Review Article
Volume 3 Issue 5 - 2019
Iridoids. Some Monotepenes and Other Natural Products for Development of Potential Therapies in Alzheimer’s Disease-A Review
1Scientific Director, DR Kulvinder Kaur Centre for Human Reproduction 721, G.T.B. Nagar Jalandhar-144001, Punjab, India
2Scientific Director, Ex-Rotunda-A Centre for Human reproduction 672, Kalpak Garden, Perry Cross Road, Near Otter’s Club, Bandra (W)- 400040 MUMBAI, INDIA
3Consultant Neurologist, Swami Satyanand Hospital Near Nawi Kachehri, Baradri, Ladowali road, Jalandhar PUNJAB
2Scientific Director, Ex-Rotunda-A Centre for Human reproduction 672, Kalpak Garden, Perry Cross Road, Near Otter’s Club, Bandra (W)- 400040 MUMBAI, INDIA
3Consultant Neurologist, Swami Satyanand Hospital Near Nawi Kachehri, Baradri, Ladowali road, Jalandhar PUNJAB
*Corresponding Author: Kulvinder Kochar Kaur, Scientific Director, Ex-Rotunda-A Centre for Human Reproduction 721, G.T.B. Nagar, Jalandhar-144001, Punjab, India.
Received: May 14, 2019; Published: July 19, 2019
Abstract
Alzheimers disease (AD) is a progressive deadly and neurodegenerative disease that is characterized by memory loss, cognitive impairments and dementia .Various hypothesis have been proposed for the pathogenesis based on the pathological changes in the brain of AD patients during the last few decades. Currently only few drugs that function as acetyl cholinesterase inhibitors or N-methyl-D –Aspartate (NMDA) receptor antagonists are av ailable for alleviating symptoms. Iridoids area class of monoterpenoid compounds constructed from 10-carbon skeleton of isoprene building units. These compounds in their aglycones and glycosated forms exist in nature for contributing to mechanisms related to plant defenses and diverse plant-animal interactions. Recent studies have shown that iridoids and structurally related monoterpenes display a wide range of pharmacological effects which make them potential modulators of AD. In this review a critical evaluation of natural products is done by assessing pivotal in vivo and in vitro published data. A mechanistic approach of scrutiny addressing their effects in Alzheimer’s brain including Ϯ protein phosphorylation signaling, amyloid beta formation, aggregation, toxicity and clearance along with various effects from antioxidant anti-inflammatory mechanisms are discussed. The drug likeness of these compounds and future compounds to consider in their potential as potential leads are considered. Further latest in silIco studies enhancing the AD potential of other natural products are addressed.
Keywords: AD; Alzheimer’s brain; Iridoids; Other monoterpenes; AChE inhibitor; Aquaporin 4
Introduction
Alzheimer’s disease (AD) has become one of the highest prevalent age associated diseases which affect mostly aged population. Of the average 5.5 million Americans with AD in 2017, 5.3 million that comprised about 96% of the patients’ population, were either 65 years or older [1]. AD also accounts for 70% of all cases of dementia, with a global prevalence of approximately 47 million in 2015 [2]. Further the projected figures for dementia by the year 2050 is 131.5million which underscores how rapid there is an increase of AD. Once life expectancy continues to rise all over the world in parallel with the economy developing, risk of AD and its cost along with social burden will be realized further in the future. This disease is characterized by progressive cognitive defects along with irreversible neuronal damage. Till date no cure for AD exists and the average lifespan between the manifestation of clinical symptoms and death is roughly 8.5years [3]. The memory deficiency is also associated with behavioral changes that makes patient care a big challenge in AD.
Therapeutic options for AD are very limited, that are mainly directed at the cholinergic system by utilizing cholinesterase inhibitors like donezil, galantamine and rivastigmine. Limited benefit has been found on use of N-methyl-D-aspartate (NMDA) receptor antagonists which include memantine which have been tried [4,5] Ealier role of natural products in ameliorating different neurodegenerative diseases has been reviewed [6]. Marked understanding that has got advanced with regards to therapeutic potential of polyphenolic compounds like flavonoids [7-12]. Caffeic acid derivatives [13] and aromatic diterpenoids [14]. Still one class that has not got much attention as being potential therapy for AD is the monoterpene class. Thus here we have tried to do a systematic review regarding the same.
Methods
Thus we did a systematic review using pubmed database and further supplemented data obtained from google scholar regarding utilization of monoterpenes,iridoids any small molecules in the treatment of alzheimers disease. The MeSH terms utilized were ‘alzheimer’s disease (AD); other natural compounds Alzheimer brain; iridoids in AD.;monoterpenes in AD, Pathophysiology of AD; aquaporin 4; amyloid beta; natural therapy for AD and enhancement of treatment potentiality of AD drugs.
Results
We found a total of 1649 articles out of which we utilized 127 articles for this review. No meta-analysis was done.
Biochemistry of Iridoids
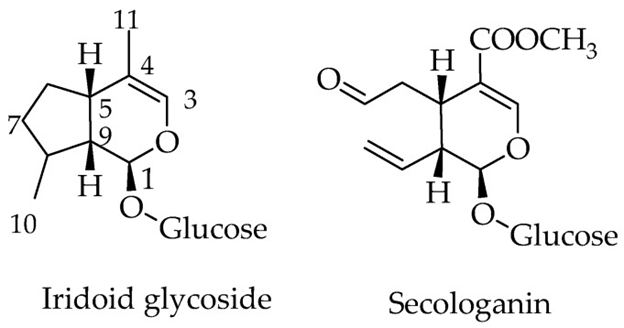
The monoterpenoid natural products, made up of 10 carbon skeleton are referred to as iridoids. The structural marker of these compounds is the cis –fused cyclopental [c] pyran system,which is present in nature in the form of glycosides, aglycones, as secoiridoids or bisiridoid forms (figure 1). For secoiridoids the C7-C8 bond of the iridoid skeleton is cleaved following a series of oxidation steps to give rise to compounds like secologanin,that also serves as a precursor for the synthesis of alkaloids.
The monoterpenoid natural products, made up of 10 carbon skeleton are referred to as iridoids. The structural marker of these compounds is the cis –fused cyclopental [c] pyran system,which is present in nature in the form of glycosides, aglycones, as secoiridoids or bisiridoid forms (figure 1). For secoiridoids the C7-C8 bond of the iridoid skeleton is cleaved following a series of oxidation steps to give rise to compounds like secologanin,that also serves as a precursor for the synthesis of alkaloids.
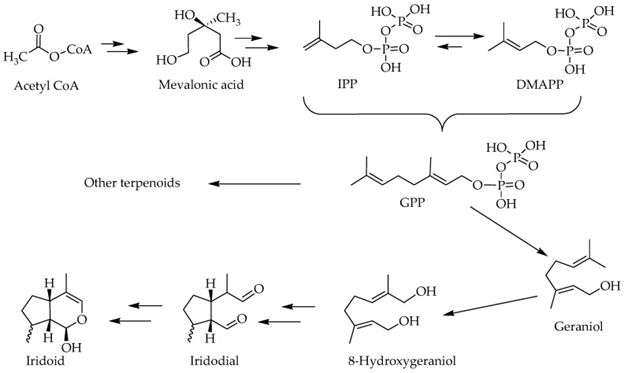
We have earlier reviewed the biosynthetic pathways of terpenoids [15,16], which involves some pivotal intermediates like mevalonic acid. Despite the initiating primary metabolite is a 2 carbon [C] metabolite, acetyl Co A, the basic skeleton of all terpenoids by definition is 5-C isoprene units in the form of isopentenyl pyrophosphate (IPP) and dimethyl allyl pyrophosphate (DMAPP). The precursor of all terpenoids in the further steps of reaction is the geranyl pyrophosphate (GPP), which is made from two isoprene units (figure 2). The sesquiterpenes (15C), Diterpenes (20C) and triterpenes (30C) are the typical examples of terpenoids which arise by condensation of these isoprene units via a range of both cyclic and acyclic monoterpenes of biological significance [17], while the 8-hydroxy geraniol unique pathways => iridoids and their derivatives (figure 2).
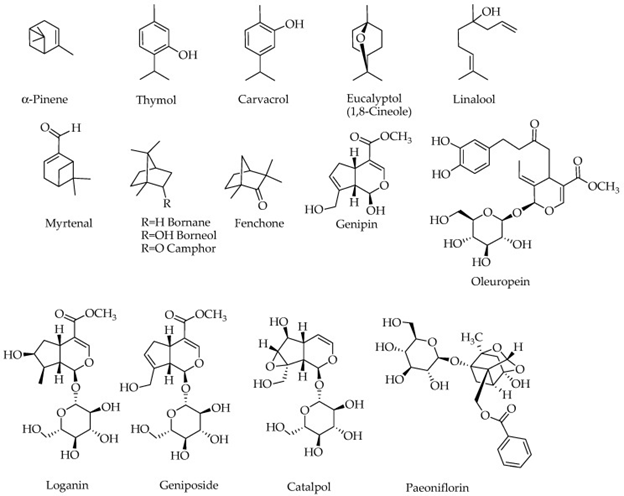
The compounds which have been examined for being potential for removing biochemical and behavioral symptoms of alzheimer’s disease (AD) are represented in figure 3.
Figure 3: Courtesy ref no-75-Structures of compounds with potential effects on a
lzheimer’s brain. Compounds containing a sugar moiety (oleuprein, loganin, g eniposide,
catalpol and paeoniflorin) are highly polar and hence not components of essential oils.
General functions of Iridoids along with other Monoterpenes
There has been a question asked for centuries, i.e. why plants and animals produce secondary metabolites. Basic reasons given are the role in cell-cell communication within the organism, or plant-animal based interactions [15,18,19]. As far as chemical defense for herbivores and pathogens, role of Iridoids is well defined, since these compounds have been shown to be bitter and demonstrate good sets of biological activities [20]. Animals like butterfly accumulate these chemical agents for defense for pathogens on requirement [21,22]. In mammals biological activities of Iridoids have been studied for their role in type 2 diabetes mellitus [T2DM] [reviewed in 16,17]. Here role of monoterpenes, but basically Iridoids was studied by using invitro and in vivo studies for use in AD. Further studies that helped in enhancing AD treatment potential were explored.
Potential for treating AD
a. in vitro Protective Effects
The Amyloid beta (Aβ) formation, aggregation and function have been the main areas than can be targeted in AD for in vitro studies. Marumoto., et al. examined the β-secretase (recombinant human BACE 1) inhibitory activities of some monoterpenes. Although inhibitory activities of these monoterpenes had been confirmed, their activity was moderate (above 50nM) with geranyl acetone being the most effective (IC50 value of 51.9+_3.9µM) followed by (+)-camphor (95.9+_11.0µM), (-)- fenchone (106.3 +_14.9µM), (+)- fenchone (117.0+_18.6µM) and (-)- camphor (134.1+_16.4µM). Lot of in vitro studies have been dedicated regarding studying the inhibitory activities of monoterpenes against Aβ-induced cytotoxicity in neuronal cells in vitro. This Aβ-induced cytotoxicity along with oxidative stress got suppressed with the use of borneaol in the SH-SY5Y (human neuroblastoma cells) [23], while similar effects were demonstrated by 1,8-cineole (eucalyptol) in PC12 (rat pheochromocytoma cells) [24]; and genipin in cultured hippocampal neurons [25]. Antioxidant effect of monoterpenes is also obvious when they ameliorate effect of the H2O2-induced oxidative stress, as seen with catalpol in astrocytes [26] and α-pinene and 1, 8-cineole in PC12 cells [27]. When Cultured primary cortical neurons were exposed to Aβ, they could be rescued by geniposide, both from toxicity and oxidative stress [28]. Another experiment showed the mechanism of action of how geniposide protected primary cultured cortical neurons, the geniposide induced Ϯ protein phosphorylation along with phosphorylation of Akt Ser 473 site and GSK-3β at Ser-9 site got inhibited by leptin antagonists [29]. Role of leptin as potential mechanism of iridoids action on Alzheimer’ brain is dicussed subsequently.
The Amyloid beta (Aβ) formation, aggregation and function have been the main areas than can be targeted in AD for in vitro studies. Marumoto., et al. examined the β-secretase (recombinant human BACE 1) inhibitory activities of some monoterpenes. Although inhibitory activities of these monoterpenes had been confirmed, their activity was moderate (above 50nM) with geranyl acetone being the most effective (IC50 value of 51.9+_3.9µM) followed by (+)-camphor (95.9+_11.0µM), (-)- fenchone (106.3 +_14.9µM), (+)- fenchone (117.0+_18.6µM) and (-)- camphor (134.1+_16.4µM). Lot of in vitro studies have been dedicated regarding studying the inhibitory activities of monoterpenes against Aβ-induced cytotoxicity in neuronal cells in vitro. This Aβ-induced cytotoxicity along with oxidative stress got suppressed with the use of borneaol in the SH-SY5Y (human neuroblastoma cells) [23], while similar effects were demonstrated by 1,8-cineole (eucalyptol) in PC12 (rat pheochromocytoma cells) [24]; and genipin in cultured hippocampal neurons [25]. Antioxidant effect of monoterpenes is also obvious when they ameliorate effect of the H2O2-induced oxidative stress, as seen with catalpol in astrocytes [26] and α-pinene and 1, 8-cineole in PC12 cells [27]. When Cultured primary cortical neurons were exposed to Aβ, they could be rescued by geniposide, both from toxicity and oxidative stress [28]. Another experiment showed the mechanism of action of how geniposide protected primary cultured cortical neurons, the geniposide induced Ϯ protein phosphorylation along with phosphorylation of Akt Ser 473 site and GSK-3β at Ser-9 site got inhibited by leptin antagonists [29]. Role of leptin as potential mechanism of iridoids action on Alzheimer’ brain is dicussed subsequently.
As far as Aβ-induced cytotoxicity is concerned in the central cortical neurons, the role of insulin-degrading enzyme (IDE) has been the point of focus recently. Besides degrading and clearing Aβ, the IDE has a key role in the regulation of Aβ activity. Zhang., et al. [30] using primary cortical neurons, in a culture media showed that geniposide increases the phosphorylation of peroxisome proliferator activated receptor gamma (PPAR-γ). Action of geniposide in activating the IDE promoter was also demonstrated to be mediated through glucagon-like peptide 1(GLP1) receptor, while other pathways involved in inhibitor studies showed phosphatidyl inositol-3 kinase, PI3K, proto oncogene tyrosine protein kinase Src(c-Src), PPAR-γ, protein kinase A(PKA), and epidermal growth factor receptor (EGFR) were confirmed [30]. Moreover geniposide has been shown in SH-SY5Y cells to ameliorate cytotoxicity of Aβ in addition to its oligomer assembly and cytotoxicity [31]. SH-SY5Y cells that were treated with other toxicants like formaldehyde were shown to be protected by geniposide [32]. Paeniflorin’s protective effect in PC-12 cells from Aβ was similar to geniposide in that its activity correlated with upregulation of the protein kinase B (Akt) phosphorylation, B cell lymphoma 2(Bcl2) protein expression, decreasing Bax protein expression which was inhibited by LY294002 [33]. Paeniflorin’s perspective effect in PC12 cells from the 6-hydroxy dopamine –induced apoptosis was also correlated with increased antioxidant capacity (GSH glutathione reduced form) and decrease of nuclear factor kappa-light chain enhancer of activated B cells (NFKB) translocation [34]. A lot of other studies showed protective effect of paeoniflorin against Aβ-induced cytotoxicity
In PC12 CELLS [33,34] and SH-SY5Y cells [35], along with glutamate induced cytotoxicity in PC 12 cells [36]. Hydrogen peroxide (H2O2)-induced cytotoxicity in PC12 cells could be inhibited by geniposide through the P13K –dependent pathway as seen from a study using a selective inhibitor LY294002 [37]. Liu., et al. in some cell system also demonstrated that the effect of geniposide reversed the oxidative stress induced by H2O2 which involves an increased levels of Bcl2 by activating mitogen activated protein kinase(MAPK), Mitogen activated protein kinase kinase(MEK) and rapidly increased fibrosarcoma protooncogene serine/threonine protein (c-Raf) phosphorylation in addition to phosphorylation of the p90 variant of the ribosomal s 6 kinase (p90RSK) [37]. The requirement of the PI3K and GLP1 receptor activation has also been verified in the PC-12 cells protection from the H2O2 induced cytotoxicity [38].
Antiinflammatory effects of these compound in the CNS came from findings in vitro which showed that inhibition of NO release from lipopolysaccharide (LPS) stimulate microglia by genipin along with decrease in microglial cells activation [25]. Besides NO reduction, genipin also ameliorated the LPS induced tumor necrosis alpha (TNF-α), interleukin -1β (IL-1), prostaglandin –E2(PGE2),inhtracellular reactive oxygen species(iROS),and NFκB activation in microglial cells in vitro [5].
In an organotypic cultured hippocampal tissues,the scopolamine induced functional changes was shown to get inhibited by loganin along with inhibition of acetyl cholineasterase(AChE),butyl cholinesterase(BChE) and β-secretase (BACE 1) [39]. An effect on BACE 1 on inhibitory activity of loganin was also reported by Yoan., et al. [40]. A direct effect on one of the most prevalent AD direct, ACh E, for loganin with IC50 value in sub-micromolar range was exciting [41]. Molecular docking studies have shown that loganin’s –non-competitive type of interaction produces a negative binding energies for cholinesterases along with BACE1, which suggests high affinity and tighter binding capacity for the active site of the enzyme [41]. As BChE (though to a lesser extent) is also inhibited, logan appears to target ACh E, BChE,and BACE1, which are all important in AD pathology. The Aβ-induced inflammatory changes in PC12 cells could also be inhibited by loganin as seen by a decrease in the level of TNF-α and protein expression of iNOS and cyclooxygenase -2(COX2) [42]. These effects got corrected with inhibition of NFκB also, besides the closely related regulatory pathways including the phosphorylation of MAPK’s (ERK1/2 (Extracelluar signal related kinase ½), p38, and JNK(c-Jun –N-terminal kinase [42].
A lot of other studies have demonstrated that monoterpenes possesss direct inhibitory effects against ACh E activity. A study by Kaufmann., et al. [43] on 8-cineole, cavacrol, myrtenal and verbenone, although showed the best activity in this study, it was seen at relatively high (concentrations (IC50=170µM for myrtenal). On the other hand, oleuropein, thymol and cavacrol have been observed to have a much greater activity but the best affinity (IC50<5µM) was got when a carbamate moiety was added to carvacrol through a synthesis approach [44]. In the latter case, there has also been a drive to improve biological activity of existing anti Alzheimer’s drugs by incorporating the monoterpene skeleton through synthesis. For example with the help of a docking –based design, galantamine camphane hybrids have been shown to display over 100 fold better activity in ACh E inhibition than galantamine [45].
The gross inhibition of cytotoxicity in neuronal cells induced by Aβ and other toxic agents have been shown to be ameliorated. The reactive oxygen species (ROS), proinflammatory cytokines and many mediators could also be reduced, while mitochondrial deterioration was inhibited. At the molecular level, a range of antioxidant proteins and enzymes could be increased by natural products in addition to anti-apoptotic genes and proteins, while proapoptotic genes and proteins appear to be decreased.
b. Action in In Vivo Studies
Along with the overwhelming in vitro data, animal studies on iridoids and some other monoterpenes have shown potential therapeutic effects for treating AD [35, 36, 46-57]. Neuroprotective effect of carvacrol in vivo got studied by Zhong., et al. [56,57] using the intracerebral haemorrhage mouse model, where a significant decrease in the aquaporin 4(AQP4) dependent oedema was observed. Aqp4 is a water channel in the brain which plays a great role in the development of cerebral oedema. The structural, physiological and pathological significance of AQP4 has been deeply reviewed [58,59]. Considering the pathophysiological role of AQP4 in a variety of CNS disorders including ischemic stroke [60], neuroinflammation [61], and autoimmune neurodegenerative diseases [62], the reversal of cerebral oedema induced through the AQP4 activity by monoterpenes is an important finding.
Along with the overwhelming in vitro data, animal studies on iridoids and some other monoterpenes have shown potential therapeutic effects for treating AD [35, 36, 46-57]. Neuroprotective effect of carvacrol in vivo got studied by Zhong., et al. [56,57] using the intracerebral haemorrhage mouse model, where a significant decrease in the aquaporin 4(AQP4) dependent oedema was observed. Aqp4 is a water channel in the brain which plays a great role in the development of cerebral oedema. The structural, physiological and pathological significance of AQP4 has been deeply reviewed [58,59]. Considering the pathophysiological role of AQP4 in a variety of CNS disorders including ischemic stroke [60], neuroinflammation [61], and autoimmune neurodegenerative diseases [62], the reversal of cerebral oedema induced through the AQP4 activity by monoterpenes is an important finding.
A number of studies have targeted oxidative stress and associated disorders by inducing the pathology with D-(+)-galactose injection into experimental animals. Catalpol has been shown to decrease the level of Aβ in the cerebral cortex along with involvement in learning and memory, while the level of antioxidant defenses (SOD and GPx) were boosted [46]. In senescent mice treated with D-galactose, Zhang., et al. [47] further showed neuroprotection by catalpol as seen by increased levels and activity of choline acetyl transferase (CHAT). Furthermore catalpol, in this model has been shown to reverse the decreased levels of muscarinic acetyl choline (Ach) receptor M1 while simultaneously suppressing the level of inflammatory and oxidant markers (TNF-α, IL-1 and advanced glycation end products (AGEs) [48]. Improvement of memory detail along with antioxidant markers (glutathione –S –transferase (GSH-ST), Glutamine synthesis (GS) and creatine kinase (CK) have also been shown for catalpol [49,50].
In other experiments, Aβ was directly injected into the brain to study IR reviewed recently [17] the biochemical and behavioral changes in animals. Catalpol was among the iridoids showing activity in the model where prevention of the Ach neuronal damage was found from the increased level of choline CHAT activity [63]. Geniposide further ameliorated the Aβ induced neuronal abnormalities that included cellular densities and synaptic protein levels in the transgenic mice models [50]. The paeoniflorin effect in a memory improvement and protection of animals from Aβ via mechanisms that included enhanced antioxidant defenses (e.g. GSH) and calcium homeostasis had also been published [56,57].
APP/PSI transgenic mouse model of AD was used by Zhang., et al. [50] for studying the potential benefit of geniposide. The insulin deficiency by streptozotocin (STZ) in these wild type transgenic animals appeared to enhance the GSK-3β level/activity which was suppressed by geniposide administration in a dose dependent manner. The dose employed here were very small (5, 10, and 20mg/kg). Data was also in line with the broader effect of geniposide in signal transduction pathways relate to IR reviewed recently [17]. The GSK-3β has a direct role in Ϯ protein hyperphosphorylation [64]. Role of Akt in the regulation of GSK-3β is well understood and on getting phosphorylated in => inactivation which appeared to be modulated by geniposide. Geniposide can also regulate the phosphorylation of Ϯ protein both in the insulin –dependent and independent manner in primary cultured cortical neurons. It also increases the phosphorylation of Akt at Ser 473 site and Thr 308 sites [63]. Thus this double effect of geniposide is seen both in T2DM, and AD from its effect on the phosphorylation of Ϯ protein through the PI3K-GSK-3β kinase pathway. Upto date, hyperphosphorylated Ϯ protein is one of pathological hallmark of AD since it is the principle component of neurofibrillary targets (NFT’s) [64]. The structural integrity of Ϯ protein is regulated by a cascade of phosphorylation –related pathways, thus both kinases and phosphatases have important role in stable NFT formation. The GSK-3β is the pivotal player in the kinase –mediated hyperphosphorylation of Ϯ protein, its regulation by geniposide appears to give some idea into how possibly iridoids act. The crosstalk between DM and AD was brought into focus by Gao., et al. [52] who proved that there is potential role of geniposide via GSK-3β regulation.Liu., et al. also showed that geniposide reduced the Aβ 1-42 level while increasing the expression of IDE in Aβ-treated STZ –induced DM rats. Further experiment done on transgenic mice model, geniposide improved learning and memory in addition to anti-inflammatory effect (via decreasing RAGE-dependent) signaling in activation of ERK and IκB/NFκB and the production of TNF-α, IL-1β) and decreasing the Aβ level in the cerebrum [65]. Others that improve learning and memory in transgenic model of AD are linalool which could suppress proinflammatory proteins like p38, MAPK, NOS-2, COX2 and IL-1β [53]. Effect of paeonIflorin in the transgenic mouse model of AD was studied by Gu., et al. as well [65]. Besides improving the memory deficit, a decrease in inflammation (NFκB, TNF-α, IL-1β, IL-6) and apoptotic (caspase-3)markers were seen. Just like geniposide, paeoniflorin also modulates GSK-3β signaling in transgenic animal model of AD [55].
Other behavioral models include the scopolamine –induced AD model where loganin demonstrated beneficial effects via the route of administration [50]. The neuroprotective effects of monoterpenes in other in vivo models has also been shown. For e.g., oleuropein could finish the pentylene tetrazole (PTZ-induced seizures in mice or colchicines –induced learning and memory deficits [53].
Mechanism of Action of Iridoids/Other Monoterpenes in AD
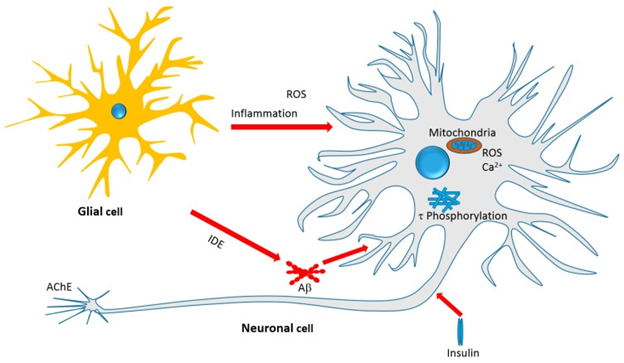
The general mechanism of action of monoterpenes,especially iridoids in the AD brain is shown in figure 4. The role of Aβ in the pathophysiology and as therapeutic target for AD has been reviewed earlier [67]. Recent reviews from the laboratory of Habtemariam S have also demonstrated that many polyphenolic compounds like flavonoids, diterpenoids and cinamate derivatives show therapeutic potential for AD via multiple mechanism of actions that involve Aβ [6-14]. Thus the formation, aggregation and toxicity of Aβ can all serve as targets for developing therapeutic drugs. The direct role of monoterpenes in the formation and aggregation of Aβ is not that clear and the activity is seen at moderate concentrations, might not be of therapeutic relevance. But still direct effect on APP processing enzymes has been observed. The major form of pathological Aβ in the brain are Aβ1-42, that get formed via the amyloidogenic β-secretase dependent pathway. The selective inhibition of this enzyme by monoterpenes, without much action on the non-amyloidogenic marker enzyme (α-secretase) is interesting. Further evidence suggests that monoterpenes finish the Aβ-induced cytotoxicity in cultured neuronal cells and various animal models of AD [6-14]. On aggregation the Aβ oligomers induce neurotoxicity, that further => cell death, impaired synaptic function and behavioral deficits, seen commonly in AD animal models. Thus one major target of iridoids along with selected monoterpenes seems to be mediated via mechanisms that are related to Aβ formation and/or toxicity.
The general mechanism of action of monoterpenes,especially iridoids in the AD brain is shown in figure 4. The role of Aβ in the pathophysiology and as therapeutic target for AD has been reviewed earlier [67]. Recent reviews from the laboratory of Habtemariam S have also demonstrated that many polyphenolic compounds like flavonoids, diterpenoids and cinamate derivatives show therapeutic potential for AD via multiple mechanism of actions that involve Aβ [6-14]. Thus the formation, aggregation and toxicity of Aβ can all serve as targets for developing therapeutic drugs. The direct role of monoterpenes in the formation and aggregation of Aβ is not that clear and the activity is seen at moderate concentrations, might not be of therapeutic relevance. But still direct effect on APP processing enzymes has been observed. The major form of pathological Aβ in the brain are Aβ1-42, that get formed via the amyloidogenic β-secretase dependent pathway. The selective inhibition of this enzyme by monoterpenes, without much action on the non-amyloidogenic marker enzyme (α-secretase) is interesting. Further evidence suggests that monoterpenes finish the Aβ-induced cytotoxicity in cultured neuronal cells and various animal models of AD [6-14]. On aggregation the Aβ oligomers induce neurotoxicity, that further => cell death, impaired synaptic function and behavioral deficits, seen commonly in AD animal models. Thus one major target of iridoids along with selected monoterpenes seems to be mediated via mechanisms that are related to Aβ formation and/or toxicity.
Role of ROS in Aβ-induced neurotoxicity is well proven from proofs that link redox metals like copper, zinc, and iron coordinating the generation of toxic free radicals and/or ROS [68-71,]. Being inhibitors of ROS generation via direct metal chelation and ROS scavenging, the role of polyphenol as potential therapeutic agents for AD has been deeply studied. The studies of Habtemariam S on catechol functional group and the flavonoid skeleton as optimized structural moieties for biological effects have been well studied [72-76], reviewed in [75,76,77-81]. The monoterpenes however lack this structural moiety unless additional skeleton as that shown in oleuprein is added (see figure 3). Their effect on finishing the Aβ toxicity along with neurotoxicity that is induced by H2O2 which suggest a mechanism of action beyond direct ROS scavenging. This may include boosting antioxidant defenses and this connection, various studies have demonstrated an enhanced antioxidant status in the AD brain following monoterpene therapy.
The other mechanism that is well-defined for monoterpenes in AD brain appears to be linked to anti-inflammatory action. In view of neuroinflammation as the major pathological hallmark of AD. Role of inflammatory cells activation in the brain, primary astrocyte and microglial cells, have been studies in last few decades [82-85]. All of the best characterized inflammatory markers like TNF, IL-1, COX and NOS have been shown to be decreased by compounds that have been studied. Since inhibition of proinflammatory cytokines like TNF provide a favourable outcome in AD [86]. The inhibitory effect of different monoterpenes on proinflammatory levels in the Alzheimer’s brain is in line with their potential therapeutic benefit in AD. Of the regulators of cytokines in their proinflammatory effect is NF κB that has been shown to play pivotal role in AD [87]. Different functions of leptin are emerging in recent year, which include modulation of the immune response and wide range of neuronal regulation from neuroprotection to cognition [88], besides known function in body weight and fat regulation. Role of leptin receptor mediated regulation in the cerebral cortex and hippocampus and dysregulation in AD has been observed [89]. Additionally neurons,immune cells in the brain like astrocytess and glial cells also express leptin receptors and thus get regulated by this hormone [90,91] With the evidence that show leptin has a neuroprotective effect under pathological conditions along with in vitro and in vivo experiments [92], the monoterpene’s modulatory effects on this system seems to be an important development. Since leptin antagonist finished the effect of geniposide on Ϯ phosphorylation and phosphorylation of Akt at Ser-473 site and GSK-3β at Ser-9 in the Alzheimer’s brain, it is possible that partly iridoid action is mediated via leptin regulation.
Just like formation, the degradation of Aβ peptides and plaques need to be tightly regulated for avoiding pathological disorder development like AD. The mechanisms involved in Aβ degradation and clearance Aβ proteases, low density lipoprotein receptor related protein 1 and apoprotein E systems [93]. From the protease enzymes, neprilysin (also called membrane metallo-endopeptidase) is a zinc dependent metalloproteinase which cleaves Aβ and has shown good correlation with Aβ accumulation [94]. The endothelin-converting enzyme, and angiotensin converting enzyme also function as Aβ degradating enzymes. The role of IDE in Aβ degradation has been clarified recently and its dysregulation is now known to contribute to the pathology of AD [93,95]. IDE is considered to be the main extracellular protease enzyme for the degradation of Aβ [96], and its expression with neprilysin,in the hippocampus has been shown to reduce with increasing age [97]. Thus upregulation of the Aβ degradating enzyme is among the therapeutic approaches for AD [98,99]. In the brain glial cells like microglia and astrocytes are the main source of IDE secretion [100], and their dysregulation could thus contribute to the AD pathology; while promotion of IDE secretion from these cells could be implicated in AD therapy via increasing Aβ clearance. The microglial cells and astrocytes are also primary phagocytes in the brain, which recognize Aβ through the membrane receptors to remove through phagocytosis [101]. Upregulating IDE is a tricky therapeutic approach, as IDE also selectively degrades insulin and its inhibitors, that are needed to improve glucose homeostasis (like in DM). Role of iridoids in this aspect are very interesting as geniposide has been shown to upregulate IDE [30] while showing potent antidiabetic effect [17]. Since IDE degrades the monomeric form of Aβ, it prevents the formation of oligomers or aggregates which is a prerequisite to Aβ cytotoxicity in neuronal cells. Thus a clear line of proof is now present for geniposide and/or other iridoids which showed a promise in the Alzheimer’s brain.
The double effects of iridoids in DM and AD is also manifested from the possible mechanism of action related to the Ϯ protein phosphorylation pathway. The formation of intracellular NFT’s is a result of aggregation of the hyperphosphorylated Ϯ protein phosphorylation pathway. The major component of the neuronal cytoskeleton , Ϯ protein is closely associated with microtubules and helps in a number of neuronal functions from axonal growth to neurite outgrowth[64,102].The function of Ϯprotein in stabilizing the microtubule to help in normal neuronal functions is governed by its phosphorylation that is regulated by a number of cellular kinases and phosphatases[103].As a result Ϯprotein dysregulation is among the pathiological hallmark of AD as NFT’s and hence serves as a target for drug therapy. Hyperphosphorylation of Ϯprotein quickly initiates the formation of helic Ϯprotein filaments and aggregates as is seen in the NFT’s and AD. This in turn =>microtubule disassembly and destabilization [104].
The signaling cascades in Ϯ protein hyperphosphorylation has been shown to involve the GSK-3β, which directly act on the protein (to phosphorylate it) and make it to disassociate with the microtubules [104]. Thus downregulating GSK-3β by drugs is essential in AD not only as in ROS generation from the mitochondria.E.g. GSK-3β has been shown to downregulate the transcription factor Nrf2 following oxidative damage [105]. GSK-3β itself is regulated by other kinases like Akt which phosphorylates GSK-3β at different sites to negatively regulate its activity. Moreover activation of PI3K triggers the activation of Akt which phosphorylates GSK-3β at different sites => to Ϯ protein phosphorylation or NFT formation in AD. The p 38 MAPK has also emerged as another kinase involved in Ϯ protein phosphorylation and hence can be targeted by these drugs [106] Medina., et al. reviewed the signal transduction pathway s and possible pharmacological regulations [107]. The findings of iridoids regulationg Ϯ protein phosphorylation by inhibiting GSK-3β and regulation of the associated system,mainly the PI3K/Akt signaling is a marked documentation of record of this compounds. The pioneering compound in this regard is geniposide [29,30,52]. Other natural products like phenolics that include resveratrol [108], curcumin [109], hyperforin [110] and capsaicin [111] have been shown to display inhibitory effect against of Ϯ protein hyper phosphorylation along with affect in vivo models of AD. Thus iridoids with structural features distinctively different from polyphenols appear to share common feature of mechanism in their potential AD modulations.
In total it appears that the iridoids and some monoterpenes target the various cellular and biochemical features of AD pathology as shownin figure 4. They target oxidative stress by boosting antioxidant defenses; inhibit the Aβ cascades especially neurotoxicity; inhibit Ϯprotein phosphorylation and thus NFT’s formation;promote the clearance of toxic proteins (Aβ) through IDE; modulate the insulin signaling pathway and insulin resistance as antidiabetic agents; and show a range of anti-inflammatory effects by suppressing the numerous pivotal proinflammatory proteins. Another important development is the direct effect of monoterpenes on AChE enzyme and further possible opportunity of potency optimization through chemicals.
Figure 4: Courtesy ref no.75Therapeutic targets of Iridoids and other Monoterpenes discussed in this
review. Antiinflammatory effect, amelioration of oxidative stress, mechanisms related to Aβ formation,
aggregation and clearance, τ-protein phosphorylation and aggregation, and neurotoxicity associated
with mitochondrial dependent and independent mechanisms are among the therapeutic targets.
Structural Perspectives and Drug likeness
Different measures both qualitative and quantitative of drug like parameters in wide ranges have been utilized for finding leads in drug discovery researches along with improving the efficacy of known biochemical compounds. Here in silico drug –likeness predictors, the undesired properties of small molecular weight as assessed by poor ADMET (absorption, distribution, metabolism, excretion and toxicity characteristics) have been used for screening [113]. In this regard monoterpenes (unless glycosylated, figure 3) act as components of essential oils with list solubility profile in water decreases within the poor drug –likeness profile Thus absorption, distribution, metabolism and excretion profiles were not as expected with that of an ideal drug molecule. Thus all in vitro and in vivo data thus far suggested that they are absorbed and distributed to tissues although at much slower rate than expected ideally [114,115]. Human trials confirmed the observations that iridoid glycosides like geniposide, with sugar attachment have been shown to be metabolized by intestinal bacteria to release their aglycone like genipin [116-118], that also give rise to conjugated products (like glucuronic acid) [119]. By glycosylation, water solubility gets increased for releasing bioactive aglycone in the intestine, that is one method of increasing bioavailability for natural products [120]. Even for glycosides like geniposides however, the absolute oral bioavailability following oral administration remains poor ~9.67% [121]. Anyway both in vitro and in vivo experiments have given good results for finishing the biochemical and behavioral markers of AD.
Different measures both qualitative and quantitative of drug like parameters in wide ranges have been utilized for finding leads in drug discovery researches along with improving the efficacy of known biochemical compounds. Here in silico drug –likeness predictors, the undesired properties of small molecular weight as assessed by poor ADMET (absorption, distribution, metabolism, excretion and toxicity characteristics) have been used for screening [113]. In this regard monoterpenes (unless glycosylated, figure 3) act as components of essential oils with list solubility profile in water decreases within the poor drug –likeness profile Thus absorption, distribution, metabolism and excretion profiles were not as expected with that of an ideal drug molecule. Thus all in vitro and in vivo data thus far suggested that they are absorbed and distributed to tissues although at much slower rate than expected ideally [114,115]. Human trials confirmed the observations that iridoid glycosides like geniposide, with sugar attachment have been shown to be metabolized by intestinal bacteria to release their aglycone like genipin [116-118], that also give rise to conjugated products (like glucuronic acid) [119]. By glycosylation, water solubility gets increased for releasing bioactive aglycone in the intestine, that is one method of increasing bioavailability for natural products [120]. Even for glycosides like geniposides however, the absolute oral bioavailability following oral administration remains poor ~9.67% [121]. Anyway both in vitro and in vivo experiments have given good results for finishing the biochemical and behavioral markers of AD.
Thus despite their predicted poor drug-likeness profile, iridoids along with other monoterpenes have exhibited potent activity to get seriously considered as potential lead compounds for further studies. Kumar., et al. screened 646 small molecules of natural origin having reported pharmacological and functional values through in silico docking studies to predict safer neuromodulatory molecules with potential to modulate acetyl choline metabolism .Further the potential of the predicted molecules to inhibit AchE activity and their ability to protect neurons from degeneration was determined via in vitro assays. They predicted quercetin,caffeine, ascorbic acid and gallic acid to be potential AChE inhibitors based on in silico AChE interaction studies. They confirmed the AChE inhibitory potential of these molecules through in vitro AChE inhibition assay and compared results with donezepil and begacestal. Herbal molecules significantly inhibited enzyme activity and inhibition of quercetin and caffeine did not show any significant difference from donezepil. Moreover the tested molecules did not show any neurotoxicity against primary (E18) hippocampal neurons.
They found quercetin and caffeine significantly improved neuronal survival and efficiently protected hippocampal neurons from Hg Cl2 induced neurodegeneration, which other molecules that included donezepil and begacestal did not do. Thus concluding that quercetin and caffeine have the potential as ‘’disease modifying drugs’ and might find an application in AD [123]. Getting inspired from , ‘’HDL bionics’’ Zhang 2019 proposed that biologically reassembled nanodrugs, donepezil-loaded apolipoprotein A-1(aPOA-1) reconstituted HDL (rHDL/Do) that concurrently executed dual-missions of Aβ –targeting clearance and AChE inhibition in AD therapy Once prepared (rHDL/Do) nanodrug achieved high drug encapsulation efficiency of 90.47%, and mimicked the configurations and properties of natural lipoproteins aiming to significantly increase BBB penetration and modulate Aβ induced neuronal damage both in vitro and in vivo. Surface Plasmon resonance (SPR) analysis confirmed that (rHDL/Do) facilitated microglial-mediated Aβ intake and degradation ,showing low KD value with Aβ affinity (2.45x 10-8 of Aβ monomer and 2.78 10-8 of Aβ oligomer). In AD models, daily therapy with (rHDL/Do) efficiently inhibited AChE activity,ameliorated neurological variation ,promoted Aβ clearance and rescued memory loss at a safe level.
These collective findings showed that a biological nanodrug was provided which had the capacities to penetrate BBB, Aβcapture and degradation via microglial cells, and cholinergic dysfunction amelioration after controlled donepezil release.Thus (rHDL/Do) nanodrugs could offer a promising strategy to synergize both symptom control and disease modification in AD therapy [124]. Further Hassan., et al. 2019 designed a computational and enzyme inhibitory mechanistic approach to fetch the promising drugs from the pool of FDA approved drugs against AD. The binding interaction patterns and conformation of screened drugs within active region of AChE were confirmed through moleculardocking profiles. The possible association of selected drugs with AD genes were predicted by pharmacogenomics analysis and confirmed through molecular docking profiles. The possible associations of selected drugs with AD genes were predicted by pharmacogenomics analysis and confirmed through data mining.
The stability behavior of docked complexes (Drugs-AChE) were checked by in vitro analysis. Taken together, Cintapride displayed a comparable results with standard and can be used as possible therapeutic agent in treatment of AD [125]. A series of isoflavone analogs were designed, synthesized and evaluated as multitargeted ligands for treatment of AD. Wang., et al. evaluated some ligands that had multifunctional profiles, including potent blockage of histamine 3 receptor (H3R) in vitro and found excellent inhibition of Ach E, neuroprotective effects and anti inflammatory properties. Of these derivatives compound 9b showed the highest ability to block H3R (IC50=0.27µM) and good inhibitory activity against AChE (IC50=0.08µM). Moreover compound 9b exhibited obvious neuroprotective effect on SH-SY5Y by preventing copper induced neuronal damage and potent anti-inflammatory activity by inhibiting the production of inflammatory factors on BV2 cells. A molecular modeling study revealed that 9b acts as a mixed –type inhibitor that interacts simultaneously with H3R and AChE, Further in vivo data showed that compound 9b did not cause acute toxicity in mice at doses up to 1000mg/kg, and had desirable pharmacokinetic profiles, as well as good BBB permeability (log BB=1.24+-0.07). Further studies showed that chronic oral therapy with 9b significantly improved cognitive function in scopolamine –induced AD mice in the step down passive avoidance test. Taken together this study showed that compound 9b is a promising multifunctional drug candidate for the therapy of AD [126].
Conclusions and Further Prospects in coming future
The advantage of using compounds that are of natural origin (like monoterpenes) is that their association is with common foods along with beverages which are already being used by humans. As neuromodulators, especially in AD, the benefit of some essential oils in crude mixtures of small molecular weight fragment compounds including monoterpenes have been reported in different publications [127]. But the drug-likeness of these molecules has not been in favour of developing drugs given their poor water solubility and bioavailability. The iridoids glycosides seem to give a better bioavailability profile and pharmacology as seen from their activity profile in vivo and in vitro. This fact that both glycosylated and the aglycones are active in vitro suggests that the glycosides being a better bioavailable compounds might be preferable candidates. Important is to understand that research on this class of compounds is still in infancy and further work is required for optimizing their pharmacological profile through medicinal chemistry. Effect of some monoterpenes, for example, could be increased by over 100 times on adding other functional groups like carbamate moiety. Naturally, human clinical trials would give not only important data regarding efficacy but also pharmacokinetic profile which are required desperately for these compounds. This study would be needed once a lead compound is found on further research .In the meantime, all the available data now suggest that small molecules of the iridoids class and related monoterpenes could be considered as potential leads to AD therapy.
References
- Alzheimer’s Association.Alzheimer’s Disease Facts and Figures http://w.w.w./org/facts(accessed on 22nd deceember 2017).
- Alzheimer’s Disease International.World Alzheimer Report 2016.Improving healthcare for People Living with Dementia:Coverage ,quality and Costs Now and in the Future. Available online: http://w.w.w.alz.co.uk/ world report 2016(accessed on 22nd deceember 2017)
- Jost BC and Grosberg GT. “The natural history of Alzheimer’s Disease;A brain bank study”. J Am Geriatr Soc 43 (1995): 1248-1255.
- Haas C. “Strategies, development, and pitfalls of thera [eutic options for Alzheimer’s Disease”. J Alzheimers Dis 28 (2012): 1248-1255.
- Moreira PI., et al. “Terapeutic options in Alzheimer’s Disease”. Expert Rev Neurother 6.6 (2006): 897-910.
- Elufioye TO., et al. “Plants –derived neuroprotective agents:Cutting the cycle of cell death through multiple mwhanisms”. ECAM 2017 (2017): 357401.
- Braidy N., et al. “Neuroprotective effects of citrus fruit-derived flavonoids, nobiletinand tangeretin in Alzheimer’s and Parkinson’s Disease”. CNS Neurol Disord Drug Targets 16 (2016): 387-397.
- Habtemariam S. “Rutin as a natural therapy for Alzheimer’s Disease:Insights into its mechanism of action”. Curr Med Chem 23 (2016): 860-73.
- Habtemariam S and Lentini G. “The therapeutic potential of rutin for diabetes:An Update”. Mini Rev Med Chem 15 (2015): 524-28.
- Nabavi SF., et al. “Apigenin as neuroprotective :Of mice and men”. Pharmacological Res 2017.
- Nabavi SF, Braidy N, Habtemariam S,Sureda A,Manayi A, Nabavi SM .Neuroprotective effects of fisetin in Alzheimer’s and Parkinson’s Diseases:From chemistry tomedicine. Current Top Med Chem 2016;16:1910-15.
- Nabavi SF, Braidy N, Habtemariam S,Orhan LE,Daglia M,Manayi A Nabavi SM. Neuroprotective effects of Chrysin: From chemistry tomedicine.Neurochem Int 2015;90:224-231.
- Habtemariam S.Protective effects of caffeic acid and the Alzheimer’s brain:An update. Mini Rev Med Chem 2017;17:667-674.
- Habtemariam S. The therapeutic potential of rosemary(Rosmarinus officianilis)diterpenes for Alzheimer’s Disease.ECAM 2016;2016:2680409.
- Tholl D.Biosynthesis and biological functions of terpenoids in plants.Adv Biochem Eng Biotechnol 2015;148:63-106.
- Kulvinder Kochar Kaur,Allahbadia GN,Singh M.Monoterpenes-a class of terpenoid Group of Natural Products-as a source of natural Antidiabetic agents in the future-A Review.2019;3(4):1-21.
- Habtemariam S.Antidiabetic potential of monoterpenes:A case of small molecules punching above their weight.Int J Mol Sci 2018;19:4.
- Pichersky E,Raguso RA.Why do plants produce so many terpenoid compounds?New Phytol 2016.
- Singh B,Sharma RA.Plant terpenes:Defense responses,phylogenetic analysis,regulation and clinical applications.Biotech 2015;5:129-51.
- Biere A,Marak HB,Van Damme JM.Plant chemical defense against herbivores and pathogens.Generalized defense or trade offs?Oecologia 2004;140:430-441.
- Reudler JH,Lindsrtedt C,Pakkanen H,Lehtinen I,Mappes J.Cost and benefits of plantallelo chemicals in herbivore diet in a multi enemy world. Oecologia 2015;179:1147-58.
- Mason PA,Deane-BowersM.Localization of defensive chemicals in two congeneric butterflies(Euphydras,Nymphalidae)J Chem Ecol 2017;43:480-486.
- Hur J,Pak SC,Koo BS,Jeon S.Borneol alleviates oxidative stress via uprgulation of Nrf2 and Bcl2 in SH-SY5Y cells.Pharm Biol 2013;51:30-35.
- Khan A,Vaibhav K,Javed H,Tabassum R,Ahmed ME,Khan MM,Khan MB,Srivastava P,etal.1,8-cineole(eucalyptol)mitigates inflammation in amyloid beta toxicated PC12 cells:Relevance to Alzheimer’s Disease.Neurochem Res 2014;39:344-352.
- Yamazaki M,Sakura N,Chiba K,Mohri T.Prevention of the neurotoxicityof the amyloid beta protein by genipin .Biol PharmBull 2001;24:1454-55.
- Bi J,Jiang B,Liu JH,Lei C,Zhang XL,An LJ,Protective effects of catalpol against H2O2 induced oxidative stress in astrocytes primary cultures.Neurosci Lett 2008;69:291-296.
- Porres-MartinezM,Gonzalez-Burgos E,Carretero ME,Gomez-Serranillos MP.In vitro neuroprotective potential of the monoterpenes α-pinene and 1,8-cineole(eucalyptol) against H2O2 induced oxidative stress in PC12 cells.Z.Naturforsch C 2016;71:191-199.
- Zhao C,Lv C,Li H,Du S,Liu X,Li Z,Xin W,Zhang W.Geniposide protects primary cortical neurons against oligomeric A β1-42 induced neurotoxicity through a mitochondrial pathway.PLoSONE 2016;11:e01552551.
- Liu J,Liu Z,Zhang Y,Yin F.Leptin signaling plays a critical role in the geniposide induced decrease of tau phosphorylatio,Acta Biochim Biophys Sin 2015;47:1018-22.
- Zhang Y,Xia Z, Liu J, Yin F. Cell signaling mechanisms by which geniposide regulates insulin degrading enzyme expression in primary cortical neurons.CNS Neurol Disord Drug Targets 2015;14:370-377.
- Sun P,Ding H,Liang M,Li X, Mo WC,Wang X, ,Liu Y,etal.Neuroprotective effects of geniposide in in SH-SY5Y cells and primary hippocampal neurons exposed to A β 42.Biomed Res Int 2014;2014:284314.
- Sun P,Chen JY,Li J,Sun MR,Mo WC,Liu KL,Meng YY,Liu Y,etal.The protective effect of geniposide on human neuroblastoma cells in the presence of formaldehyde.BMC Complement Alter Med 2013;13:152.
- Liu L,Wang SY,Wang JG.Role of PI3K/Aktpathway in effect of paeoniflorin against A β 25-35 induced PC 12 cell injury .Zhongguo Zhong Yao Za Zhi 2014;39:4045-49.
- Dong H,Li R,Yu C,Xu T,Zhang X,Dong M,Paeoniflorin inhibition of 6-hydroxy dopamine –induced apoptosis in PC 12 cells via suppressing reactive oxygen species-mediated PKCδ/NFκB pathway.Neuroscience 2015;285:70-80.
- Wang K,Zhu L,Zhu X,Zhang K,Huang B,Zhang J,Zhang Y,et al.Protective effect of paeoniflorin on A β 25-35 induced SH-SY5Y cell injury by preventing mitochondrial dysfunction. Cell Mol Neurobiol 2014;34:227-34.
- Sun R, Wang K,Wu D,Li X,Ou Y.Protective effect of paeoniflorin against glutamate –induced neurotoxicity in PC12 cells via Bcl2/Bax signal pathways.Folia Neuropathol 2012;50:270-76.
- Liu HJ,Yin F,Guo LX,Deng XH,Hu YH.Neuroprotection of geniposide against hydrogen peroxide induced PC12cellinjury:Involvement of PI3 kinase signal pathway.Acta Pharmacol Sin 2009;30:159-63.
- Liu J,Yin F,Zheng X,Jing J,Hu Y.Geniposide,a novel agonist for GLP1 receptor ,prevents PC 12 cells from oxidative damage via MAP kinase pathway.Neurochem Int 2007;51:361-369.
- Hwang ES,Kim HB,Lee S,Kim MJ,LeeSO,Han SM,Park JH.Loganin enhances long term potentiation and recovers scopolamine –induced learning and memory impairments.Physiol Behav 2017;171:243-48.
- Youn K,Jeong WS,Jun M.β-Secretase(BACE1) inhibitory property of loganin isolated from Corni fructus.Nat Prod Res2013;27:1471-74.
- Bhakta HK,Park CH,Yokozawa T,MinBS,Jung HA,Choi JS.Kinetics and molecular docking studies of loganin ,mirnoside and 7-O-galloyl-D-sedoheptulose derived from Corni fructus as cholinesterase and .β-Secretase 1 inhibitors .Arch Pharm Res 2016;39:794-805.
- Kim H,Youn K,Ahn MR,Kim O,Jeong WS,Ho CT,Jun M.Neuroprotective effects of loganinagainst A β25-35 induced injury via the NFκB dependent signaling pathway in PC 12 cells Food Facts 2015;6:1108-16.
- Kaufman D,Dogra AK,Wink M.Myrtenal inhibits acetyl cholinesterase,a known Alzheimer’s target.J Pharm Pharmacol 2011;63:1368-71.
- Kurt BZ,Gazioglu L,Dag A,Salmas RE,Kayak G,Durdagi S,Sonnez F.Synthesis,anticholinesterase activity and molecular modeling study of novel carbamate-substituted thymol/carvacrol derivatives.Bioorg Med Chem 2017;25:1352-63.
- Stavrakov G,Philipova I,Zheleva-Dimitova D,Valkova I,Salamonava F,Konstantinov S,Doytchinova i.Docking –based design and synthesis of galantamine-camphanr hybrids as inhibitors of acetyl cholinesterase.Chem Biol Drug Des 2017;90:709-718.
- Huang Z,Wu J,Xiang S,Sheng S,Jiang Y,Yang Z,Hua F.Catalpol preserves neural function and attenuates the pathology of Alzheimer’s disease in mice .Mol Med Rep 2016;13:491-96.
- Zhang X,Jin C,Li Y,Guan S,Han F,Zhang S.Catalpol improves cholinergic function and reduces inflammatory cytokines in the senescent mice induced by D-galactose.Food Chem Toxicol 2013;58:50-55.
- Zhang XL,An LJ,Bao YM,Wang JY,Jiang B. D-galactose administration induces memory loss and energy metabolism disturbance in mice :Protective effects of catalpol. Food Chem Toxicol 2008;46:2888-94.
- Zhang X, ZhangA , Jiang B, Bao YM, Wang JY, An LJ.Further pharmacological evidence of the neuroprotective effect of catalpol from Rehmannia glutinosa.Phytomedicine 2008;15:484-90.
- Zhang Y,Yin F,Liu L,Guo L,Xia Z,Zidichouski J.Geniposide attenuates insulin –deficiency induced acceleration of β-amyloidosis in an APP/PSI transgenic model of Alzheimer’s disease.Neurochem Int 2015;89:7-16.
- Lv C,Wang L,Liu X,Yan S,Yan SS,Wang Y,Zhang W,Multi-faced neuroprotective effects of geniposide depending on the RAGE –mediated signaling in an Alzheimer’s mouse model.Neuropharmacology 2015;89:175-184.
- Gao C,Liu Y,Jiang Y,Ding J,Li L. Geniposide ameliorates learning memory deficits ,reduces tau phosphorylation and decreases apoptosis via GSK pathway in streptozocin induced Alzheimer’s rat model.Brain Pathol 2014;24:261-269.
- Sabogal-Guaqueta AM,OsorioE,Cardona-Gomez GP.Linalool reserves neuropathologica and behavioral impairments in old triple transgenic Alzheimer’s mice . Neuropharmacology 2016;102:111-120.
- Pourkhodadad S,Alirezaei M,Moghaddasi M,Ahmadvand H,Karami M,Delfan B,Khanipour Z.Neuroprotective effect of oleuropein against cognitive dysfunction induced by colchicines in hippocampal CA1 areas in rat .J Physiol Sci 2016;66:397-405.
- Zhang HR,Peng JH,Cheng XB,Shi BZ,Zhang MY,Xu RX.Paeoniflorin attenuates amyloidogenesis and the inflammatory responses in a transgenic mouse model of Alzheimer’s disease.Neurochem Res 2015;40:1583-1592.
- Zhong SZ.Ma SP,Hong ZY. Paeoniflorin activates A β(1-42) induced hippocampal neuron injury in rats.Yao Xue Xue Bao 2013;48:1353-1357.
- Zhong SH,Ge QH,Li Q,Qu R,Ma SP. Paeoniflorin attenuates A β(1-42)mediated neurotoxicity by regulating calcium homeostasis and ameliorating oxidative stress in hippocampus of rats.J Neurol Sci 2009;280:71-78.
- Ho JD,Yeh R,Sandstrom A,Chorny I,Harries WE,Robbins RA,Miercke LJ,Stroud RM.Crystal structure of human aquaporin 4 at 1.8A and its conductance.Proc Natl.Acad Sci USA 2009;106:7437-7442.
- Potokar M Stenovec M,Jorgacevski J,Holen T,Kreft M,Ottersen OP.Zorec R,Regulation of AQP4 surface expression via vesicle mobility in astrocytes.Glia 2013;61:917-28.
- Vella J,Zammit C,Giovanni GD,Muscat R,Valentino M.The central role of aquaporin in thepathophysiology of ischemic stroke.Front Cell Neurosci 2015;9:108.
- Fakuda AM,Badault J.Aquaporin 4:A player in cerebral oedema and neuroinflammation .J Neuroinflamm 2012;9:279.
- Badault J,Fukuda AM,Julliene A,Petry KG. Aquaporin and brain diseases. Biochim Biophys Acta2014;840:1554-65.
- Wang JH,Xie H,Zhao TK,Kang B.Catalpol regulates cholinergic nerve system function through effect on choline acetyl transferase not M receptor affinity .Biomed Pharmacothee 2015;69:291-96 .
- Oliveira J,Costa M,De Almeida MSC,Da Cruz E Silva OAB,Henriques AG.Protein phosphorylation is a key mechanism in Alzheimer’s disease.J Alzheimers’ disease 2017;58:953-978.
- Gu X,Cai Z,Cai M,Liu K,Liu D,Zhang Q,Tan J,Ma Q,Protective effects of paeoniflorin on inflammation and apoptosis in the cerebral cortex of a transgenic model of Alzheimer’s disease.Mol Med Rep 2016;66:397-405.
- Kwon SH,Kim HC,LeeSY,Jang CG.Loganin improves learning and memory impairments induced by scoplolamine in mice .Eur J Pharmacol2009;619:44-49.
- Leon R,Garcia AG,Marco-Contelles J.Recent advances min the multitargeted directed ligands approach for the treatment of Alzheimers’ disease.Med Res Rev 2013;33:139-189.
- Bousejra-El Garah F,Bijani C,Coppel Y,Faller P,Hureau C.Iron(II)binding to amyloid β,the Alzheimers’ peptide .Inorg Chem 2011;50:9024-30.
- Reybier K,Ayala S,Alies B,Rodriques JV,Bustos-Rodriques S,La Penna G,Collin F,Gomes CM,etal.Free superoxide is an intermediate in the production of H202 by copper(I)-Aβ peptide and O2.Angew Chem Int Ed Engl 2016;55:1085-1089.
- Zawisza I,Rozga M,Bal W.Affinity of copper and zinc to proteins and peptidesrelated to neurodegenerative conditions(Aβ,APP α-synuclein,PrP).Coord Chem Rev 2012;256:352-368.
- Miglironi C,Porciatti E,Luczkowski M,Valensin D.Structural characterization of Cu2+,Ni2+and Zn2+binding sites of model peptides associated with neurodegenerative diseases. Coord Chem Rev 2012;256:2297-2307.
- Habtemariam S,Varghese GK.A novel diterpene skeleton:Identification of a highly aromatic ,cytotoxic and antioxidant 5-methyl-10demethyl-abietane –type diterpene from Premna serratiofloia.Phyther Res 2015;29:80-85.
- Habtemariam S.Investigation into the antioxidant andantidiabetic potential of Moringastenopetala :Identification of the active principles.Nat Prod Commun 2015;10:475-478.
- Habtemariam S,Varghese GK.Extractability of rutin in herbal tea preparations of of Moringastenopetala leaves.Beverages 2015;1:169-182.
- Habtemariam S.Iridoids and other monoterpenes in the Alzheimer’s Brain:Recent Development.Molecules 2018;23:117.9;
- Wu X,Cai H,Pan L,CuiG,Qin F,Li Y,Cai Z.Small molecules Natural products and Alzheimers’ disease Curr Top Med Chem 2019;19(3):187-204.
- Tang YW,Shi CJ,Yang HL,Cai P,Liu QH,Yang XL,Kong LY,Wang XB.Synthesi s and evaluation of isoprenylation –resveratrol dimer derivatives against Alzheimer’s disease.Eur J Med 2019; 163:307-319.
- Park HJ,Jung JH, Kwon H,Yu J,Jo E,Kim H,Park SJLeeYC,Kim DH,Ryu JH.The ethanol extract of Zizhphus juice va.spinosa seeds ameliorates the memory deficits in Alzheimer’s disease model.Ethnopharmacol 2019;233:73-79.
- Qi C,Qiao Y,Gao W,Liu M,Zhou Q,ChenC ,Lai Y,Xue Y,Zhang J,etal.New 3,5-dimethylorsellinic acid –based monoterpenoids with BACE 1 and Ach E Inhibitory activities from Aspergillus terreus.Org BiomolChem 2018;16(46):9046-52.
- Qi C,Qiao Y,Gao W,Liu M,Zhou Q,ChenC ,Lai Y,Xue Y,Zhang J,etal.New 3,5-dimethylorsellinic acid –based monoterpenoids with BACE 1 and Ach E Inhibitory activities from Aspergillus terreus.Org BiomolChem 2018;16(46):9046-52.
- Ras saghi N,Karimi A,Ebadi A.The potential of natural products vs neurodegenerative disorders :In silico study of antoflavanocoumarian as BACE 1 Inhibitor.Comput Biol Chem 2018;77:307-17.
- LiC,Zhao R,Gao K,Wei Z,Yin MY,Lau LT,Chui D,Yu AC.Astrocytes :Implications for neuroinflammatory pathogenesis of Alzheimer’s disease.Curr Alzheimer Res 2011;8:67-80.
- Garwood CJ,Ratcliffe LE,Simpson JE,Heath PR,Ince PG,Whatson SB.Astrocytes in Alzheimer’s disease and other age related dementia :A supporting player for the histocomability glycoprotein HLA-DR.Neuropathol Appl Neurobiol 2017;43:281-98.
- Heijmakers L,Heinen Y,Van Dam AM,Lucassen PJ,Korosi A,Microglial priming and Alzheimer’s disease:A possible role for(early)immune challenges and epigenetics?Front Hum Neurosci 2016;10:398.
- Heppner FL,Ransohoff RM,Becher B.Immune attack:The role of inflammation in Alzheimer’s disease. Nat RevNeurosci 2015;16:358-372.
- Shamim D,LasKowski M.Inhibition of inflammation mediated through the tumor necrosis factor-αbiochemicalpathway can lead to favourable outcomes in Alzheimer’s disease..J Cent Nerv Syst Dis 2017;9:
- Shi ZM,Han YW,Han XH,Zhang K,Chang YN,Hu ZM,Qi HZ,etal.Upstream regulators and downstream effectors of NFκB in Alzheimer’s disease.J Neurol Sci 2016;366:127-134.
- Garza JC,Guo M,Zhang W,Lu XY.Leptin increases adult hippocampal neurogenesis in vivo and in vitro.J Biol Chem 2008;283:18238-18247.
- Khemka VK,Bagchi D,Bandhopadhyay K,Bir A,Chattopadhya M,Biswas A,Basu D,ChakrabortyS.Altered serum level of adipokines and insulin in probable Alzheimer’s disease.J Alzheimers’ disease 2014;41:525-533.
- Lieb W,Beiser AS,Vasan RS,Tan ZS,Au R,Harris TB,Roubenoff R,etal.Association of plasma leptin levels with incident Alzheimers’ disease and MRI measures of brain aging .JAMA 2009;302:2565-2572.
- Kim JG,Suyama S,Koch M,Jin S,Argene-Arizon P,,Argente J,Liu ZW,etal.Leptin signaling in astrocytes regulates hypothalamic neuronal circuits and feeding.Nat Neurosci2014;17:908-910.
- Lu J,Park CS,LeeSK,Shin DW,Kang JH.Leptin inhibits 1methyl-4henylphyridinium-induced cell death in SH-SY5Y cells.Neurosci Lett 2006;407:240-43.
- Cook DG,Leverenz JB,McMilan PJ,Kulstad JJ,Ericksen S,Roth RA,Schellenberg GD,Jin LW,etal.Reduced hippocampal insulin deg rading enzyme in late onset alzheimers’ disease is associated with apolipoprotein E-epsilon 4 allele.Am J Pathol2003;162:313-319.
- Iwata N,Tsubuki S,Takaki Y,Shirotani K,Lu B,GerWQ,Gerard NP,Gerard C,etal. Metabolic regulation of brain A β by neprilysin.Science 2001;292:1550-52.
- Vekrelis K,Ye Z,Qiu WQ, Walsh D,Hartly D,Chesneau V,Rosner MR,Selkoe DJ.Neurons regulate extracellular levels of amyloid-beta protein via proteolysis by insulin degrading enzyme.J Neurosci 2000;20:1657-65.
- Qiu WQ , Walsh D,YeZ, Vekrelis K,Zhang J,Podlisny MB, Rosner MR,Safavi A.etal. Insulin degrading enzyme regulate extracellular levels of amyloid-beta protein by degradation.J Biol Chem 1998;273:32730-738.
- Caccaino A,Oddo S.Sugarman MC,Akbari Y,La Ferla FM.Age and region-dependent alterations in Aβ-degrading enzymes:Implications for Aβ induced disorders.Neurobiol Aging 2005;26:645-654.
- El-Amouri SS,Zhu H,Yu J,Marr R,Verma IM,Kindy MS.Neprilysin:An enzyme candidate to slow the progression of Alzheimers’ disease . Am J Pathol 2008; 172:1342-54.
- Turner AJ,Nalivaeva NN.New insights into the role of metalloproteinasesin neurodegeneration and neuroprotection.Int Rev Neurobiol 2007;82:113-125.
- Son SM,Cha MY,Choi H,Kang S,Choi H,Lee MS,Park A,Mook Jung I.Insulin degrading enzyme secretion from astrocytes is mediated by an autophagy based unconventional secretory pathway in Alzheimers’ disease .Autophagy 2016;12:784-800.
- Ries M,Sastre M.Mechanism of Aβ clearance and degradation by glial cells.Front Aging Neurosci 2016;8:160.
- Guzman-Marti Am J Pathol2003;162:nez L,Farias GA,Maccioni RB.Tau oligomers as potential targets for Alzheimers’ disease diagnosis and novel drugs .Front Neurol 2013;4:167.
- Mi K,Johnson GV.The role of tau phosphorylation in the pathogenesis of Alzheimers’ disease .Curr Alzheimers’ Res 2006;3:449-63.
- Alonso AC,Zaidi T,Grundke-Iqbal I,Iqbal K,.Role of abnormally phosphorylated tau in the breakdown of microtubules in Alzheimers’ disease . Proc Natl.Acad Sci USA 1994;91:5562-66.
- Cho JH,Johnson GV.Primed phosphorylation of tau in Thr 231 by glycogen synthase kinase 3 beta(GSK3beta)plays a critical role in regulating tau’s ability to bind and stabilize microtubules.J Neurochem 2004;88:349-58.
- Rojo AJ,Sagarra MR,Cuadrado A.GSK-3β downregulates the transcription factor Nrf2 after oxidant damage:Relevance to exposure of neuronal cells to oxidative stress.J Neurochem2008;105:192-202.
- Munoz L,Ammit AJ.Targeting p38 MAPK pathway for the treatment of Alzheimers’ disease.Neuropharmacology 2010;58:561-568.
- Medina M,,Garrido JJ,Wandosell FG,.Modulation of GSK-3 as therapeutic strategy on tau pathologies .Front Mol Neurosci 2011;4:24.
- Jhang KA,Park JS,Kim HS,Chong YH.Resveratrol ameliorates tau hyperphosphorylation at Ser 396 site and oxidative damage in rat hippocampal slices exposed to Vandalate:Implications of ERK1/2 and GSK-3 β signaling cascades,J A gric Food Chem 2017;65:9626-34.
- Sun J,Zhang X,Wang C,Teng Z,Li Y,Curcumin decreases hyperphosphorylation of tau by downregulating caveolin -1/GSK-3β in N2a/APP695 swe cells and APP/PSI double transgenic Alzheimers’ disease mice.Am J Clin Med 2017;45:1667-82.
- Huang W,Cheng P,Yu K,Han Y,Song M,Li Y.Hyperforin attenuates aluminium –induced Aβ production and tau phosphorylation via regulating Akt/GSK-3β signaling pathway in PC 12 cells. Biomed Pharmacother 2017;96:1-6.
- Xu W,Liu J,Ma D,Yuan G,Lu Y,Yang Y.Capsaicin reduces Alzheimers’-associated tau changes in the hippocampus of type 2 diabetes rats.PLoSONE 2017;12:eo172477.
- Tian S,Wang J,Li Y,Li D,Xu L,Hou T.The application of in silico drug -
- likeness predictions in pharmaceutical reaearch.Adv Drug Deliv Res 2015;86:2-10.
- Liu ZQ,Jiang ZH,Liu L,Hu M.Mechanisms responsible for poor availability of paeoniflorin :Role of intestinal disposition and interactions with sinomenine.Pharm Res 2006;23:2768-2780.
- Cheng C,Lin JZ,LiL,Yang JL,Jia WW,Huang WH,Du FF,Wang EQ.etal.Pharmacokinetics and disposition of monoterpenes glycosides derived from Paeonia lactiflora roots(Chishao) after intravenous dosing of antiseptic XueBijling injection in human subjects and rats .ActaPharmacol Sin 2016;37:530-544
- Kohlert C,Schindler G,Marz RW,Abel G,Brinkaus B,DerendorfH,Cordova C,Grafe EU,Veit M.Systemic availability and pharmacokinetics in thymol in humans .J Clin Pharmacol 2002;42:731-737.
- Miller JA,Lang JE,Ley M,Nagle R,Hsu CH,Thompson PA,Cordova C,Waer A Chow HH.Human breast disposition and bioactivity of limonene in women with early stage breat cancer.Cancer Prev Res 2013;6:577-84.
- Cheng S,Lin LC,Lin CH,Tsai TH.Comparative bioavailability of geniposide following oral administration of geniposide,Gardenia jasminoides Ellis fruits and Gardenia herbal formulation in rats .J Pharm Pharmacol 2014;66:705-12.
- Chen C., et al. “Simultaneous determination of geniposide and its metabolites genipin and genipinine in culture of of Aspergillus niger by HPLC”. Biomed Chromatogr 22 (2008): 753-57.
- Han H,Yang L,Xu Y,Ding Y,Annie Blngh SW,Zhang T,Wang ZT. “Identification of metabolites of geniposide in rat urine using ultra –performance liquid chromatography combined with electrospray ionization cuadrupole time of –flight tandem mass spectrometry”. Rapid Commun Mass Spetrom 25 (2011): 3339-50.
- Habtemariam S and Belai A.”Natural therapies of inflammatory bowel disease:the case of rutin and its aglycone ,quercetin”. Mini Rev Med Chem 17(2017).
- YuB,., et al. “Effect of borneolon the pharmacokinetics of geniposide in cortex ,hippocampus, hypothalamus and striatum of conscious rats by simultaneous microdialysis is coupled with UPLC-MS”. J Pharm Biomed Anal 77 (2013): 128-132.
- Kumar A., et al. “Computational and In vitro validation of natural molecules as potential acetyl cholinesterase inhibitors and neuroprotective agents”. CurrAlzheimer Res 16.2 (2019): 116-127.
- Zhang H., et al. “Reassembly of native components with donezipil to execute dual-mission in Alzheimer’s disease therapy”. J Control Release 296 (2019): 14-28.
- Hassan M,Raza H,Abbrasi MA,Moustafa AA,Seo SY. “The exploration of novel Alzheimer’s therapeutic agents from the pool of FDA approved medicines using drug repositioning, enzyme inhibition and kinetic mechanism approaches”. Biomed Pharmacother 109 (2019): 2513-2526.
- Wang D., etal. “Design, Synthesis and evaluation of isoflavone analogs asmultifunctional agents for the treatment of Alzheimer’s disease”. Eur J Med Chem 168 (2019): 207-220.
- Maggio A., et al. “Essential oils and pure volatile compounds as potential drugs in Alzheimer’s disease therapy:An updated review of the literature”. Curr Pharm Des 22 (2016): 4011-27.
Citation:
Kulvinder Kochar Kaur., et al. “Iridoids. Some Monotepenes and Other Natural Products for Development of Potential Therapies in Alzheimer’s Disease-A Review”. Nutrition and Food Toxicology 3.5 (2019): 741-756.
Copyright: © 2019 Kulvinder Kochar Kaur., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.



































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.