Research Article
Volume 3 Issue 3 - 2018
Microorganisms Associated with Plain Cake from Eateries and Parties in Jos Metropolis, Nigeria.
Department of Plant Science and Technology, University of Jos, Plateau Nigeria
*Corresponding Author: Ibejekwe AN, Department of Plant Science and Technology, University of Jos, Plateau Nigeria.
Received: August 01, 2018; Published: September 25, 2018
Abstract
Microorganisms associated with plain cake spoilage was evaluated. Fifteen samples of cakes were collected from eateries and parties and cultured. Bacteria and Fungi were isolated using Nutrient and Czapek agar respectively. Three bacterial species and five fungal species were isolated from the spoilt cake samples. The bacterial isolated were, Bacillus laterosporus, Staphylococcus epiderdimis and Staphylococcus aureus. Fungal isolated were, Rhizopus stolonifer, Aspergillus fumigatus, Aspergillus niger, Aspergillus flavus, Penicillum citrinum and, Penicillum chrysogenum. The organisms isolated were listed in order of frequency of occurrence. It then means that most cakes sold in shops and served in parties were not properly prepared and preserved thus, there is a need to employ standard conditions in baking.
Introduction
According to food historians, the first culture to show evidence of baking skills and interest were the ancient Egyptians. They were probably fed up with game meat and wanted to try something new. However, they were more bread-like and instead of sugar, they were sweetened with honey. The word cake is of viking origin, from the norse word "kaka".
Cakes have their part to play in ancient beliefs and superstitions, some which still carries on to modern times. In olden times, people used cakes as offerings to their gods and spirits around the world. The Chinese celebrate Harvest Moon festival and have moon cakes to honour their moon goddess. This tradition continues up to today. Russians have sun cakes called blini which are thin pancakes to pay their respect to a deity called Maslenitsa. Ancient Celts rolled cakes down a hill during the Beltane festival held on the first day of spring to imitate solar movement. With such a rich history and connection of humans with cake, it is no wonder that they remain such an important part of our lives, (www. historyofcake.com). www.food timeline.org
Bakery products are valuable source of nutrients in our diet providing us with calories and protein requirement. Cake is a good example of bakery products made from cereals (Saranraj, et al., 2011). Cake is a cherished food source for people throughout the whole world. Cakes are baked with, flour, sugar, fat, eggs, preservatives and its composition makes it rich food resource. Cakes are used for special occasions such as wedding, birthdays, parties and special events. Despite these aesthetic properties of cakes, they are still subjected to spoilage problems, these include physical, chemical and microbiological spoilage. Properly handled cakes generally lacks sufficient amounts of moisture to slow growth of any microorganism, except moulds. As normal cooking temperature destroy most fungal spores, (Saranraj., et al. 2011).
Bakery products are subjected to spoilage problems. Microbiological spoilage, especially mould cause serious problem for bakeries. Storage of baked goods, cake inclusive under low humid condition retards mould growth. Eurotium species are usually the first fungi to colonize this product. Aspergillus and Penicillium spp which can produce toxins also thrives, members of the genus Bacillus also brings about spoilage. These losses could be due to many individual cases such as packaging, sanitary practices, manufacturing storage conditions and product turnover (Saranraj., et al. 2011). Rachel., et al. (2004) also attested to the fact that microbial spoilage of these product can be caused by bacteria, yeast and moulds and also enzymatic spoilage caused by lypoxygenase.
Material and Methods
Collection of Material
Fifteen samples of plain cakes were collected from eatries and parties from Jos metropolis
Fifteen samples of plain cakes were collected from eatries and parties from Jos metropolis
Isolation and Identification of Microorganisms
The test Microorganisms were Isolated from the samples of cakes gotten from eateries and parties in Jos metropolis
The test Microorganisms were Isolated from the samples of cakes gotten from eateries and parties in Jos metropolis
Isolation and Enumeration of Microorganisms
Isolation of spoilage microorganisms of plain cake was carried out using the method described by Ayanda., et al. (2013). The samples were soaked in sterile water for 30 minutes to dissolve. Serial dilution of the sample were made to 108. 1ml of each dilution was innuculated on each labeled plate and 20ml of nutrient agar was poured into plates and incubated at 37° for 24 to 48 hours.
Isolation of spoilage microorganisms of plain cake was carried out using the method described by Ayanda., et al. (2013). The samples were soaked in sterile water for 30 minutes to dissolve. Serial dilution of the sample were made to 108. 1ml of each dilution was innuculated on each labeled plate and 20ml of nutrient agar was poured into plates and incubated at 37° for 24 to 48 hours.
Fungi isolation was carried out using Czapek agar, which was prepared according to manufacturer’s instruction. 20ml of the prepared agar was poured into each plate and allowed to solidify. Surface growth method was used, and incubated at ambient temperature for 4 to 7 days. Sub-culturing was carried out to obtain pure isolates.
Gram Staining
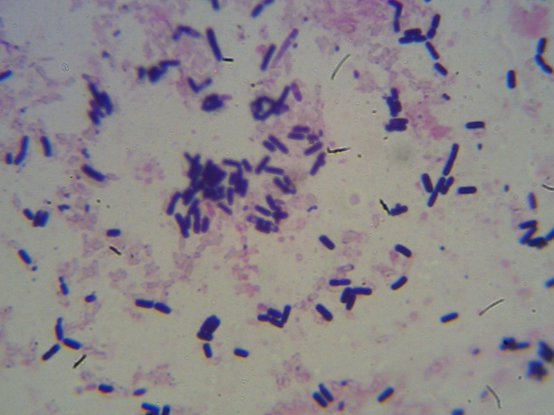
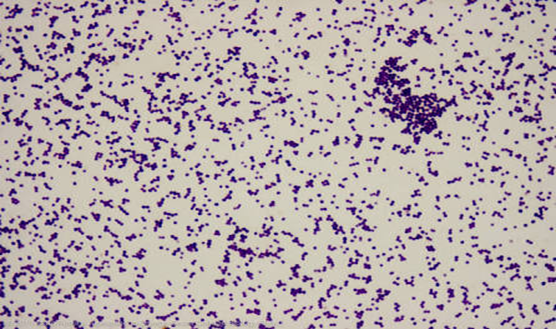
This technique is used to differentiate gram positive bacteria from gram negative bacteria based on their cell wall composition. For gram positive, their cell wall was purple because they retain the primary stain which is crystal violet, while gram negative bacteria were stained pink, because they retain counter stain. The procedure is as follows: A smear was prepared using 18-24hours old culture. One looped of sterile distilled water was transferred on clean grease free glass slide and colony from 24hours old culture was transferred and emulsified on it to make thin preparation. The smear was air dried and the edge of the glass slide was passed over Bunsen flame for about three [3] times to heat fix the smear. Primary stain (crystal violet) was added to the smear and left for about 60seconds. The stained smear was rapidly washed with distilled water, the smear grams iodine solution was added for another 60 seconds and washed with distilled water, then the smear was decolourised with 95% alcohol for 5seconds and washed with water. Finally, the smear was counterstained with saffranin dye for 60seconds and washed with distilled water. The smear was then observed under x 100 oil immersion objective lens of the microscope.
This technique is used to differentiate gram positive bacteria from gram negative bacteria based on their cell wall composition. For gram positive, their cell wall was purple because they retain the primary stain which is crystal violet, while gram negative bacteria were stained pink, because they retain counter stain. The procedure is as follows: A smear was prepared using 18-24hours old culture. One looped of sterile distilled water was transferred on clean grease free glass slide and colony from 24hours old culture was transferred and emulsified on it to make thin preparation. The smear was air dried and the edge of the glass slide was passed over Bunsen flame for about three [3] times to heat fix the smear. Primary stain (crystal violet) was added to the smear and left for about 60seconds. The stained smear was rapidly washed with distilled water, the smear grams iodine solution was added for another 60 seconds and washed with distilled water, then the smear was decolourised with 95% alcohol for 5seconds and washed with water. Finally, the smear was counterstained with saffranin dye for 60seconds and washed with distilled water. The smear was then observed under x 100 oil immersion objective lens of the microscope.
Biochemical Tests
Biochemical test was performed for identification purposes and confirmatory tests and the organisms used for inoculation of the test media was sourced from fresh cultures. Specific biochemical tests performed include:- catalase test, carbohydrate fermentation test, urease test, indole production test, citrate utilization test, mortility, oxidase test and coagulase test (Cheesbrough, 2009).
Biochemical test was performed for identification purposes and confirmatory tests and the organisms used for inoculation of the test media was sourced from fresh cultures. Specific biochemical tests performed include:- catalase test, carbohydrate fermentation test, urease test, indole production test, citrate utilization test, mortility, oxidase test and coagulase test (Cheesbrough, 2009).
Coagulase test
This test detects the presence of enzyme coagulase. A smear of 24 hours old culture of the organism under study was made on a slide, some drops of blood were added to the smear and left to stand for about 1 hours. As positive reaction to this test is indicated by clotting and coagulation of the blood plasma (suggesting that the enzymes coagulase was produced by the organism). However, absence of clotting or coagulation is a negative reaction.
This test detects the presence of enzyme coagulase. A smear of 24 hours old culture of the organism under study was made on a slide, some drops of blood were added to the smear and left to stand for about 1 hours. As positive reaction to this test is indicated by clotting and coagulation of the blood plasma (suggesting that the enzymes coagulase was produced by the organism). However, absence of clotting or coagulation is a negative reaction.
Oxidase test
This test detects the presence of cytochrome C oxidase that is able to reduce oxygen. A smear is made on a slide, a piece of filter paper soaked with a few drops of oxidase reagent strip, was placed on the smear for 4 minutes. A positive reaction the oxidase test is indicated by a deep purple colour while the other colours or no change in colour is indicated of a negative reaction.
This test detects the presence of cytochrome C oxidase that is able to reduce oxygen. A smear is made on a slide, a piece of filter paper soaked with a few drops of oxidase reagent strip, was placed on the smear for 4 minutes. A positive reaction the oxidase test is indicated by a deep purple colour while the other colours or no change in colour is indicated of a negative reaction.
Catalase test
This test detects the presence of enzymes catalase, which contents hydrogen peroxide to water and oxygen. It is used to differentiate bacteria species based on their positive or negative reaction with hydrogen peroxide solution. A smear was made on a slide and 3 drops of 3% hydrogen peroxide solution was added to it. Production of effervescent oxygen bubbles indicated reaction.
2H202 + Catalase 2H20202
This test detects the presence of enzymes catalase, which contents hydrogen peroxide to water and oxygen. It is used to differentiate bacteria species based on their positive or negative reaction with hydrogen peroxide solution. A smear was made on a slide and 3 drops of 3% hydrogen peroxide solution was added to it. Production of effervescent oxygen bubbles indicated reaction.
2H202 + Catalase 2H20202
Urease test
This test detects the enzyme that splits Urea to Ammonia and Carbondioxide in bacterial. It is used to distinguish Proteus spp, Klebsiella spp, which produce urease, also E.coli that do not produce urease. When Urea is added to the smear preparation of the organism to be identified, evolution of CO2 is a positive reaction while a negative reaction results if there is no evolution of carbondioxide gas.
This test detects the enzyme that splits Urea to Ammonia and Carbondioxide in bacterial. It is used to distinguish Proteus spp, Klebsiella spp, which produce urease, also E.coli that do not produce urease. When Urea is added to the smear preparation of the organism to be identified, evolution of CO2 is a positive reaction while a negative reaction results if there is no evolution of carbondioxide gas.
Citrate test
This test is used on the identification of enteric bacteria. Enterobacter spp are positive to this test, while Escherichia coli will give a negative reaction. The test is used to check the ability of organisms to utilize citrate as a sole carbon source. The isolate was inoculated aseptically using a sterile wire loop to streak on simmon’s citrate Agar medium. It was then incubated for 48hours. After incubation at 38°c, a blue colouration on the medium indicates a positive reaction, while no change in the colour of the medium, indicates a negative reaction to citrate test.
This test is used on the identification of enteric bacteria. Enterobacter spp are positive to this test, while Escherichia coli will give a negative reaction. The test is used to check the ability of organisms to utilize citrate as a sole carbon source. The isolate was inoculated aseptically using a sterile wire loop to streak on simmon’s citrate Agar medium. It was then incubated for 48hours. After incubation at 38°c, a blue colouration on the medium indicates a positive reaction, while no change in the colour of the medium, indicates a negative reaction to citrate test.
Sugar fermentation test
This was carried out to identify the isolate to species level. The production of turbidity and gas from carbohydrates (lactose, glucose, sucrose, manitol) was used to identify isolates to species level.
This was carried out to identify the isolate to species level. The production of turbidity and gas from carbohydrates (lactose, glucose, sucrose, manitol) was used to identify isolates to species level.
One gram of each sugar was dissolved in 50 ml of distilled water. The sugar solutions were filtered using membrane filtration technique and the filtered solutions were used for analysis. Nutrient broth was prepared by dissolving 3.3 gram of the nutrient powder in 250 ml of distilled water and 2.5ml of phenol red was added to the nutrient broth. With the aid of sterile needle and syringe, 8ml of the broth was transferred into sterile test tubes. Durham tubes were inserted into the test tubes containing the nutrient broth. They were autoclaved at 121°C for 15minutes. After cooling, 2ml each of the filtered solutions were added to the sterilized nutrient broth and were inoculated with appropriate purified colonies under an asceptic conditions. They were incubated anaerobically at 37°C for 48 hours. A colour change was observed.
Motility test
This test is performed to distinquish the motile organisms from the non-motile ones. Isolates was inoculated aseptically using a sterile wire loop to stab the test organism in nutrient broth. It was then incubated for 48 hours. Diffuse hazy growth that spreads throughout the medium, making it look opaque indicate motility and when growth is confine to the line of inoculation it indicate non- motility.
This test is performed to distinquish the motile organisms from the non-motile ones. Isolates was inoculated aseptically using a sterile wire loop to stab the test organism in nutrient broth. It was then incubated for 48 hours. Diffuse hazy growth that spreads throughout the medium, making it look opaque indicate motility and when growth is confine to the line of inoculation it indicate non- motility.
Isolation and Identification of Fungi
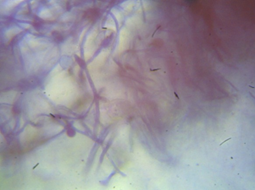
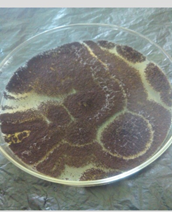
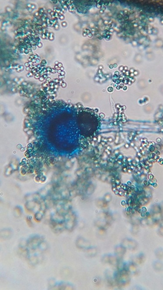
The morphological and cultural characteristics with special reference to the sporulation of the fungi isolates was used to identify them. Where the plates were observed daily for growth rate of each of the cultural isolates. The colour and morphology of the colonies was recorded. A wet mount of each fungus was prepared by suspending a loopful of isolate in a few drops of lacto-phenol cotton blue solution on a microscope slide and then cover with a slip then viewed under x40 magnification.
The morphological and cultural characteristics with special reference to the sporulation of the fungi isolates was used to identify them. Where the plates were observed daily for growth rate of each of the cultural isolates. The colour and morphology of the colonies was recorded. A wet mount of each fungus was prepared by suspending a loopful of isolate in a few drops of lacto-phenol cotton blue solution on a microscope slide and then cover with a slip then viewed under x40 magnification.
Results
The Biochemical Reactions of Bacteria Isolates
Three species of Bacteria were isolated; Bacillus laterosporus, Staphylococcus aureus and Staphylococcus epidermidis. Bacillus laterosporus – an aerobic filamentous, spore bearing gram positive rod. Staphylococcus aureus and Staphylococcus epidermidis are non-motile gram positive cocci that are catalase positive, where Staphylococcus aureus is coagulase positive, Staphylococcus epidermidis is coagulase negative. See table 3.
Three species of Bacteria were isolated; Bacillus laterosporus, Staphylococcus aureus and Staphylococcus epidermidis. Bacillus laterosporus – an aerobic filamentous, spore bearing gram positive rod. Staphylococcus aureus and Staphylococcus epidermidis are non-motile gram positive cocci that are catalase positive, where Staphylococcus aureus is coagulase positive, Staphylococcus epidermidis is coagulase negative. See table 3.
Identified Fungi Isolates.
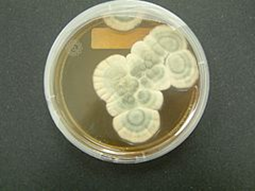
Two genus were identified (Zygomycetes and Deuteromycetes) and they are common contaminant on various substrates like grains, fruits etc. the general cultural and microscopic characteristics of the species are showed in table 4, adapted from fungi Atlass (Samson., et al. 2004).
Two genus were identified (Zygomycetes and Deuteromycetes) and they are common contaminant on various substrates like grains, fruits etc. the general cultural and microscopic characteristics of the species are showed in table 4, adapted from fungi Atlass (Samson., et al. 2004).
Frequency of Occurrence of the Isolated Bacteria and Fungi
It was observed for bacteria species, that Bacillus laterosporus had the highest occurrence of 62.5%, where Staphylococcus epidermidis and Staphylococcus aureus had 25% and 12.5% respectively. Rhizopus stolonifer had 29.41%, Aspergillus fumigatus 23.53% where Aspergillus flavus, Aspergillus niger, Penicillius citrinum and Penicillium chrysogenum had 11.76% respectively for fungi species. Table 5 showed the result.
It was observed for bacteria species, that Bacillus laterosporus had the highest occurrence of 62.5%, where Staphylococcus epidermidis and Staphylococcus aureus had 25% and 12.5% respectively. Rhizopus stolonifer had 29.41%, Aspergillus fumigatus 23.53% where Aspergillus flavus, Aspergillus niger, Penicillius citrinum and Penicillium chrysogenum had 11.76% respectively for fungi species. Table 5 showed the result.
| Microorganisms | Gram reaction | Coagulase | Catalase | Citrate | Oxidase | Motility | Urease | Gas | Lactose | Glucose | Mannitol | Arabinose | Xylose |
| Staphylococcus aureus | + | + | + | - | - | - | + | + | + | + | + | ||
| Staphylococcus epiderdimis | + | - | + | - | - | - | |||||||
| Bacillus laterosporus | + | - | - | - | + | - | + | + | - | - |
Table 1: Biochemical Reaction of Bacteria Isolates.
Key:
+ = Positive
- = Negative
Key:
+ = Positive
- = Negative
| Cultural characteristics on Czapek agar | Microscope examination of solid culture | Probable identify |
| Blue, green colour, wooly surfaces growth and the reverse is yellow | Short conidiophores, smooth-walled and conical shaped terminal vesicles. | Aspergillus fumigatus |
| White fluffy growth of colonies with elevated mycelia that turned black after 36 hours | Single celled spore and flattened conidia in chains developing at the end of the sterigma, arising from the terminal bud of the septate hyphae. | Aspergillus niger |
| Colonies usually consist of a dense yellow green conidiophores becoming dark yellow-green | Conidia heads typically radiates, later splitting into loose columns conidia globose to subglobose phialides borne directly on the vesicle. | Aspergillus flavus |
| Whitish colony becoming grayish-brown and brown black sporangia, often over 20mm high smooth or slightly rough walled stolons and blackish brown at maturity. | Columella is brown, spores are short ellipsoidal, zygospores brownish-black, sporangiospores irregular in shape often polygonal or avoid. | Rhizopus stolonifer |
| Growing restrictedly within 7days 1-5cm in diameter consisting of a dense felt of conidiophores appearing leathery blue green reverse normally yellow | It has septate, branching fungal mycelia. Its conidiophores are one stage branched smooth walled terminating in a whorl of 6 – 10 phialides, phialides, flask shaped, conidia produced in columns, globose to sub globose. | Penicillium citrinum |
| Rapid growth, lobed margin, pale green blue changing to darker green shades, odour is fruity usually velvety and flat | Septate hyphae, conidiophores simple or branched. | Penicillium chrysogenum |
Table 2: Morphological Characteristics of the Fungal Isolates.
| Organism Isolated | No of Occurrence | % Frequency |
| Bacteria Isolates | ||
| Staphylococcus aureus | 1 | 12.5 |
| Bacillus laterosporus | 5 | 62.5 |
| Staphylococcus epidermis | 2 | 25 |
| Fungi Isolates | ||
| Rhizopus Stolonifer | 5 | 29.41 |
| Aspergillus niger | 2 | 11.76 |
| Aspergillus fumigatus | 4 | 23.53 |
| Aspergillus flavus | 2 | 11.76 |
| Penicillium citrinum | 2 | 11.76 |
| Penicillium chrysogenum | 2 | 11.76 |
Table 3: Frequency of Occurrence of Microorganism Isolated from Plain Cakes.
Note:
Frequency of occurrence = No of a specie isolated x 100
The total no of microorganism (Bacteria or fungi)
Note:
Frequency of occurrence = No of a specie isolated x 100
The total no of microorganism (Bacteria or fungi)
Discussion
The bacteria isolated were; Bacillus laterosporus, Staphylococcus aureus, and Staphylococcus epidermidis., These organisms are different from those stated in the literatures as possible spoilage microorganisms caused by bacteria in cake when compared with those isolated by Bailey., et al. 1993, Saranraji., et al. 2012 but Seiler., et al. 2000, attested to the fact that cakes and desserts contributed 3 percent of the Staphylococcus aureus food poisoning in Britain in 1969 and 1972.
The mould isolated were Aspergillus fumigatus, A.niger, A.flavus, Penicillium citrinum, Penicillium chrysogenum and Rhizopus stolonifer. This is in accordance with the mould species isolated from cake by Jarvis (2001). This also means that many species of Aspergillius, Penicillium and Mucor are more resistant to heat than are other mould spores fairly resistant to dry heat. Frazier., et al. 1999.
Conclusion
This research work has a public health implications where the possible Micoorganims; bacteria and fungi, that invades cakes and thereby causing spoilage of the cakes were evaluated. It then means that most cakes sold in shops and served in parties, that deteriorates within days,were not properly prepared and preserved. There is an urgent need to educate bakers on the need to adopt standard baking conditions,to reduce and eradicate food poisoning by this Microorganisms.
References
- OI Ayanda., et al. “Isolation, Characterization and Extracellular Enzyme detection of Microbial Isolate from deteriorated apple (Malus domestica) fruits”. International International Journal of Biological and Chemical Sciences 7.2 (2013): 641-648.
- Barros HR., et al. “Antioxidant capacity and mineral content of pulp and peel from commercial cultivars of citrus from Brazil”. Food Chemistry 134.4 (2012): 1892-1898.
- Bailey CP and Holy AV. “Bacillus spore contamination associated with commercial bread manufacture”. Food Microbiology 10.4 (1993): 287-294.
- Cheesbrough M. District Laboratory practice in Tropical countries, Cambridge University, United Kingdom. 2.2 (2009): 65-67.
- Frazier WC and Westhoff DC. Food Microbiology. Third edition. Hill Book Company New York, (1999): 104.
- Rachel N., et al. “Early detection and differentiation of spoilage of bakery products”. Sensors and Actuators B: Chemical 106.1 (2004): 20-23.
- Robert A and Samson JI pitt –mk. “Integration of Modern Taxonomic methods for Penicillium and Aspergillus classification”. Canadian Research Council press 20 (2000): 524- 526.
- Samson RA., et al. Introduction to Food and Airborne Fungi, Central bureau yoor schiminel cultures,Utrech, The Netherlands,Seventh edition (2004).
- Saranraj P and Geetha M. “Microbial spoilage of bakery products and its control by preservatives”. International Journal of Pharmaceutical and biological Archives 3.1 (2011): 38-48.
- Seiler DAL. “Preservation of bakery products”. Institution of Food Science and Technology Proceedings. 17 (2000): 35-40.
- https://www.historyofcake.com.,history and importance of cakes.retrievedJuly 2016.
- https;//www.foodtimeline.org.,history and importance of cakes.retrieved July 2016.
Citation:
Ibejekwe AN and Nyam MA. “Microorganisms Associated with Plain Cake from Eateries and Parties in Jos Metropolis, Nigeria.”
Nutrition and Food Toxicology 3.3 (2018): 652-660.
Copyright: © 2018 Ibejekwe AN and Nyam MA. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.






































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.