Research Article
Volume 3 Issue 3 - 2018
Antioxidant Content and Capacity of Moringa Leaf
Department of Food and Animal Sciences, Alabama Agricultural and Mechanical University, United States
*Corresponding Author: Dr. Martha Verghese, Department of Food and Animal Sciences, Alabama Agricultural and Mechanical University,
4900 Meridian St., Normal, AL, United States.
Received: October 27, 2018; Published: November 06, 2018
Abstract
Herbal teas have been used as medicinal aids for centuries, having been suggested to act as natural antioxidants, possibly preventing disease. Moringa oleifera, commonly known as moringa, is used around the world for its medicinal properties. Moringa, especially the leaves, is a rich source of marco- and micronutritents and is suggested to possess many health benefits. The objectives of this study were to determine the effects of processing on antioxidant content, capacity, and inhibition of metabolizing enzyme activities in moringa leaf extracted with 80% ethanol, 80% methanol, and water (60°C and 25°C). Total phenolic content (TPC), total flavonoid content (TFC), free radical scavenging activity by 1,1-diphenyl-2-picryhydrazyl (DPPH), trolox equivalent antioxidant capacity (TEAC), ferric reducing antioxidant power (FRAP), oxygen radical absorbance capacity (ORAC) and inhibition of lipid and carbohydrate metabolizing enzyme activities (lipase, a- glucosidase and a-amylase) were determined in moringa leaf extract.
The highest TPC was observed in aqueous extract at 60°C of moringa leaf (427.7 mg GAE/g) and the lowest seen in methanolic extract of moringa leaf (35.41 mg GAE/g). For TFC, the aqueous extraction yielded higher content compared to the ethanolic extract (84.5 mg CAE/g; 50.6 mg CAE/g). After 90 minutes, ethanolic extract of moringa leaves showed a higher DPPH % inhibition compared to the aqueous extract (78.10%; 59.79%). Ethanolic extract of moringa leaf was able to reduce a greater amount of ferric than the aqueous extract (206.06%; 55.839%). Aqueous extract was able to inhibit a-amylase more effectively than the ethanolic extract (38.83%, 10.10%). Ethanolic extract was able to inhibit a-glucosidase more effectively than the aqueous extract (73.84%, 54.25%). Ethanolic extract was able to inhibit lipase more effectively than the aqueous extract (64.60%, 41.27%). Results from the current study show that both aqueous and ethanolic extracts of moringa leaves have antioxidant activity, which could potentially be used for health benefits and to prevent or treat chronic disease resulting from oxidative stress.
Keywords: Antioxidant; steeping; Herb; Tea; Enzymes
Abbreviations: TPC – Total phenolic content; TFC – Total flavonoid content; DPPH – 1,1-diphenyl-2-picrylhydrazyl; TEAC – Trolox equivalent antioxidant capacity; FRAP – Ferric reducing antioxidant capacity ORAC – Oxygen radical absorbance capacity; GAE – Gallic acid equivalents; CAE – Catechin equivalents; MLM – Moringa leaf extracted with 80% methanol; MLE – Moringa leaf extracted with 80% ethanol; MLR – Moringa leaf extracted with room temperature (25°C) water; MLS – Moringa leaf extracted with heated (60°C) water; AAPH - 2,2'-azobis(2-methylpropionamidine) dihydrochloride
Introduction
Traditional and herbal teas have been consumed for centuries as disease preventing agents. While traditional tea is derived from the plant Camellia sinensis, which is the most widely consumed beverage in the world second to water [1], herbal teas are increasing in popularity and being used for their medicinal properties. Studies suggest a positive relationship between consumption of herbal tea and reduction of select chronic diseases. Health benefits associated with herbal teas include hypotensive, anti-diabetic and anti-mutagenic properties [2-5]. A number of the health beneficial properties of herbal teas are attributed to antioxidants present. Natural antioxidants are receiving an enormous amount of attention from nutritionists, food manufacturers, medical experts and consumers due to their purported health benefits. Herbal teas, such as moringa, are suggested to be one such source of natural antioxidants.
Originating in the western and sub-Himalayas, Africa, India and Pakistan [6-8] various parts of the moringa plant (Moringa oleifera), including the leaf, bark and essential oils, have been utilized for many years as a supplement or consumed as a tea or vegetable. Reportedly high in beta-carotene, vitamin C and minerals, moringa leaves have been deemed “as virtually ideal dietary supplement(s)” [9,8]. The leaves are also suggested to have high antioxidant capacity, which scavenges free radicals that may lead to chronic diseases such as diabetes, heart disease and cancer [10].
Moringa has also been shown to have anti-diabetic effects in STZ-induced rats [11]. Because of the high amounts of carbohydrates and lipids, the western diet has been associated with the promotion of diseases of civilization, including obesity and diabetes. Lipase, a-glucosidase and a-amylase are enzymes that aid in the metabolism of fats (lipase) and carbohydrates (a-glucosidase; a-amylase). Pancreatic lipase, composed of 449 amino acids, is a crucial enzyme for the absorption of dietary triglycerides [12]. a-glucosidase (small intestine) and a-amylase (pancreas) are enzymes that play a key role in carbohydrate digestion. The inhibition of these key metabolizing enzymes could result in retardation of lipid and carbohydrate absorption; possibly reducing the risk of chronic diseases [13-14].
To date, there has been some research conducted on the phytochemical content and potential health benefits of moringa leaf, but there has been limited research considering the most effective phytochemical extraction method and effects of processing on the leaf.
The use of supplements and other herbal tea derived products is constantly increasing. Because of this, there is a possible niche in the market for other non-Camellia senesis derived teas. Though there are some herbal products on the market, there is still a need to incorporate products from different plant sources and investigate their antioxidant properties. The objectives of this study were to determine the effects of processing on antioxidant content, capacity, and inhibition of metabolizing enzyme activities in moringa leaf extracted with 80% ethanol, 80% methanol, and water (60°C and 25°C).
Materials and Methods
All chemicals were obtained from Sigma Chemical Company, St. Louis, Mo. and Fisher Scientific Company, Waltham, Mass. Moringa leaves were obtained from a privately owned farm in India.
Determination of Phytochemicals in Moringa
Sample preparation and extraction of phenolics: Extraction of moringa leaves was performed using established methods. Leaves were ground to a fine powder using a coffee grinder (Hamilton Beach, Glen Allen, VA). Five grams of leaf powder was added to 50mL of 80% methanol, 80% ethanol, or water (60°C or 25°C). The mixtures were stirred for 2 hr on an orbital shaker then centrifuged at 3000 xg for 20 mins. The supernatant was collected, filtered, and evaporated to dryness. The extraction was reconstituted with respective solvent and stored at -80°C until further analysis. Moringa leaf extracts were identified as follows: MLM (moringa leaf extracted with 80% methanol); MLE (moringa leaf extracted with 80% ethanol); MLR (moringa leaf extracted with room temperature (25°C) water); MLS (moringa leaf extracted with heated (60°C) water).
Sample preparation and extraction of phenolics: Extraction of moringa leaves was performed using established methods. Leaves were ground to a fine powder using a coffee grinder (Hamilton Beach, Glen Allen, VA). Five grams of leaf powder was added to 50mL of 80% methanol, 80% ethanol, or water (60°C or 25°C). The mixtures were stirred for 2 hr on an orbital shaker then centrifuged at 3000 xg for 20 mins. The supernatant was collected, filtered, and evaporated to dryness. The extraction was reconstituted with respective solvent and stored at -80°C until further analysis. Moringa leaf extracts were identified as follows: MLM (moringa leaf extracted with 80% methanol); MLE (moringa leaf extracted with 80% ethanol); MLR (moringa leaf extracted with room temperature (25°C) water); MLS (moringa leaf extracted with heated (60°C) water).
Determination of Total Phenolic and Flavonoid Contents in Moringa Extracts. Total phenolics in moringa leaf extracts were determined by the Folin-Ciocalteau method and reported as gallic acid equivalents (GAE) as described by [15]. Total flavonoids in moringa leaf extracts were determined by a colorimetric methods described by [16-17] with modifications and reported as catechin equivalents (CAE).
Antioxidant Potential of Moringa
Antioxidant activities including ferric reducing antioxidant power (FRAP), 1,1 diphenyl 2-picrahydrazyl (DPPH) radical scavenging ability, trolox equivalent antioxidant capacity (TEAC), and oxygen radical absorbance capacity (ORAC) of moringa leaf extracts were determined. FRAP of moringa leaf extracts was determined by the methods described by [18]. DPPH radical scavenging ability of leaf extracts was determined following methods described by [19] and modified by [20]. Trolox equivalent antioxidant capacity (TEAC) of moringa leaf extracts was performed following methods by [21] using the 2,2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radical. Oxygen radical absorbance capacity of moringa was compared to that of trolox using methods described by [22].
Antioxidant activities including ferric reducing antioxidant power (FRAP), 1,1 diphenyl 2-picrahydrazyl (DPPH) radical scavenging ability, trolox equivalent antioxidant capacity (TEAC), and oxygen radical absorbance capacity (ORAC) of moringa leaf extracts were determined. FRAP of moringa leaf extracts was determined by the methods described by [18]. DPPH radical scavenging ability of leaf extracts was determined following methods described by [19] and modified by [20]. Trolox equivalent antioxidant capacity (TEAC) of moringa leaf extracts was performed following methods by [21] using the 2,2’-azino-bis (3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radical. Oxygen radical absorbance capacity of moringa was compared to that of trolox using methods described by [22].
Determination of Lipid and Carbohydrate Metabolizing Enzyme Inhibition.
Inhibition of lipase, a-glucosidase and a-amylase by moringa was determine by methods described by [23] and [24], respectively.
Inhibition of lipase, a-glucosidase and a-amylase by moringa was determine by methods described by [23] and [24], respectively.
Statistical analysis
Results are presented as means ± SEM using SAS system version 9.3 ANOVA was used to determine any significant differences among the treatment groups. Significance was determined at p ≤ 0.05. The means were separated using Tukey’s Studentized Range Test.
Results are presented as means ± SEM using SAS system version 9.3 ANOVA was used to determine any significant differences among the treatment groups. Significance was determined at p ≤ 0.05. The means were separated using Tukey’s Studentized Range Test.
Results and Discussion
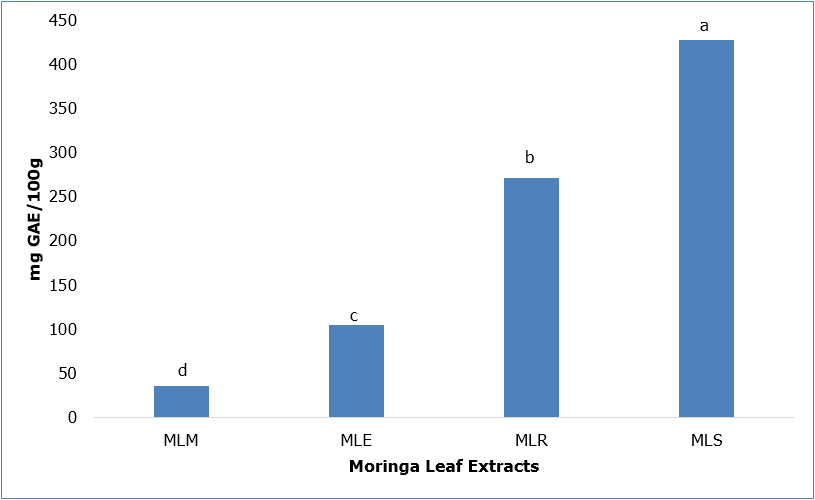
There are a number of herbal products on the market currently and some herbal tea products and supplements include information of the product’s antioxidant potential and content on the primary packaging. The antioxidant content and potential of moringa leaf extracted with various solvents was determined. Figure 1 shows the total phenolic content of moringa leaf extracted with 80% methanol, 80% ethanol, 25°C water and water at 60°C. Overall, moringa leaf extracted with heated water at 60°C (MLS) had a significantly (p ≤ 0.05) higher content compared to MLR, MLE and MLM. Water was able to extract more phenolic compounds from moringa leaf compared to organic solvents; suggesting the presence of more water soluble phytochemicals in the leaf. Moving forward with other assays in the present study, MLS and MLE were selected as these extracts of moringa leaf exhibited significantly higher phenolic content compared to their polar or non-polar counterparts.
Abbreviations: GAE- gallic acid equivalents, MLM- moringa leaf extracted with 80% methanol, MLE- moringa leaf extracted with 80% ethanol, MLR- moringa leaf extracted with room temperature water (25ºC), MLS- moringa leaf extract with heated water (60ºC)
Bars (n=3) expressed as means ± SEM. Means within treatments without a common letter(abc) differ significantly at p ≤ 0.05.
Figure 1: Total Phenolic Content of Moringa Leaf Extracts.
Bars (n=3) expressed as means ± SEM. Means within treatments without a common letter(abc) differ significantly at p ≤ 0.05.
Figure 1: Total Phenolic Content of Moringa Leaf Extracts.
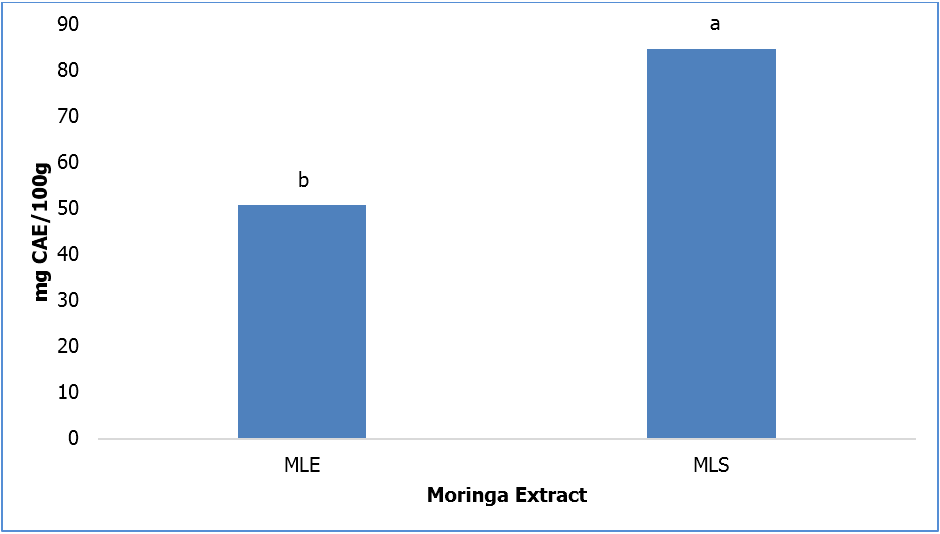
Figure 2 shows the total flavonoid content of MLS and MLE. Following a similar trend as the TPC results, moringa extracted with water heated at 60°C had a significantly (p ≤ 0.05) higher TFC compared to MLE.
Abbreviations: CAE- catechin equivalents, MLE- moringa leaf extracted with 80% ethanol, MLS- moringa leaf extract with heated water (60ºC)
Bars (n=3) expressed as means ± SEM. Means within treatments without a common letter(abc) differ significantly at p ≤ 0.05.
Figure 2: Total Flavonoid Content of Moringa Leaf Extracts.
Bars (n=3) expressed as means ± SEM. Means within treatments without a common letter(abc) differ significantly at p ≤ 0.05.
Figure 2: Total Flavonoid Content of Moringa Leaf Extracts.
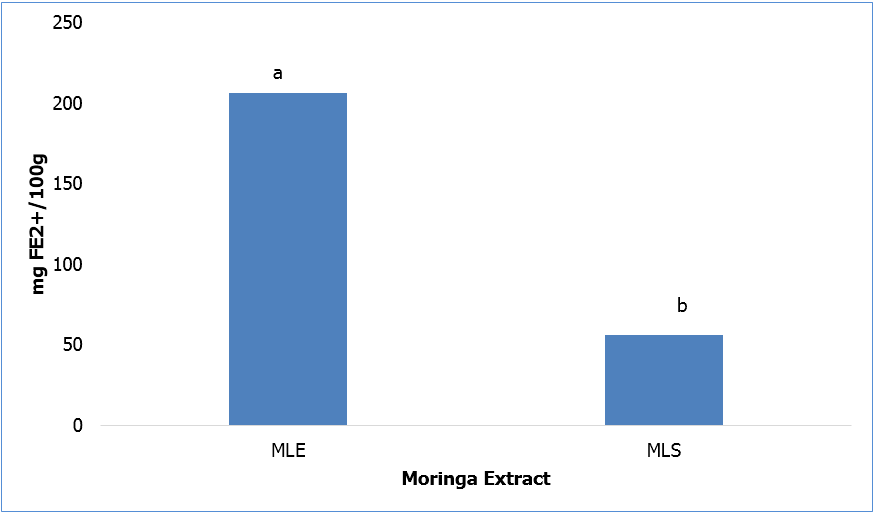
FRAP results are displayed in Figure 3. The ability of MLE to reduce ferric to ferrous iron was significantly (p ≤ 0.05) greater than that of moringa extracted with heated water.
Abbreviations: FE2+- Ferrous sulphate, MLE- moringa leaf extracted with 80% ethanol, MLS- moringa leaf extract with heated water (60ºC)
Bars (n=3) expressed as means ± SEM. Means within treatments without a common letter(abc) differ significantly at p≤0.05.
Figure 3: Ferric Reducing Antioxidant Power of Moringa Leaf Extracts.
Bars (n=3) expressed as means ± SEM. Means within treatments without a common letter(abc) differ significantly at p≤0.05.
Figure 3: Ferric Reducing Antioxidant Power of Moringa Leaf Extracts.
Table 1 displays the antioxidant capacity of moringa leaf extracts including DPPH radical scavenging, trolox equivalent antioxidant capacity and oxygen radical absorbance capacity. DPPH is a stable free radical that is deep purple in color. This assay measures the ability of biological samples to reduce 1,1-diphenyl-2-picryl hydrazyl radical to 1,1-diphenyl-2-picryl hydrazine, therefore a reduction in purple color indicates a reduction in free radicals. At an extract concentration of 0.1mg/ml MLE had a significantly (p≤0.05) higher DPPH radical scavenging ability compared to MLS. TEAC of moringa leaf extracts, reported as trolox equivalents (TE)/100g of sample, is also displayed in Table 1. Trolox is a water-soluble analog of vitamin E; this assay compares the ability of the moringa extracts to scavenge the ABTS radical (ABTS + to ABTS -) to that of vitamin E.
| Moringa Extract | DPPH Radical % Scavenging | TEAC (mm TE/100g) | ORAC (mm TE/100g) |
| MLE | 78.10 ± 0.34a | 222.18 ± 11.55a | 1309.45 ± 115.93a |
| MLS | 59.79 ± 6.28b | 256.50 ± 4.44a | 138.07 ± 17.41b |
Abbreviations: MLE- moringa leaf extracted with 80% ethanol, MLS- moringa leaf extract with heated water (60ºC), : DPPH - 1,1 diphenyl 2-picrahydrazyl , TEAC- Trolox Equivalent Antioxidant Capacity, ORAC – Oxygen Radical Absorbance Capacity, TE- trolox equivalents.
Columns (n=3) expressed as means ± SEM. Means within treatments without a common letter(abc) differ significantly at p ≤ 0.05.
Table 1: Antioxidant Potential of Moringa Leaf Extracts.
Columns (n=3) expressed as means ± SEM. Means within treatments without a common letter(abc) differ significantly at p ≤ 0.05.
Table 1: Antioxidant Potential of Moringa Leaf Extracts.
There were no significant (p ≤ 0.05) differences in TEAC between MLE and MLS. ORAC values of moringa extracts are also displayed in Table 1. ORAC measures the ability of moringa extracts to protect fluorescein from oxidation, caused by AAPH- "2,2’-azobis(2-methylpropionamidine) dihydrochloride, compared to that of trolox. The ORAC value of MLE (1309.45) was nearly 10-fold higher than that of MLS (138.07).
Table 2 shows carbohydrate and lipid metabolizing enzyme inhibition by moringa leaf extracts. The Western diet is of growing concern to researchers, as it contains a high amount of carbohydrates and fats; which contribute heavily to the pathogenesis of obesity and diabetes. Lipase, α-glucosidase and α-amylase are enzymes that aid in the metabolism of fats (lipase) and carbohydrates (α-glucosidase; α-amylase). Inhibition of the aforementioned enzymes could lead to the prevention of diabetes and obesity. The ethanolic extraction of moringa leaf inhibited α-glucosidase and lipase more effectively than MLS; oppositely, MLS had a significantly (p ≤ 0.05) higher α-amylase inhibition compared to MLE.
| Moringa Extract | a-glucosidase (% Inhibition) | a-amylase (% Inhibition) | Lipase (% Inhibition) |
| MLE | 73.84a | 10.10b | 64.60a |
| MLS | 54.25b | 38.83a | 41.27b |
Abbreviations: MLE- moringa leaf extracted with 80% ethanol, MLS- moringa leaf extract with heated water (60ºC)
Columns (n=3) expressed as means ± SEM. Means within treatments without a common letter(abc) differ significantly at p ≤ 0.05.
Table 2: Carbohydrate and Lipid Metabolizing Enzyme Inhibition by Moringa Leaf Extracts.
Columns (n=3) expressed as means ± SEM. Means within treatments without a common letter(abc) differ significantly at p ≤ 0.05.
Table 2: Carbohydrate and Lipid Metabolizing Enzyme Inhibition by Moringa Leaf Extracts.
Discussion
TPC, TFC, FRAP, TEAC and ORAC were evaluated in various extractions of moringa leaf. Leaves were extracted with 80% methanol, 80% ethanol, room temperature (25°C) water or heated (60°C) water. Leaves were extracted with heated water to simulate tea steeping. In the present study, heating increased extractability of polyphenols. The present results align with Su and others, who reported that phytochemical yield increased with steeping temperature and time of oolong tea extracts. The same authors also observed a reduction in phenolics after steeping at 100°C for 10 mins [25]. A similar trend was noted in TFC for the current study.
In the present study, the ethanolic extract of the moringa leaf had higher FRAP, DPPH and ORAC antioxidant potentials. Ethanolic extractions of moringa were shown to isolate niazinin derivatives, which are suggested to have health benefitting properties [26,8]. It was also suggested that the ethanolic extract of moringa improves antioxidant status by enhancing the activity of antioxidant enzymes [27-28].
The trolox equivalent antioxidant capacity assay measures the ability of extracts to inhibit the ABTS radical, compared to a water-soluble vitamin E analog, trolox. Results from the present study suggest that moringa leaf extracted with water inhibited more ABTS compared to the ethanolic extract of moringa. A study conducted by Floegel and others aligns with the present study by suggested that the ABTS radical is inhibited at a higher percentage by hydrophilic extracts [29].
Moringa leaf extracts inhibited carbohydrate and lipid metabolizing enzymes in the present study. Moringa is an immense source of flavonoids such as kaempferol, and isoquercitrin [30-31, 28]. A study conducted by Tadera and others suggested that the enzyme inhibitory activity of flavonoids is driven by the unsaturated C ring and hydroxyl substitution on the B ring [14], which possibly gave rise to the enzyme inhibition of moringa leaf extracts in the present study.
Conclusion
Though there are a number of herbal products on the market, very few utilize moringa leaves as an ingredient. The results of the study suggest that moringa leaf extracts may benefit consumers by improving their antioxidant status and inhibiting carbohydrate and lipid metabolizing enzymes, possibly leading to the prevention of chronic disease.
References
- Kuroda Yukiaki and Yukihiko Hara. "Antimutagenic and anticarcinogenic activity of tea polyphenols." Mutation Research/Reviews in Mutation Research 436.1 (1999): 69-97.
- De Vos Sven and Remi De Schrijver. "Lipid metabolism, intestinal fermentation and mineral absorption in rats consuming black tea." Nutrition research 23.4 (2003): 527-537.
- Haslam Edwin. "Thoughts on thearubigins." Phytochemistry64.1 (2003): 61-73.
- Büyükbalci Aynur and Sedef Nehir El. "Determination of in vitro antidiabetic effects, antioxidant activities and phenol contents of some herbal teas." Plant Foods for Human Nutrition 63.1 (2008): 27-33.
- Carabajal Monica Patricia Antonella., et al. "Evaluation of antioxidant and antimutagenic activity of herbal teas from native plants used in traditional medicine in Argentina." South African Journal of Botany 110 (2017): 258-265.
- Somali MA., et al. "Chemical composition and characteristics ofMoringa peregrina seeds and seeds oil." Journal of the American Oil Chemists' Society 61.1 (1984): 85-86.
- Mughal M., et al. "Drumstick (Moringa pterygosperma Gaertn.): a unique source of food and medicine." J. Econ. Taxon. Bot 23 (1999): 47-61.
- Anwar Farooq., et al. "Moringa oleifera: a food plant with multiple medicinal uses." Phytotherapy Research: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives 21.1 (2007): 17-25.
- Makkar HPS and, and K Becker. "Nutrional value and antinutritional components of whole and ethanol extracted Moringa oleifera leaves." Animal feed science and technology63.1-4 (1996): 211-228.
- Luqman, Suaib., et al. "Experimental assessment of Moringa oleifera leaf and fruit for its antistress, antioxidant, and scavenging potential using in vitro and in vivo assays." Evidence-Based Complementary and Alternative Medicine 2012 (2012).
- Gupta, Rajnish, et al. "Evaluation of antidiabetic and antioxidant activity of Moringa oleifera in experimental diabetes." Journal of Diabetes 4.2 (2012): 164-171.
- Slanc Petra, et al. "Screening of selected food and medicinal plant extracts for pancreatic lipase inhibition." Phytotherapy Research: An International Journal Devoted to Pharmacological and Toxicological Evaluation of Natural Product Derivatives 23.6 (2009): 874-877.
- Drent ML. "Lipase inhibition: a novel concept in the treatment of obesity." International journal of obesity and related metabolic disorders: journal of the International Association for the Study of Obesity 17.4 (1993): 241-244.
- Tadera Kenjiro., et al. "Inhibition of α-glucosidase and α-amylase by flavonoids." Journal of nutritional science and vitaminology 52.2 (2006): 149-153.
- Shackelford L., et al. "Determination of Total Phenolics, Flavonoids and Antioxidant and Chemopreventive Potential of Basil (Ocimum basilicum L. and Ocimum tenuiflorum L.)" D. Gajula,'M. Verghese," J. Boateng," LT Walker." International Journal of Cancer Research 5.4 (2009): 130-143.
- Zhishen, Jia., et al. "The determination of flavonoid contents in mulberry and their scavenging effects on superoxide radicals." Food chemistry64.4 (1999): 555-559.
- Adom Kafui Kwami and Rui Hai Liu. "Antioxidant activity of grains." Journal of agricultural and food chemistry 50.21 (2002): 6182-6187.
- Benzie Iris FF and JJ Strain. "[2] Ferric reducing/antioxidant power assay: Direct measure of total antioxidant activity of biological fluids and modified version for simultaneous measurement of total antioxidant power and ascorbic acid concentration." Methods in enzymology. Vol. 299. Academic Press 1999. 15-27.
- Brand-Williams., et al. "Use of a free radical method to evaluate antioxidant activity." LWT-Food science and Technology 28.1 (1995): 25-30.
- Lee Ki Won., et al. "Cocoa has more phenolic phytochemicals and a higher antioxidant capacity than teas and red wine." Journal of agricultural and food chemistry 51.25 (2003): 7292-7295.
- Miller, Nicholas J and Catherine A Rice-Evans. "Factors influencing the antioxidant activity determined by the ABTS•+ radical cation assay." Free radical research 26.3 (1997): 195-199.
- Huang Dejian, et al. "High-throughput assay of oxygen radical absorbance capacity (ORAC) using a multichannel liquid handling system coupled with a microplate fluorescence reader in 96-well format." Journal of agricultural and food chemistry 50.16 (2002): 4437-4444.
- Mosmuller EWJ., et al. "A new spectrophotometric method for the detection of lipase activity using 2, 4-dinitrophenyl butyrate as a substrate." Biocatalysis 5.4 (1992): 279-287.
- Apostolidis E., et al. "Inhibitory potential of herb, fruit, and fungal-enriched cheese against key enzymes linked to type 2 diabetes and hypertension." Innovative Food Science & Emerging Technologies 8.1 (2007): 46-54.
- Su Xinguo., et al. "Polyphenolic profile and antioxidant activities of oolong tea infusion under various steeping conditions." International journal of molecular sciences 8.12 (2007): 1196-1205.
- Gilani Anwar H., et al. "Pharmacological studies on hypotensive and spasmolytic activities of pure compounds from Moringa oleifera." Phytotherapy research 8.2 (1994): 87-91.
- Paliwal R., et al. "Anti-nephrotoxic effect of administration of Moringa oleifera Lam in amelioration of DMBA-induced renal carcinogenesis in Swiss albino mice." Biology and Medicine3.2 (2011): 27-35.
- Umesha, Sharanaiah., et al. "Antioxidant and antidiabetic activities of medicinal plants: A short review." Int J Res Phytochem Pharmacol 3.1 (2013): 40-53.
- Floegel., et al. "Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods." Journal of food composition and analysis 24.7 (2011): 1043-1048.
- Rajanandh MG and J Kavitha. "Quantitative estimation of β-sitosterol, total phenolic and flavonoid compounds in the leaves of Moringa oleifera." International Journal of PharmTech Research 2.2 (2010): 1409-1414.
- Moyo Busani., et al. "Nutritional characterization of Moringa (Moringa oleifera Lam.) leaves." African Journal of Biotechnology 10.60 (2011): 12925-12933.
Citation:
Martha Verghese., et al. “Antioxidant Content and Capacity of Moringa Leaf”. Nutrition and Food Toxicology 3.3 (2018): 672-
679.
Copyright: © 2018 Martha Verghese., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.



































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.