 4.1 ± 0.3c | 14.5 ± 0.5e |
| Red beans | 313 ± 6d | 920 ± 18c | 4.0 ± 0.1d | 10.0± 0.3a | 3.3 ± 0.5b | 10.2 ± 0.4b |
| White beans | 303 ± 6cd | 1005± 20d | 3.0 ± 0.1c | 11.1± 0.4a | 3.1 ± 0.4b | 11.3 ± 0.2c |
| Green beans | 243 ± 5b | 703 ± 14b | 2.9 ± 0.1c | 10.1± 0.2a | 2.5 ± 0.1a | 7.8 ± 0.5a |
| Black beans | 208 ± 4a | 605 ± 12a | 4.0 ± 0.1d | 10.2± 0.2a | 2.2 ± 0.2a | 6.7 ± 0.3a |
| Red spotted beans | 290 ± 6c | 918 ± 18c | 1.9 ± 0.1b | 11.1± 0.5a | 3.0 ± 0.2b | 10.3 ± 0.4b |
| Yellow beans | 200 ± 2a | 603 ± 13a | 1.0 ± 0.1a | 10.2± 0.2a | 2.0 ± 0.1a | 6.7 ± 0.4a |
Table 2: Some functional properties of dry legume seeds at 60 and 90°C.
Means ± standard deviation; n = 2; values in the same column followed with different letters are significantly different at p < 0.05. WAC water absorption capacity; WSI water solubility index; SP swelling power.
Both solubility and swelling power of dry seeds starches varied with temperature and it can be generally concluded that solubility and swelling power increase with temperature increment (Lai and Varriano-Marston 1979). Solubility of legume starch for all the 8 varieties in this study ranged between 10 to 17%. Lower value of solubility and swelling power of starch at low temperature is due to the crystalline nature of starch. Generally, starch absorbs less water at temperature lower than the gelatinization temperature. When starch is heated at higher temperature, the granule crystalline structure is disorganized and begins to swell. As the swelling increased amylose is leach out of the granules to increase the soluble fraction.
Results of the water absorption capacity (WAC) of legume starches are also shown in Table 2. Starch from Yellow bean, Black bean and Green bean varieties registered lower WAC than other varieties studied with respective values of 200, 208 and 243 g/100g at 60°C and 603, 605 and 703 g/100g at 90°C. It is known that water binding by starches is a function of several parameters including size, shape, conformational characteristics, steric factors, hydrophilic-hydrophobic balance in the starch molecule, lipids and carbohydrates associated with the proteins, thermodynamic properties of the system (energy of bonding, interfacial tension, etc.), physicochemical environment (pH, ionic strength, vapor pressure, temperature, presence/absence of surfactant etc.), solubility of starch molecules and others (Chou and Morr, 1979). Red bean and red spotted bean exhibited non-significant difference with respective values at 60 and 90°C of 313 and 920 g/100gC, and 290 g/100g to 918 g/100g. In addition, white bean, cowpea and Bambara starches registered respective values of 303, 335 and 390 g/100g at 60°C and 1005, 1103 and 1203 g/100 at 90°C. Sathe and Salunkhe (1981) obtained the value of 293g/100g at 21°C for water absorption of Northern green bean starch. Comer and Fry (1978) have reported cold water absorption of the purified pea starch to be 92-105 %.
Pasting Behavior
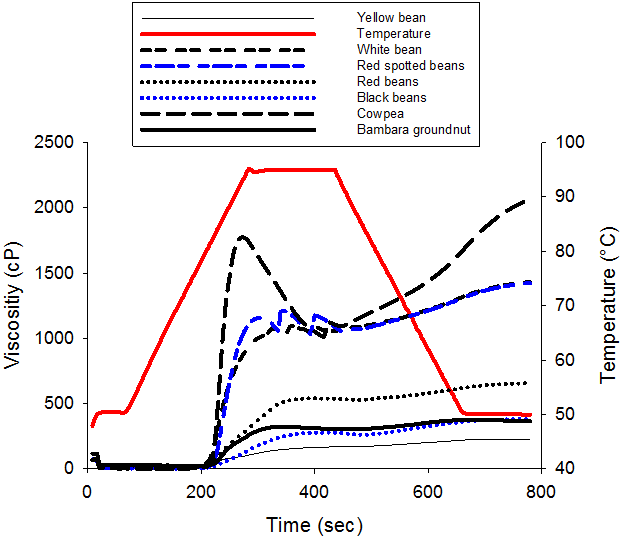
The pasting characteristics of the legume starches are shown in figure 1. The curve showed the behavior characteristic of starch with viscosity remaining fairly constant when temperature increases from 1 to 60-70°C. Over this limit temperature called pasting temperature, the viscosity increases exponentially to a pic viscosity over which the viscosity decreases to a final viscosity. As the temperature decreases, the viscosity of the solution increases to a final value. The pasting profile varied with starch legume seeds varieties and the starch concentration (Table 3). The pasting temperature of legumes seeds starches varies significantly (P < 0.05) and ranged from 71.7 to 79.9°C. These values were significantly (p<0.05) lower than reported values in literature (Sandhu and Lim, 2008) 50.2-52.5°C for black gram (Vigna mung), chickpea (Cicer arietinum), field pea (Pisum sativum), lentil (Lens culinaris), mung bean (Phaseolus aureus), and pigeon pea (Cajanus cajan). The peak temperatures (range xx – xx) were closed to 79.5°C and 75.8-80.3°C reported for four cultivars of field pea starches (Ratnayake., et al. 2001) and some Indian black gram starches (Singh., et al. 2004), respectively.
The pasting characteristics of the legume starches are shown in figure 1. The curve showed the behavior characteristic of starch with viscosity remaining fairly constant when temperature increases from 1 to 60-70°C. Over this limit temperature called pasting temperature, the viscosity increases exponentially to a pic viscosity over which the viscosity decreases to a final viscosity. As the temperature decreases, the viscosity of the solution increases to a final value. The pasting profile varied with starch legume seeds varieties and the starch concentration (Table 3). The pasting temperature of legumes seeds starches varies significantly (P < 0.05) and ranged from 71.7 to 79.9°C. These values were significantly (p<0.05) lower than reported values in literature (Sandhu and Lim, 2008) 50.2-52.5°C for black gram (Vigna mung), chickpea (Cicer arietinum), field pea (Pisum sativum), lentil (Lens culinaris), mung bean (Phaseolus aureus), and pigeon pea (Cajanus cajan). The peak temperatures (range xx – xx) were closed to 79.5°C and 75.8-80.3°C reported for four cultivars of field pea starches (Ratnayake., et al. 2001) and some Indian black gram starches (Singh., et al. 2004), respectively.
The pasting profile parameters are represented in table 3. Peak viscosity PV (the highest viscosity attainable during heating) corresponds to the point when the numbers of swollen, but still intact starch granules are maximal. It indicates the water binding capacity of the starch granules (Shimelis., et al. 2006) and it also frequently correlated with final product quality. We showed in this study that the Peak viscosity of the solution not only varied with legume variety, but also with the concentration of the starch slurry (Table 3). Dry legume starches presented PV values range from 168 to 1774 Cp at 8% starch varying from one variety to another. PV value was found to be lowest for Yellow bean (168Cp) and highest for Bambara bean and cowpea (1774Cp). However, at 4 and 6% starch concentration, no significant difference of PV was observed among varieties with respective values of 44 Cp and 295 Cp. Higher PV as well as lower PT reflects the capacity of starch granules to swell freely before physical breakdown or rupture as a result of higher temperature and mechanical agitation (Ashogbon and Akintayo, 2013b). Peak viscosity (PV) is accompanied immediately by a reduction in viscosity to a minimum call trough viscosity (TV).
The breakdown viscosity (BV) is a measure of the vulnerability of cooked starch to disintegration. BV of the starch samples varied significantly (P < 0.05) with the concentration of starch and variety. BV ranged between 63 and 670cp at 8% concentration. The lowest BV was for Yellow bean and green bean, followed by Black bean (64 Cp) and Red bean (94 Cp) starches while the highest BV were for Cowpea and Bambara starches. The higher the breakdown viscosity, the lower is the ability of the starch sample to withstand heat and shear stress during cooking(Ashogbon and Akintayo, 2013b).Therefore, Cowpea and Bambara starches possess less ability to resist heat and shear stress when compared to Common bean varieties in this study. Similar higher BV values were reported for starches from 2 Cowpea cultivars (Ashogbon and Akintayo, 2013b) and 13 improved Indian black gram cultivars (Singh., et al. 2004).
Final viscosity (FV) which indicates the ability of the starch to form a viscous paste varied significantly with concentration and variety of starch. At 8%, FV varied from 226 (Yellow bean) to 2060cp (Cowpea and Bambara). The high FV for the legume starches indicates that their paste could easily form a more rigid gel (Zhang., et al. 2005). Increase in FV is attributed to the re-association of amylose molecules during cooling and responsible of the starch retrogradation (Miles., et al. 1985). In addition, the differences in pasting characteristics of starches have been associated to factors among which Amylopectine molecular structure and Amylose contents play important role (Juliano., et al. 1987). The differences in the degree of randomly limited branching in amylose concentration might have also contributed to varietal differences (Ashogbon and Akintayo, 2012). Other reasons for differences may be inherent to differences in starch structure or may be due to different degree of interactions between starch and its associated compounds during pasting (Zhang and Hamaker, 2008).
| Variety | Starch concentration (%) | PT (°C) | PV (Cp) | TV (Cp) | BV (Cp) | FV (Cp) |
| White bean | 4 | 71,7 | 44 | 34 | 10 | 55 |
| 6 | 75,8 | 295 | 289 | 6 | 321 | |
| 8 | 78,15 | 1097 | 930 | 167 | 1430 | |
| Yellow bean | 4 | 71,7 | 44 | 34 | 10 | 55 |
| 6 | 71,7 | 295 | 289 | 6 | 321 | |
| 8 | 78,15 | 168 | 105 | 63 | 226 | |
| Black bean | 4 | 75 | 44 | 34 | 10 | 55 |
| 6 | 78,5 | 295 | 251 | 44 | 321 | |
| 8 | 79 | 277 | 213 | 64 | 379 | |
| Red bean | 4 | 76,55 | 44 | 34 | 10 | 55 |
| 6 | 78,15 | 295 | 251 | 44 | 321 | |
| 8 | 77,45 | 539 | 445 | 94 | 653 | |
| Red spotted bean | 4 | 76,55 | 44 | 34 | 10 | 55 |
| 6 | 78,15 | 295 | 289 | 6 | 321 | |
| 8 | 79 | 1211 | 1085 | 126 | 1423 | |
| Green bean | 4 | 76,55 | 44 | 34 | 10 | 55 |
| 6 | 78,15 | 295 | 289 | 6 | 321 | |
| 8 | 79,9 | 321 | 258 | 63 | 363 | |
| cowpea | 4 | 76,55 | 44 | 34 | 10 | 55 |
| 6 | 78,15 | 295 | 289 | 6 | 321 | |
| 8 | 78,2 | 1774 | 1104 | 670 | 2060 | |
| Bambara groundnut | 4 | 72,5 | 44 | 34 | 10 | 55 |
| 6 | 78,15 | 295 | 289 | 6 | 321 | |
| 8 | 78,2 | 1774 | 1104 | 670 | 2060 |
Table 3: Pasting properties of dry legume starches.
Conclusion
The pasting and functional properties of dry legume starches from different varieties vary significantly. The swelling power of starch isolate from seven common varieties of the legume beans studied fall on the group of highly restricted-swelling starches. This characteristic is desirable for starch extracts to be used for the manufacture of value-added products such as noodles and composite blends with cereals. Despite of the differences among the legume beans, the isolated starches had similar physico-chemical, pasting and functional properties. The significant differences in the functional properties of the legume bean starches especially their pasting properties indicated that these differences observed could be used in their selection for specific food processing applications.
Overall, the physico-chemical, pasting and functional properties obtained indicate that dry seeds starches have useful technological properties for many applications. It can be used in the food processing industry and non-food applications of starch. Research and development studies on African varieties of beans which are constantly released from various research centers will help to meet new demands in the Africa. Finally, in order to boost the small scale bean production and develop new market opportunities to stimulate economic growth in the continent. Further research and development programs in these aspects which can be integrated with other currently ongoing research activities are necessary.
References
- AACC. Approved Methods of the AACC. 18th Ed., American Association of Cereal Chemists, St. Paul, MN. Approved method 26-30, 40-44. (1983).
- Adebowale KO and Lawal OS. Microstructure, functional properties and retrogradation behavior of mucuna beans (Mucunapruriens) starch on heat moisture treatments. Food Hydrocoll. 17 (2003): 265-272.
- Alfredo Fernandez-Quintela., et al. “Nutritional Evaluation and Metabolic Eþects in Rats of Protein Isolates Obtained from Seeds of Three Legume Species”. Journal of the Science of Food and Agriculture 78 (1998): 251-260.
- Anderson RA., et al. “Gelatinization of corn grits by roll and extrusion cooking”. Cereal Science Today14 (1969): 11-12.
- AOAC. “fficial Methods of Analysis of AOAC International. Association of Official Analytical Chemists (AOAC) International, Williams, S. (ed). 14th ed., USA. Official Method 14.022, 14.031. (1984).
- AOAC. Analysis of the Association of Official Analytical Chemists, (AOAC) International, William, H. (ed). 17th ed., Gaithersburg, MD, USA: Official Method 923.03, 923.05, 962.09, 979.09. (2000).
- Ashogbon AO and Akintayo T. “Morphological and functional properties of starches from cereal and legume: A comparative study”. International Journal of Biotechnology and Food Science 1.4 (2013a): 72-83.
- Ashogbon AO and Akintayo E T. “Isolation and characterization of starches from two cowpea (Vigna unguiculata) cultivars.” International Food Research Journal 20.6 (2013b): 3093-3100.
- Ashogbon AO and Akintayo ET. “Morphological, functional and pasting properties of starches separated from rice cultivars grown in Nigeria. International Food Research Journal 19.2 (2012): 665-671.
- Ashogbon AO., et al. “Morphological, hydrolytic and thermal properties of legume starches”. Pakistan Journal of scientific and Industrial Research 54 (2011): 155-174.
- Barampama Z and Simard ER. “Nutrient composition, protein quality and antinutritional factors of some varieties of dry beans (Phaseolus vulgaris, L.) grown in Burundi”. Food Chemistry 47 (1993): 159-167.
- Beuchat LR. “Functional and electrophoretic characteristics of succinylated peanut flour protein”. Journal of Agricultural and Food Chemistry 25 (1977): 258-261.
- Bishnoi S and Khetarpaul N. “Variability in physico-chemical properties and nutrient composition of different pea cultivars”. Food Chemistry 47 (1993): 371-373.
- Buléon A., et al. “Starch granules: Structure and biosynthesis.” International Journal of Biological Macromolecules 23.2 (1998): 85-112.
- Chou DH and Morr CV. “Protein-water interactions and functional properties”. Journal of the American Oil Chemists’ Society 56.1 (1979): 534.
- Comer FW and Fry MK. “Purification, modification, and properties of air-classified pea starch”. Cereal Chemistry 55 (1978): 818.
- Daniel JR and Weaver CM. Carbohydrates: Functional properties. In: Christen GL, Smith JS. Eds., Food chemistry: Principles and applications. California: Science technology system, (2000): 63-66.
- David J and William AA. Starch modifications. Ergan hand book series: Starches. American Association of Cereal Chemists, (1999): 31- 48.
- De Godinez CM., et al. “Apparent digestibility of bean protein evaluated in humans, rats and in vitro assays”. Nutrition Research 12.2 (1992):235-246.
- Deffenbaugh BL and Walker EC. “Comparison of starch pasting properties in the Brabender Viscoamylograph and the Rapid Visco-Analyzer (RVA)”. Cereal Chemistry 66 (1989):493-499.
- Dengate HN. “Swelling, pasting, and gelling of wheat starch. In: Advances in cereal science and technology, (Y. Pomeranz, ed.) American Association of Cereal Chemists (AACC), St. Paul, MN. (1984): 49-71.
- Dickman SR and Bray RH. “Calorimetric determination of phosphate”. Industrial Engineering and Chemical Electrolyte Design 12.11 (1940): 665-668.
- Doughty J and Walker A. “Etude FAO: Alimentation et Nutrition”. Food and Agriculture Organization of the United Nations (FAO), Rome, Italy. (1982).
- E Helmy Rahma., et al. “Physicochemical characterisation of mung bean (Phaseolus aureus) protein isolates.” Journal of the Science of Food and Agriculture 80 (2000): 477-483.
- EARO. Crop protection research program strategy. Ethiopian Agricultural Research Organization (EARO). Addis Ababa, Ethiopia. (2000).
- Eliasson AC. Carbohydrates in food. II. Series: Food science and technology. Marcel Dekker, Inc. Madison Avenue, New York. (1996). 355-357.
- Galvez FCF and Resurreccion AVA. “Reliability of the focus group technique in determining the quality characteristics of mung bean noodles”. Journal of Sensory Studies 7 (1992): 315-326.
- Galvez FCF and Resurreccion AVA. “The effects of decortication and method of extraction on the physical and chemical properties of starch from mung bean (Vigna radiate (L.) wilczec)”. Journal of Food Processing and Preservation 17 (1993): 93-107.
- Halbrook WU and Kurtzman RHJr. “Water uptake of bean and other starches at high temperatures and pressures”. Cereal chemistry 52 (1975): 156.
- Jacobs H., et al. “Influence of annealing on the pasting properties of starches from varying botanical sources”. Cereal chemistry 72 (1995): 480-487.
- Juliano BO., et al. “Varietal differences in properties among high amylose rice starches”. Starch/Starke 39 (1987): 390-393.
- Lai CC and Varriano-Marston E. “Studies on the characteristics of black bean starch”. Journal of Food Science 44 (1979): 528-530.
- Leach HW., et al. “Structure of the starch granules. I. Swelling and solubility patterns of various starches”. Cereal Chemistry 36 (1959): 534-541.
- Liang X and King JM. “Pasting and crystalline property differences of commercial and isolated rice starch with added amino acids”. Journal of Food Science 68.3 (2003): 832-836.
- Lii CY and Chang SM. “Characterization of red bean (Phaseolus radiatus var. aurea) starch and its noodle quality”. Journal of Food Science 46.1 (1981): 78-81.
- Lineback, D. R. and Rasper, U. F. In: Pomeranz, Y. (Ed.). “Wheat Carbohydrates, in wheat: Wheat Chemistry and Technology”. Vol. I, 3rd Edn. American Association of Cereal Chemists. St. Paul, MN (1988): 277-372.
- Lineback DR and Ke CH. “Starches and low-molecular -weight carbohydrates from chick pea and horse bean flours”. Cereal Chemistry 52 (1975): 334-347.
- Loh J. “The effect of shears and strain on pasting behavior of food Starches”. Journal of food Engineering 16 (1–2) (1992): 75-89.
- Miles M J., et al. “The role of amylose and amylopectin in the gelation and retrogradation of starch”. Carbohydrate Research 135 (1985): 271-281.
- Naivikul O. The carbohydrates present in flour obtained from various types of legumes. Ph.D. thesis, North Dakota State University, Fargo, N.D.USA (1977).
- Ngatchic Metsagang JT., et al. “Evaluation of some selected blood parameters and histopathology of liver and kidney of rats fed protein-substituted mucuna flour and derived protein rich product”. Food and Chemical Toxicology 57 (2013): 46–53.
- Pahane Majeste Mbiada., et al. “Production, nutritional and biological value of bambara groundnut (Vigna subterranea) milk and yoghurt”. Journal of Food Measurement and Characterization. Food Measure (2017). DOI 10.1007/s11694-017-9541-2.
- Pomeranz Y. “Functional properties of food components, 2nd ed”. (1991): 27-28, Academic Press, New York.
- Ratnayake WS., et al. “Composition, molecular structure, and physicochemical properties of starches from four field pea (Pisumsativum) cultivars”. Food Chemistry 74.2 (2001): 189-202.
- Saikia P., et al. “Chemical composition, antinutritional factors and effect of cooking on nutritional quality of rice bean [Vigna umbellate (Thunb; Ohwi and Ohashi)]”. Food Chemistry 67.4 (1999): 347-352.
- Sandhu KS and Lim ST. “Digestibility of legume starches as influenced by their physical and structural properties”. Carbohydrate Polymers 71.2 (2008): 245-252.
- Sathe SK and Salunkhe D. “Isolation, partial characterization, and modification of the Great Northern bean (Phaseolus vulgaris L.) starch”. Journal of Food Science 46.2 (1981): 617-621.
- Schoch TJ and Maywald EC. “Preparation and properties of various legume starches”. Cereal Chem 45 (1968): 564-573.
- Sekine M. “Measurement of dynamic viscoelastic behavior of starch during gelatinization in a xanthan-gum solution”. Japan: Chemical Abstracts. Nippon Shokuhin Kagaku Kaishi 43 (1996): 683-688.
- Shimelis AE., et al. “Physicochemical properties, pasting behavior and functional characteristics of flours and starches from improved bean (PhaseolusVulgsris L.) varieties grown in East Africa”. CIGR Journal 8 (2006): 1-8.
- Shimelis AE and Rakshit SK. “Proximate composition and physico-chemical properties of improved haricot bean (Phaseolus vulgaris L.) varieties grown in Ethiopia”. Journal of Food Science and Technology (LWT) 38.4 (2005): 331-338.
- Singh J and Singh N. “Studies on the morphological, thermal and rheological properties of starch separated from some Indian potato cultivars”. Food Chemistry 75.1 (2001): 67-77.
- Singh N., et al. “Physicochemical, thermal, morphological and pasting properties of starches from some Indian black gram (Phaseolusmungo L.) cultivars”. Starch/Starke 56.11 (2004): 535-544.
- Su HS., et al. “Microstructure and physicochemical characteristics of starches in six bean varieties and their bean paste products”. Journal of Food Science and Technology (LWT) 31.3 (1998): 265-273.
- Swinkels JJM. “Composition and properties of commercial native starches”. Starch/Starke 37.1 (1985): 1-5.
- Tester RF and Morrison WR. “Swelling and gelatinization of cereal starches I. Effects of amylopectin, amylase and lipids”. Cereal Chemistry 67.6 (1990): 551-557.
- Tharanathan RN and Mahadevamma S. “A Review: Grain legumes a boon to human nutrition”. Trends in Food Science and Technology 14.12 (2003): 507-518.
- Yang CC., et al. “Studies on the starches in Taiwan Kidney bean. Bulletin Institute of Chemistry”. Academic Sinica 27 (1980): 37.
- Yang CH and Chang WH. “Effects of protein and lipid binding to starch on the physicochemical and pasting properties of rice flour”. Journal of Food Science and Agricultural Chemistry 1 (1999): 277-285.
- Yixiang Xu., et al. “Resistant Starch Content, Molecular Structure and Physicochemical Properties of Starches in Virginia grown Corn, Potato and Mungbean”. Journal of Cereals and Oil seeds 4.1 (2013): 10-18.
- Zhang GA and Hamaker B. “Nutritional property of endosperm starches from maize mutants. A parabolic relationship between slowly digestible starch and amylopectin fine structure”. Journal of Agriculture and Food Chemistry 56 (2008): 4686-4694.
- Zhang Z., et al. “Sonication enhanced corn starch separation”. Starch/Starke 57.6 (2005): 240-245.
Citation:
Njintang Yanou Nicolas., et al. “Pasting Properties and some Functional Properties of Starches from 8 Tropical Legumes
Grown in Central Africa”. Nutrition and Food Toxicology 2.6 (2018): 477-487.
Copyright: © 2018 Njintang Yanou Nicolas., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.



































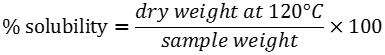
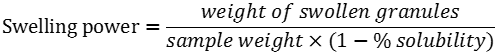
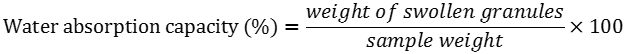
 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.