Research Article
Volume 2 Issue 6 - 2018
Phyto and Natriuretic Analysis of Delonix Regia Plant Extract for Vitamins and Mineral Contents
Department of Medical Biochemistry, Faculty of Basic Medical Sciences, College of Health Sciences, University of Abuja, P.M.B 117 FCT, Abuja-Nigeria
*Corresponding Author: Michael P Okoh, Department of Medical Biochemistry, Faculty of Basic Medical Sciences, College of Health
Sciences, University of Abuja, P.M.B 117 FCT, Abuja-Nigeria.
Received: April 17, 2018; Published: May 23, 2018
Abstract
Plants contain many bioactive compounds such as nutrients (minerals and vitamins) and, which are very important for the treatment of various diseases. Delonix regia seed powder was analyzed for the vitamins and mineral contents. Using spectrophotometer combined with titration, the vitamins content of Delonix regia was analyzed. Further, Atomic absorption spectrophotometer was used to analyze the mineral contents. The plant was show to contain low amounts of vitamin B1 (0.04 ± 0.0001mg %), B2 (0.08 ± 0.0003mg %), and B12 (0.08 ± 0.001mg %) with high vitamin B3 (766.66 ± 8.81mg) and B6 (316.46 ± 6.35mg). Vitamin C (16.0 ± 0.3mg/100g) was significantly higher in the plant compare to vitamin E (0.54 ± 0.01), vitamin A (2.81 ± 0.012), vitamin B1, B2, and B12. Delonix regia seed also contain important minerals such as Magnesium and Calcium in moderate amount (16.83 ± 8.23mg/g and 27.58 ± 0.00). Also, Chromium, Zinc, Cadmium and Iron were observed in trace amount (0.56 ± 0.0003mg/g, 0.51 ± 0.29 mg/g, 9.48 ± 0.03mg/g and 1.64 ± 0.33mg/g) respectively. In conclusion, Delonix regia seed as a phytochemical maybe necessary in the management of diseases like diabetes, Sickle cell and Aging as it may influence cellular systems with varied network, impacting on multiple targets with effects on enzymatic/or protein–protein interaction processes. Our results provide insight on the rich contents of Delonix regia extract however; further investigation on this plant is required ascertaining supplementary dosage necessary for good health in both resource poor, industrial and postindustrial environment, moving forward.
Keywords: Delonix regia; Vitamin; Minerals; Natriuretic sickle cell disease; Diabetes; Aging process
Introduction
Delonix regia belongs to the family caesalpiniaceaae, and is a legume also known as the flame of the forest. The fruits are long pods and are green, flaccid when young and later turn dark brown and hard when matured. On ripening, the mature fruits splits open into two halves revealing the elongated hard seeds [1-6]. It is commonly grown in different countries with humid temperature [7]. Delonix regia leave and seed had been reported to posses’ antimicrobial, anti-inflammatory, anti-ulcer and anti-oxidative properties [2-3].
Analysis of delonix regia showed it contains bioactive compound such as flavonoids, reducing sugars, titer-penoids, anthraquinons, amino acids, alkaloids sodium, potassium, calcium phosphorous and iron [8-9]. Plant extract of delonix regia had been reported to possess anti-bacteria, anti-malaria and anti-fungal properties [10-12]. The seed contain gum that may be useful in textile, food industries and medicine where its used as a binder in the manufacturing of tablets. The seed are used as beads, containing 18 to 27.5% fatty acid oil known as “karanga” or “pangain” oil with main use in the tanning industry [13]. The leaves, roots and seeds of the plant have been used extensively in the treatment of many disease and ailment such as diabetics.
Diabetes is a metabolic disorder characterized mainly by insulin resistance and B cells dysfunction [14] it is associated with increase in reactive oxygen species (ROS) [15], obesity especially in abdominal region [16] as a consequences of an imbalance between free radical formation, and their control [17]. Thus, micronutrients with anti-oxidative function are very important in the management of the disease and its complications. Despite numerous preventive and therapeutics method, the management of diabetes for instance, remains insufficient [18], due to the high cost of treatment thus, herbal remedy remain a viable option because of its low cost and less side effect [19]. Herein, Delonix regia seed powder was analyzed for its vitamin and mineral contents as a likely candidate for use in the management of varying diseases including diabetes.
Materials and Methods
Seed samples
Mature seeds of delonix regia were collected, removed from the pods, dried under the sun for two days. The dried seeds were pulverized using a blender, and then kept in an air tight container for further analysis.
Mature seeds of delonix regia were collected, removed from the pods, dried under the sun for two days. The dried seeds were pulverized using a blender, and then kept in an air tight container for further analysis.
Determination of mineral composition
The micro and macro contents such as magnesium (mg), calcium (Ca) Zinc (Zn), Lead (Pb), Chromium (Cr), Cadmium Cd, were determined in triplicate using atomic absorption spectrophotometer (FS240, Varians)
The micro and macro contents such as magnesium (mg), calcium (Ca) Zinc (Zn), Lead (Pb), Chromium (Cr), Cadmium Cd, were determined in triplicate using atomic absorption spectrophotometer (FS240, Varians)
Determination of vitamin composition
Vitamin (A, B1, B2, B3, B6, B12, C and E) was determined using various methods.
Vitamin (A, B1, B2, B3, B6, B12, C and E) was determined using various methods.
Determination of Vitamin A
Vitamin A was determined by colorimetric method of Kirk and sawyer (20). A measured weight (1g) of the sample was mixed with 20 mL of absolute alcohol and 3 ml of 50% KOH solution and heated for 30 minutes (100°C) under reflux, washed with distilled water, and vitamin A extracted with 3 x 50 mL of diethyl ether. The extract was evaporated to dryness at low temperature and then dissolved in 10 ml of isopropyl alcohol. 1 mL of standard vitamin A solution was prepared and the dissolved extract, transferred to separate cuvettes. The absorbances were taken using a spectrometer at 325 nm and the Molar concentration calculated.
Vitamin A was determined by colorimetric method of Kirk and sawyer (20). A measured weight (1g) of the sample was mixed with 20 mL of absolute alcohol and 3 ml of 50% KOH solution and heated for 30 minutes (100°C) under reflux, washed with distilled water, and vitamin A extracted with 3 x 50 mL of diethyl ether. The extract was evaporated to dryness at low temperature and then dissolved in 10 ml of isopropyl alcohol. 1 mL of standard vitamin A solution was prepared and the dissolved extract, transferred to separate cuvettes. The absorbances were taken using a spectrometer at 325 nm and the Molar concentration calculated.
Determination of Vitamin E
Using the Futter – Mayer colorimetric method [20], the level of vitamin E in the sample was determined. 1g of the sample was mixed with 10 ml of ethanolic sulphuric acid solution and heated for 30 minutes (100°C) under reflux. The mixture was transferred into a separating funnel and treated with 3 x 30 mL diethyl ether, recovering ether layer each time. The ether extract was evaporated for 30 minutes with a desiccator and dried at room temperature. The dried extract was dissolved in 10 mL of 95% ethanol. 1 mL of the dissolved extract and equal volume of standard vitamin E were transferred to a separate tube and 5 mL of 95% ethanol with 1ml of concentrated nitric acid solution were added, shake and allowed to stand for 5 minutes, the absorbance measured at 410 nm using a spectrometer (FX 240 Varians, Germany).
Using the Futter – Mayer colorimetric method [20], the level of vitamin E in the sample was determined. 1g of the sample was mixed with 10 ml of ethanolic sulphuric acid solution and heated for 30 minutes (100°C) under reflux. The mixture was transferred into a separating funnel and treated with 3 x 30 mL diethyl ether, recovering ether layer each time. The ether extract was evaporated for 30 minutes with a desiccator and dried at room temperature. The dried extract was dissolved in 10 mL of 95% ethanol. 1 mL of the dissolved extract and equal volume of standard vitamin E were transferred to a separate tube and 5 mL of 95% ethanol with 1ml of concentrated nitric acid solution were added, shake and allowed to stand for 5 minutes, the absorbance measured at 410 nm using a spectrometer (FX 240 Varians, Germany).
Determination of Vitamin C
Using the titrimetric method [20], a weighed sample of the extract was homogenized in 6% EDTA/TCA solution. The homogenate was filtered and used for analysis. 20 mL of 30% KI solution and 100 mL distilled H2O was added to the homogenate containing 1 mL of 1% starch solution. This was titrated against 0.1M CuSO4 solution. The end point was marked by a black coloration.
Using the titrimetric method [20], a weighed sample of the extract was homogenized in 6% EDTA/TCA solution. The homogenate was filtered and used for analysis. 20 mL of 30% KI solution and 100 mL distilled H2O was added to the homogenate containing 1 mL of 1% starch solution. This was titrated against 0.1M CuSO4 solution. The end point was marked by a black coloration.
Vitamin C content was calculated using the equation below.
Determination of Vitamin B1 and B2
1g of sample were dissolved in 100 mL of deionized water, shaken thoroughly and allowed to cool, then filtered. The filtrate was pipetted into a cuvettes and their absorbance measured at 261 nm and 242 nm (B1 and B2) using a spectrometer. The concentration of the vitamins in the extract was calculated using the formula below.
1g of sample were dissolved in 100 mL of deionized water, shaken thoroughly and allowed to cool, then filtered. The filtrate was pipetted into a cuvettes and their absorbance measured at 261 nm and 242 nm (B1 and B2) using a spectrometer. The concentration of the vitamins in the extract was calculated using the formula below.
Where A = absorbance
E = Extinction coefficient = 25 for B1 and B2
DF = Dilution Factor
E = Extinction coefficient = 25 for B1 and B2
DF = Dilution Factor
Determination of Vitamin B3 and B6
5g samples was dissolved in 20mg of anhydrous glacial acetic acid and warmed slightly for 1 minute to, which 5 mL of acetic acid anhydride was added and mixed. 3 drops of crystal violet solution was added as indicator. 0.1M concentration of Per Chloric acid (HClO4) solution was used to titrate to a greenish blue colour. Calculation: Each mL of 0.1M per chloric acid is equivalent to 0.0122g of C6H6N2O. For vitamin B6 5g of sample was dissolved in a mixture of 5ml of anhydrous glacial acetic acid and 6 mL of mercury II acetate solution. 0.05 Or 2 drops of crystal violet was added as indicator and 0.1M HCLO4 was used to titrate to a green color end point
5g samples was dissolved in 20mg of anhydrous glacial acetic acid and warmed slightly for 1 minute to, which 5 mL of acetic acid anhydride was added and mixed. 3 drops of crystal violet solution was added as indicator. 0.1M concentration of Per Chloric acid (HClO4) solution was used to titrate to a greenish blue colour. Calculation: Each mL of 0.1M per chloric acid is equivalent to 0.0122g of C6H6N2O. For vitamin B6 5g of sample was dissolved in a mixture of 5ml of anhydrous glacial acetic acid and 6 mL of mercury II acetate solution. 0.05 Or 2 drops of crystal violet was added as indicator and 0.1M HCLO4 was used to titrate to a green color end point
Calculation: Each ml of 0.1m per chloric acid is equivalent to 0.02056g of C8H11NO3
Determination of Vitamin B12 25mg of sample was dissolved in 250 mL of deionized water. The absorbance was read at 361 nm
Where A = absorbance
E = Extinction coefficient
DF = Dilution Factor = 25
E = Extinction coefficient
DF = Dilution Factor = 25
Result
In this study, Delonix regia seed extract/powder was used and analyzed for its vitamin and mineral contents. This is with a view to check the phytochemical constituents and its usefulness in managing diseases including diabetes.
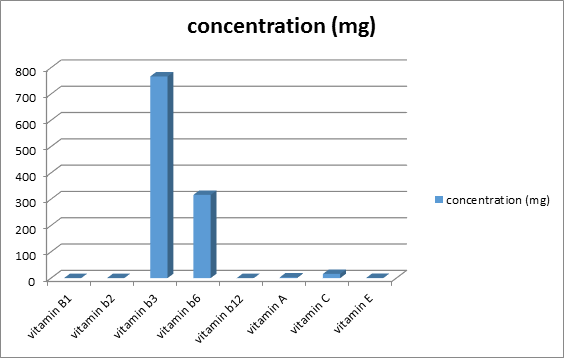
Figure 1 shows the concentration of vitamin in delonix regia seed. Each value is represented as mean ± SEM with significant different of P<0.05. Vitamins were present in the plant in varying concentration, with the B-Complex vitamins, the extract had vitamin B1 (0.04 ± 0.0001mg %), B2 (0.08 ± 0.0003mg %) and B12 (0.08 ± 0.001mg %) while vitamin B3 (766.66 ± 8.81mg) and B6 (316 ± 6.35mg) were significantly higher (P < 0.05) in the extract. On the other hand, vitamin C (16.0 ± 0.03mg/100g) was significantly higher (P < 0.05) in the plant compared to vitamin E and vitamin A (0.54 ± 0.01mg and 2.81 ± 0.012 respectively).
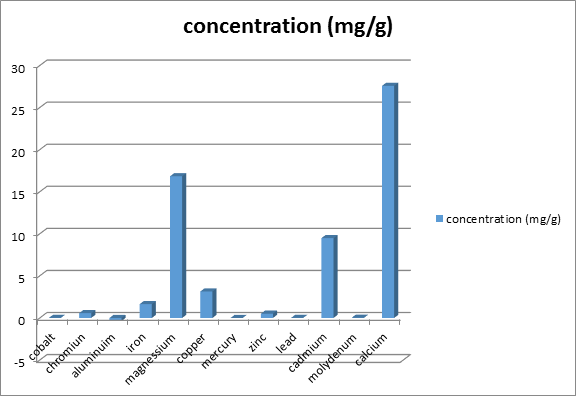
Figure 2 shows the concentration of minerals in delonix regia seed. Each value is represented as mean ±SEM with significant different of P<0.05. The Delonix regia seed contains a number of minerals in trace or moderate amounts. Among them, magnesium and calcium (16.83 ± 8.23 and 27.58 ± 0.00 respectively) and these were significantly higher to other trace element like Cr, Zn, Cd and Fe found present lower amount (0.56 ± 0.0003mg/g, 0.51 ± 0.29mg/g, 9.48 ± 0.03mg/g and 1.64 ± 0.33mg/g respectively).
Discussion
Some medicinal plants have been found to be very useful in the management of diseases like diabetes, sickle cell disease, liver diseases and in regulation of the immune function. The results presented in the figure 1 show that Delonix regia seed powder contains 766.66 ± 8.81mg of vitamin B3 (nicotinamide), which is thought to increase energy production in the cell as a precursor of nicotinamide adinosine dinucleotide (NAD) and it is associated with treatment of arthritis and early-onset Type I diabetes [21]. Moreover, nicotinamide is also being studied for its effects in improving energy deficits caused by mitochondrial dysfunctions [22]. Nicotinamide has been shown to play a role in cellular energy increase via its regulation of both glycolysis and the citric acid cycle due to the inhibition of the poly-ADP-robose polymerase. During DNA damage, poly-ADP ribose polymerase is activated, depleting the supply of NAD by transferring poly-ADP ribose subunits from NAD to various DNA repair enzymes. With such mechanism, NAD depletion ultimately leads to ATP depletion primarily due n to the decrease in the activity of both glycolysis and the Citric acid Cycle. These to a large extent, present a novel process connecting energy metabolism and protein deacetylation, which require further mechanistic investigation leading to proper understanding.
A recent study [23] suggested NAD+ activate the enzymes called sirtuins (SIR2 (silent information regulator 2) family of proteins) that are credited with the beneficial effects of Caloric Restriction (CR). These enzymes, are involved in a host of metabolic actions throughout the body, but, they seem to decline with age [23]. Earlier studies [24-25] had established that the NAD-dependent deacetylase activity of sirtuins regulates many fundamental biology such as aging processes in response to both environment and nutritional stimuli [26-28]. Thus, NAD+ boosting compounds such as delonix regia hold promise for enhancing cardiovascular health and other physiological function including aging
The de novo synthesis of NAD+ from tryptophan does not occur in all cells or tissue types. Most tissue acquires NAD via the salvage pathway using other cellular intermediates that are made available through dietary supplementation [29]. Vitamin B3 enters the salvage pathway where it acts as a NAD+ precursor. Nicotinic acid on the other hand is found to be associated with undesirable flushing at therapeutic doses [30]. Martens., et al. 2018 [23], recently established that 13 cohort with elevated blood pressure of stage 1 hypertension (120–139/80–89 mmHg), systolic blood pressure was 10 points lower after supplementation with vitamin B3. Implying such supplementation could reduce heart attack risk by as much as 25%.
Moreover, it is thought that the transcription factor, such as; nuclear factor (erythroid-derived 2)-like 2 (NRF2 a leusine zipper region), recognizes an antioxidant response element (ARE) available in approximately 250 genes [31], and are thought to be a master regulator of cell homeostasis regulating the expression of antioxidant and cyto-protective genes. These genes account for more than 1% of the human genome and include antioxidant genes, HMOX1 (coding heme oxygenase-1) and NQO1 (coding NAD(P)H quinone oxidoreductase-1), and gene encoding enzymes involved in glutathione metabolism or in the generation of nicotinamide adenine dinucleotide phosphate (NADPH), etc. Thus, supplementation with Delonix regia could help in upregulating Nrf2-mediated cytoprotective responses, which would be essential to influencing the overall health and survival of the cell and patient with diseases such as; sickle cell, heart attack and diabetics.
The precise effects of nicotinamide on treatment of diabetes especially type 2 diabetes is not fully understood although, it is established that SIRT1 (mammalian ortholog of SIR2) is involved in the pathogenesis of age-associated complications, such as type 2 diabetes and that, people who develop type 1 diabetes after puberty appears to be responsive to nicotinamide treatment as, it stimulates β-cell production [32]. Bearing that, in pancreatic β cells, both SIRT1-mediated NAD biosynthesis play important roles in the regulation of glucose-stimulated insulin secretion [32]. Currently, there are limited success in reducing high mortality rates associated with diabetics complications thus; combination of phytomedicine that targets specific epigenes such as SIRT1 may form a new approach for diabetic’s prevention, which are desperately required, moving forward.
The figure 1 also indicated that Delonix regia seed contains 316mg of vitamin B6 (pyridoxal -5’ phosphate (PLP))), an amino transferase, which acts as a coenzyme for glucose-phosporylase, glycogen utilization in liver and muscle [33]. It helps in the biosynthesis of neurotransmitters and in maintaining normal levels of homocysteine and in the regulation of the immune function (interlukin-2-production). Substrate metabolism is known to be closely linked to its cofactors: hence, for homocysteine, three vitamins do act as cofactors or coenzymes: i.e. B6, B12 and folate. Vitamin B6 is showed to be involved in the formation of cystathionine from homocysteine via the trans-sulfuration pathway. B6 is also critical for the formation of 5,10-methylentetrahydrofolate from tetrahydrofolate a derivatives of folic acid, therefore they are very related to the metabolism of folate, in conjunction with vitamin B12, which are thought to be involved in the remethylation of homocysteine to methionine. This reaction process, is dependent on the availability of vitamin B12. Deficiency of vitamin B6 in humans and animals is associated with glucose intolerance [34].
Earlier experiment, Cam., et al. 2000 [35], indicated that patients diagnosed for diabetics have lower PLP concentration compare to non-diabetic subjects [35]. However, currently there are no experimental evidence linking vitamin B6 deficiency with onset of diabetes.
Vitamin C is found to be markedly reduced in patients with diabetes compared to healthy controls [36]. Delonix regia seed contain 16.0 ± 0.33mg/100g of vitamin C, an anti-oxidant, which helps to remove free radicals generated by reactive oxygen species. Under diabetes condition for instance, oxidative stress is produced and this causes various forms of tissue damage including; liver and kidney damage. For diabetics patient antioxidants such as vitamin C have been shown to help stimulate insulin secretion, preserve β-cell proliferation thereby preventing complications that may arise from the disease conditions. Patients with type 2 diabetes for instance, are found to have conditions that increase lipid peroxidation with decreasing anti-oxidative enzymes including, vitamin C during the first two years of the disease progression [37-38]. Vitamin C is found to help in preventing other disease condition such as scurvy in humans, facilitate wound healing due to its role in the production of collagen and it lowers serum uric levels, resulting in the corresponding lowering incidence of gout [39]. Importantly vitamin C was earlier postulated to be beneficial in decreasing blood glucose and lipids in patients with type 2 diabetes and thus reducing the risk of complications arising from diabetes condition [40]. The combination of insulin and vitamin C in a recent experiment indicated it help to stop diabetes-induced blood vessel damage (endothelial dysfunction) in people with poor blood glucose control, as antioxidant such as vitamin C, help, erases damage memory from cells thus, allowing cell function to return to normal [41-42].
Mineral Content
The Figure 2 show that Delonix regia seed contain moderate amount of some divalent cations such as magnesium (mg2+) and calcium (Ca2+) (16.83 ± 8.23mg/g, 27.58 ± 0.00) and some traces amount of Chromium (Cr), zinc (Zn), cadmium (Cd) and iron (Fe) (0.56 ± 0.0003mg/g, 0.51 ± 0.29mg/g, 9.48 ± 0.03mg/g and 1.64 ± 0.33mg/g respectively).
The Figure 2 show that Delonix regia seed contain moderate amount of some divalent cations such as magnesium (mg2+) and calcium (Ca2+) (16.83 ± 8.23mg/g, 27.58 ± 0.00) and some traces amount of Chromium (Cr), zinc (Zn), cadmium (Cd) and iron (Fe) (0.56 ± 0.0003mg/g, 0.51 ± 0.29mg/g, 9.48 ± 0.03mg/g and 1.64 ± 0.33mg/g respectively).
Role of divalent cations:Mg as a divalent cations is a critical cofactor in numerous intracellular mechanisms. For instance, it is a cofactor for adenosine triphosphate (ATP), and is an important membrane-stabilizing agent that is required for the structural integrity of numerous intracellular proteins and nucleic acids. It is also a cofactor for important molecules including, guanosine triphosphatase, phospholipase C, adenylate cyclase, and guanylate cyclase etc. Mg2+ is involved in the control of muscle contraction and insulin levels in the body, as it is involve in regulating insulin action through the insulin mediated glucose uptake [43]. Moreover, Mg functions as a cofactor for more than 300 enzymes, as it is essential for all energy dependent transport system including glycolysis, oxidation, energy metabolism, neuromuscular activity and cell membrane stabilization [44]. Its deficiency is associated with hypertension, insulin resistance, which is associated with decrease in magnesium uptake [45].
Magnesium as a supplement is beneficial for metabolic profile in-patient with diabetics. Bearing the importance of Mg in-patient with diabetics, further prospective studies are needed/required-supporting role of dietary magnesium supplementation as a possible health strategy to ameliorate health effects of diabetes. Incidence of hypomagnesaemia, have been reported to be associated with diabetes and these may have resulted due to increased urinary losses [44-45] with other additional risk factors such as ketoacidosis and effects of certain medications etc.
Zinc is necessary for the formation of insulin in the pancreatic beta cells. Zinc has an anti-oxidant properties hence dietary supplementation with Zn is good as it help in warding off heart disease in people with type 2 diabetes [46]. However, excess Zn, can upset the balance of copper and iron in the body, leading to weakness of the immune system. Zn benefits includes its ability to act as an anti-inflammatory agent, and present within all bodily tissue where it is required for healthy cell division. It acts like an antioxidant within the body, fighting free radical hence, it help in slowing the aging process. Zinc also has a big impact on hormonal balance, so for this reason, Zn deficiency can result in an increased risk for infertility or diabetes hence, Zn is thought to have significant therapeutic benefits.
Conclusion
This study has shown that Delonix regia seed powder is rich in vitamins and minerals including calcium, which helps in building and maintaining strong bones and teeth. Delonix regia seed promoted, as supplement will provides easy means to meet most daily requirement of both vitamins and important minerals, in both resource poor/industrial and postindustrial environment. As it could help to manage incidences of varying types of diseases including, diabetes, cardiovascular diseases, aging processes, may reduces incidences of sickle cell disease (SCD) phenotype heterogeneity and other disease conditions in the population. Bearing, Delonix regia as a phytochemical can be developed to enhance nutriceuticals that may influence cellular systems with varied network by impacting on multiple targets with effects on enzymatic/or protein–protein interaction processes. Thus, such supplementation may have a more lasting outcome as compared to designed xenobiotics with affinity to targeted gene/or proteins.
References
- Adje E., et al. “Anthocyanin characterization of pilot plant water extracts of Delonix regia flowers.” Molecules 13.6 (2008): 1238–1245.
- Amata IA and Nwagu KM. “Comparative evaluation of the nutrient profile of the seeds of four selected tropical plants and maize”. International Journal of Applied Biology and Pharmaceutical Technology 4 (2013): 200–204.
- Rani PMJ., et al. “Screening of antioxidant tactivity,total phenolics and gas chromatograph and massspectrometer(GC-MS) study of Delonix regia”African Journal of Biochemistry Research 2.12( 2011): 341–347.
- Arora A., et al. “Fatty acid composition of Delonix regia (Gulmohar) seed oil from arid zone of Rajasthan”. Journal of Indian Council of Chemists 27 (2010): 150–152.
- Bake GG., et al. “Nutritional evaluation of varying levels of cooked flamboyant seed meal(Delonixregia) on the growth performance and body composition of Nile tilapia (Oreochromisniloticus) fingerlings. Agric.,Forest.Fish 3 (2014): 233–239.
- Mandal J., et al. “Aerobiological Investigation and In Vitro Studies of Pollen Grains from 2 Dominant Avenue Trees in Kolkata, India”. Journal of Investigational Allergology and Clinical Immunology 18.1(2008): 22–30.
- Kaga IB. “Biological and carcass characteristics of rabbits fed Delonix regia meal diets” Biological Systems 2 (2013): 120.
- Ahn HJ., et al. “Assessment of vitamin B (6) status in Korean patients with newly diagnosed type 2 diabetes”. Nutrition Research and Practice 5.1 (2011): 34–39.
- Kumar AR et al. “Phytochemical evaluation of Delonix regia, Samaneasaman and Bauhiniavariegata”. International Journal of Research in Pharmacy and Chemistry3 (2013): 768–772.
- Roy SP., et al. “Evaluation of anti-ulcer effects of ethanolic extract of Delonix regia flower.” Indian Journal of Research in Pharmacy and Biotechnology 1 (2013): 440–445.
- Zimmet P., et al. “Global and societal implications of the diabetes epidemic”. Nature 414.6865 (2001): 782-787.
- Ankrah NA., et al. “Evaluation of efficacy and safety of a herbal medicine used for the treatment of malaria.” Phytotherapy Research17.6 (2003): 697-701.
- Aqil F and Ahmad I. “Antibacterial properties of traditionally used Indian medicinal plants”. Methods and Findings in Experimental and Clinical Pathology 29.2(2007): 79-92.
- Tangvarasittichai T. “Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus”. World Journal of Diabetes 6.3 (2015): 456-480.
- Turrens JF and Boveris A. “Generation of superoxide anion by the NADH dehydrogenase of bovine heart mitochondria”. The Biochemical Journal 191.2 (1980): 421-427.
- Vincent HK and Taylor AG. “Biomarkers and potential mechanisms of obesity-induced oxidant stress in humans” International Journal of Obesity 30.3 (2006): 400–418.
- Zatalia SR and Sanusi H. “The role of antioxidants in the pathophysiology, complications, and management of diabetes mellitus”. Acta Medica Indonesiana 45.2 (2013): 141–147.
- Zimmet P., et al. “Global and societal implications of the diabetes epidemic” Nature 414.6865 (2001): 782-787.
- Venkatesh S., et al. “Anti-hyperglycemic activity of Caralluma attenuate”. Fitoterapia 74.3 (2003): 274-279.
- Kirk RS and Swayer R. Pearson’s food composition and analysis. (4th ed). 1991. Macmillian published company U.K.S.L.
- Huntingtons outreach project for education “nicotiamide” (2010).
- Helsingin T. “Energizing sick mitochondria with vitamin B3: Effective treatment for mitochondrial disease”. Science daily (2014).
- Martens CR., et al. “Chronic nicotinamide riboside supplementation is well-tolerated and elevates NAD+ in healthy middle-aged and older adults”. Natute communications 9 (2018): 1286.
- Blander G and Guarente L. “The sir2 family of protein deacetylases”. Annual Review of Biochemistry 73 (2004): 417–435.
- Imai S., et al. A universal link between NAD, metabolism, and aging. In: Guarente L, Partridge L, Wallace D, editors. The molecular biology of aging. New York: Cold Spring Habor Laboratory Press; (2007) 39–72.
- Ratajczak J., et al. “NRK1 controls nicotinamide mononucleotide and nicotinamide riboside metabolism in mammalian cells”. Nature Communications 7(2016):13103.
- Chi Y and Sauve AA. “Nicotinamide riboside, a trace nutrient in foods, is aVitamin B3 with effects on energy metabolism and neuroprotection”. Current Opinion in Clinical Nutrition & Metabolic Care 16.6 (2013): 657–661.
- Imai S. “A possibility of nutriceuticals as an anti-aging intervention: Activation of sirtuins by promoting mammalian NAD biosynthesis”. Pharmacological Research 62.1 (2010): 42-47.
- Bogan KL and Brenner C. “Nicotinic acid nicotinamide and nicotinamideriboside: a molecular evaluation of NAD (+) precursor vitamins in human nutrition”. Annual Review of Nutrition 28 (2008): 115–130.
- MacKay D., et al. “Niacin: chemical forms,bioavailability, and health effects”. Nutrition Reviews 70.6 (2012) 357–366.
- Umesh K., et al. “Reversal of hypermethylation and reactivation of glutathione S-transferase pi 1 gene by curcumin in breast cancer cell line.” Tumor Biology 39.2 (2017).
- Revollo JR., et al. “Nampt/pbef/visfatin regulates insulin secretion in β cells as a systemic NAD biosynthetic enzyme”. Cell Metabolism 6.5 (2007): 363–375.
- Poucheret P., et al. “Vanadium and diabetes” Molecular and Cellular Biology 188.1-2 (1998): 73–80.
- Franz MJ and Bantle JP. Eds.: American Diabetes Association Guide to Medical Nutrition Therapy for Diabetes. Alexandria Va., American Diabetes Association, (1999).
- Yusuf S., et al. “Vitamin E supplementation and cardiovascular events in high-risk patients. The Heart Outcomes Prevention Evaluation Study” The New England Journal of Medicine 342.3 (2000): 154–160.
- Cam MC., et al. “Mechanisms of vanadium action: insulin mimetic or insulin-enhancing agent? Can” Journal of Physiology and Pharmacology 78.10 (2000): 829–847.
- Bursell SE., et al. “High-dose vitamin E supplementation normalizes retinal blood flow and creatinine clearance in patients with type 1 diabetes”. Diabetes Care 22.8 (1999): 1245–125.
- Stampfer MJ., et al. “Vitamin E consumption and the risk of coronary disease in women” The New England Journal of Medicine 328.20 (1993): 1444–1449.
- Choi MD., et al. “Vitamin C Intake and the Risk of Gout in Men – A Prospective Study.” Archives of Internal Medicine 169.5 (2009): 502–507.
- Afkhami-Ardekani I and Shoiaoddiny-Ardekani A. “Effect of vitamin C on blood glucose, serum lipids & serum insulin in type 2 diabetes patients”. Indian Journal of Medical Research 126.5 (2007); 471-474.
- Ceriello A., et al. “Long-Term Glycemic Control Influences the Long-Lasting Effect of Hyperglycemia on Endothelial Function in Type 1 Diabetes.” The Journal of Clinical Endocrinology & Metabolism 94.8 (2009) 2751–2756.
- Kolluru GK., et al. “Endothelial Dysfunction and Diabetes: Effects on Angiogenesis, Vascular Remodeling, and Wound Healing”. International Journal of Vascular Medicine (2012).
- Barbagallo M and Dominguez LJ. “Magnesium and type 2 diabetes”. World Journal of Diabetes 6.10 (2015): 1152-1157.
- de Valk H. “Magnesium in diabetes mellitus”. Journal of Medicinal Chemistry 54.4 (1999): 139-146.
- American Diabetes Association. “Magnesium supplementation in the treatment of diabetes.” Diabetes Care 15 (1992): 1065–1067.
- Ying L., et al. “effect and mechanism of zinc supplementation in protecting against diabetics cardiomyopathy in rat model of type 2 diabetes”. Bosnian Journal of Basic Medical Sciences 15.1 (2015): 14-20.
Citation:
Michael P Okoh and Chijoke Madu. “Phyto and Natriuretic Analysis of Delonix Regia Plant Extract for Vitamins and Mineral
Contents”. Nutrition and Food Toxicology 2.6 (2018): 540-548.
Copyright: © 2018 Michael P Okoh and Chijoke Madu. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.



































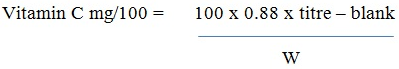
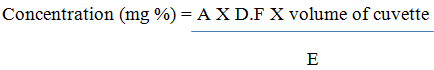
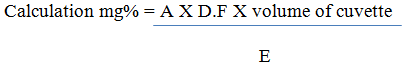
 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.