Research Article
Volume 4 Issue 2 - 2021
Safety and Efficacy Studies on Administration of Undenatured Beta Glucan in Experimental Animals
1CEO of GlycaNova AS, Fredrikstad, Norway
2Director of Clinical Research, GlycaNova AS, Fredrikstad, Norway
*Corresponding Author: Dr. Bjorn Kristiansen, CEO of GlycaNova AS, Fredrikstad Innovation Park, Fredrikstad, Norway.
Abstract
Beta-Glucan fractions from mushrooms and yeast have been incorporated into food and nutritional dietary supplement products for decades, without any evidence of toxicity to humans. One such beta-glucan fraction is the well-known Lentinan, a denatured extract from a medicinal mushroom with a molecular weight of ~500,000 Da. While these extracts are safe, they are restricted in efficacy due to low bioavailability. For optimal bioavailability, the beta-glucan must remain in its native tertiary undenatured state. This undenatured beta-glucan complex is now commercially available, but the safety of the compound has not been published.
In a series of studies, mice and rats were administered undenatured beta-glucan (Lentinex®), at different dosages, over multi-week cycles. In the first study an initial dose of 0.5 mg/mL Lentinex was tolerated well with no ill-effects. The rats had regular weight gain as observed with untreated controls. The dose of Lentinex® was increased to 0.8 mg/mLs, and then to 1.8 mg/mLs over a 12-week period, and the animals continued to increase in weight, although gains began to plateau as animals reached maturity. The safety of Lentinex was confirmed for this model.
In other studies, the effect of Lentinex on the immune system was studied in mice after oral administration. The results indicated a doubling of B-Lymphocytes, from 16% in controls to 25% after Lentinex® administration. The effect of increasing doses of Lentinex® on B-cells was also observed in a rat safety study.
The effect of Lentinex administration on pro-inflammatory cytokine production showed that there was a 4-fold increase in TNFα production by peritoneal exudate cells and a 3-fold increase by splenocytes. Both oral and i.p. lentinan treatment significantly enhanced IL-1J3 production by PEC (18-fold and 6-fold, respectively).
In a final study the effect of Lentinex on H. Pylori infected mice, while not reducing the bacterial load, showed a 256x increase in anti-body titer, and did not affect the overall health of the mice.
The current study clearly demonstrates the safety of Lentinex® when administered to rats and mice models.
Keywords: Undenatured; Beta-Glucan; Animal Safety; rats, mice; tertiary structure
Introduction
Lentinula edodes (Shiitake) is a medicinal mushroom with a long tradition of use in Asia. The major active substance in L. edodes is a (1-6, 1-3)-beta-glucan. These beta-glucans from various sources, including mushrooms and yeast have been reported to have some positive effects on the immune system in animals and humans. Chief among these active fractions is lentinan, a molecular weight fraction of ~500,000, however, these lentinans have typically been extracted from the botanical source with solvents or other treatments that destroys the tertiary structure of the beta-glucan, and significantly reduced its ability to be absorbed in mammals’ intestinal tracts.
Research conducted by different research institutions sought to determine if lentinan maintained in its tertiary structure (e.g. Lentinex®) would be safe and more efficacious in immune stimulation than the denatured lentinan. Lentinex® from shiitake mushrooms is found as β-1,3 beta-glucan with β-1,6 branching and a molecular weight typically of ≥1,00,000 Da. When the fruiting body of the shiitake mushroom is grown in proper conditions and treated by a proprietary fermentation process (1), it was shown that the beta-glucan can be isolated in its undenatured tertiary structure.
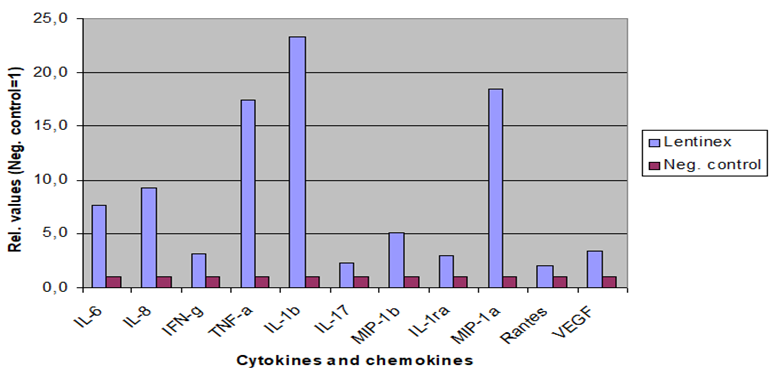
Research sponsored by GlycaNova A.S. in the United States and in Norway has supported the understanding that undenatured beta-glucan (Lentinex®) is far superior to any other denatured beta-glucan, from mushrooms or yeast, in its ability to enhance the immune system as demonstrated in Figure 1. The negative control presented in Figure 1 is denatured lentinan.
The immune stimulation by undenatured Lentinex®, as observed in in vitro and ex vivo studies, is dose dependent and affected by the molecular weight fractions, with significant stimulation observed with molecular weights of ≥500,000 Da. This is not seen with the denatured lentinan. (Figure 2)
Finally, it was confirmed that commercial lentinans are primarily denatured, and as such have little immune stimulating ability compared to undenatured lentinan.
Materials and Methods
1. Animal Safety and Efficacy Studies
1.1. Effect of Safety, Efficacy, and Growth in Rats after Lentinex Administration
Male BN/Crl rats (120-140 g, age 6 weeks) were acclimatized for one week prior to experimentation. Rats were housed 5 per cage, fed standard chow and water ad libitum. All experiments were conducted following institutional guidelines.
Lentinex® treatment started when animals reached the age of 7 weeks (Wk 7). Lentinex doses of 7.8mg/kg, 9,2mg/kg and 12 mg/kg, were administered to 10 BN rats via oral lavage, daily in 5- day cycles, with a 2-day rest between cycles. Control rats were treated from week 17 with volume matched (as per 12 mg/kg of 1.8 mg/ml) equivalents of vehicle. All rats were observed for weight loss, lethargy, toxic effects, ataxia, and behavioral changes.
Blood samples were drawn, from the lateral tail vein, after the second cycle (Wk 9) and every 2 weeks thereafter. Serum was collected and stored at -80°C and routine hematological and flow cytometric analysis were performed on whole blood samples. Routine hematology was determined on an AVIDA 120 Hematology System (Bayer) used for human analysis and was confirmed for hemoglobin and red cell counts. Blood platelets and white blood cells were interpreted as estimable for actual rat blood values, based on literature values for rat hematology in this strain and our repeated controls and diseased controls (leukemia: where platelets and white blood cells are modulated to extremes).
Peripheral blood leukocytes (PBLs) for single and multi-label flow cytometry were analyzed, and serum was analyzed for cytokines (GM-CSF, IFN-γ, IL-1α, IL-1β, IL-2, IL-4, IL-6, IL-10, TNF-α) using Bio-Plex™ Rat Cytokine 9-Plex A Panel kit. Hematological and cytokine data were analyzed using one way analysis of variance (ANOVA) and unpaired two-tailed t-test. Differences where P<0.05 were considered statistically significant.
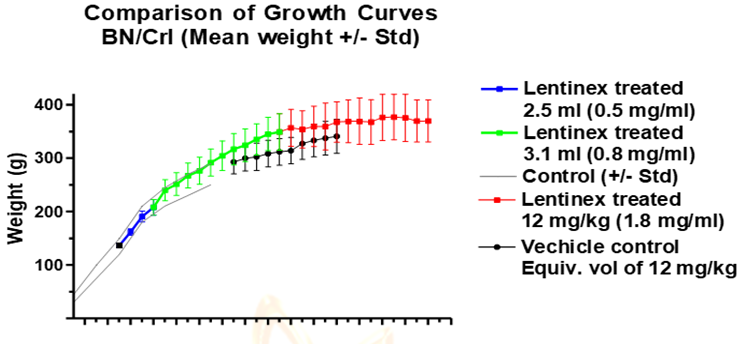
Initially (Wk 7), rats were administered 2.5 ml (0.5 mg/ml) for 3 cycles. This dose of Lentinex was tolerated well with no ill-effects observed and regular weight gain as observed with untreated controls (Figure 1). After three cycles of treatment there was no observed change in any hematological component from basal levels, so, the dose of Lentinex was increased to 3.1 mls per day and 0.8 mg/ml (Wk 10). Again, animals displayed no toxic effects and continued to gain weight as compared to untreated controls (Figure 3). From week 21 Lentinex dose was maintained at 12 mg/kg (1.8 mg/ml solution) and increased in weight although gains began to plateau as animals reach maturity. Control animals were started on Wk 17 with volume matched equivalents of vehicle. Controls started lower basal weights in comparison to age matched Lentinex treated animals and maintained similar growth rates.
Figure 3: Growth Curves of Rats Administered Increasing Amounts of Lentinex Compared to Vehicle Control
1.2. Effect of Lentinex on B-Lymphocytes In safety and efficacy studies in mice and rats, when Lentinex was administered, an increase in B-lymphocytes was observed. In a study conducted in the United States, an almost doubling of B-cells, from 16% in controls to 25% after oral intake of Lentinex was observed in mice. (O’Reilly). The effect of increasing doses of Lentinex® resulted in a significant (p<0.0001) increase in B-cells in a rat safety study, conducted in Norway (Figure 4). It is suggested that the increase in B-cell and monocytic population could be partly related to the decrease in the immunosuppressive Interleukin-10 (IL-10), which was observed after Lentinex® administration. Increases observed in B-cell and monocyte populations, in addition to increased interferon-gamma (INF-γ) levels, may suggest an immunostimulatory response after Lentinex® administration.
1.3. The Effect of Oral and Intra Peritoneal Lentinex Administration on Proinflammatory Cytokine Production
In safety and efficacy studies in mice and rats, when Lentinex was administered, an increase in B-lymphocytes was observed. In a study conducted in the United States, an almost doubling of B-cells, from 16% in controls to 25% after oral intake of Lentinex was observed in mice. (O’Reilly). The effect of increasing doses of Lentinex® resulted in a significant (p<0.0001) increase in B-cells in a rat safety study, conducted in Norway (Figure 4). It is suggested that the increase in B-cell and monocytic population could be partly related to the decrease in the immunosuppressive Interleukin-10 (IL-10), which was observed after Lentinex® administration. Increases observed in B-cell and monocyte populations, in addition to increased interferon-gamma (INF-γ) levels, may suggest an immunostimulatory response after Lentinex® administration.
Twenty-four, 5-week-old, male mice were divided into four groups. Group 1 received 2mg/kg Lentinex in 0.15 M NaCl, intraperitoneally (i.p.), group 2 received an equal volume of saline i.p., group 3 received 10mg/kg Lentinex in water by gastric gavage (oral) and group 4 received an equal volume of water orally. All mice received treatment once daily for 5 days. Twenty-four hours after the last treatment the mice were bled and euthanized for cell isolation.
Peritoneal exudates cells (PEC) were collected by peritoneal lavage as previously described (2). Cells were concentrated to 1 x 106mL in RPMI 1640 supplemented with 2 mM L-glutamine, 100units/mL penicillin and 100µg/mL streptomycin (medium) and distributed to 24 well tissue culture plates at 1mL/well. Plates were centrifuged at 1200 x g for 10 min and placed at 37°C, 5% CO2 for 2 hours. Medium was discarded and the wells washed once with warm medium and replaced with 1.5mL warm medium supplemented with 10% fetal bovine serum. Half the wells from each group were treated with E. coli lipopolysaccharide at 10ng/ml and the cells were placed at 37°C, 5% CO2 for 12 or 24 hours. The phenotype of the cells was determined by flow cytometry using antibodies to CD3 and CD14 labeled with phycoerythrin and anti-mouse immunoglobulin-G (IgG) labeled with fluorescein.
Spleens were harvested from each mouse. A portion was snap frozen for future mRNA analysis. The remaining spleens were pooled by group and crushed through a 70µm nylon screen. Splenocytes were washed and representative wells were stimulated with E. coli LPS at 10ng/ml or Concanavalin A at 5µg/mL and the cells were placed at 37°C, 5% CO2 for 12 or 24 hours. Cytokine ELISA analysis for TNFa, IL-1J3, IL-6 and IL-12 were conducted on serum and culture supernatants. Undiluted supernatants were tested in triplicate. Serum was diluted 1:10 for testing.
1.1.3. The Safety and Efficacy of Lentinex Treatment in a H. Pylori Mouse Model
A study designed to determine the effect of oral Lentinex® treatment on the colonization load of H. pylori in a mouse model was initiated.
Antibodies were determined using serum samples tested individually by ELISA using both SS1 and X47 as antigens. Following 14 days of Lentinex treatment (day 35), all the remaining mice in groups 1 (n=3), group 2 (n=10) and group 3 (n=10) were euthanized. Prior to euthanasia, mice were bled for serum and the serum was tested for the presence of antibodies to H. pylori by ELISA as described above. Serum was diluted 1:10, 1:100 and 1:1000 for testing. Sera was tested on both SS1 and X47 antigen and no differences were observed; only data for X47 is shown in Figure 5. The Lentinex® treatment resulted in a statistically significant increase in antibody responses when compared to the non-treated mice (p=0.033). Using one serum sample as a reference, an estimated titer was calculated and the differences were more striking. Lentinex® administration resulted in an average increase in antibody titer of 256-fold compared to non-treated animals.
Results and Discussion
Following the initial period of administration (Wks 7-10) with 2.5ml (0.5mg/ml) of Lentinex, there were no changes observed in hematological components. However, following administration of the first cycle of 3.1mLs (0.8 mg/ml) per day there was an increase in average platelet production in Wk 11 from basal levels which increased further (P<0.05) following 2 additional cycles (Wk 13). Subsequent to a total of 9 cycles of this dose of Lentinex, average platelet levels have declined from these initially high values. Concurrently, average red blood cell counts (RBC) increased from basal levels by Wk 13 (P<0.05) and remained higher than basal levels up to Wk 19 (P<0.05), with a slight increase in hemoglobin levels by Wk 13.
White blood cell counts (WBC) decreased initially from basal levels by Wk 11 (P<0.05). However, increased levels were observed on Wk 13 in comparison to Wk 11 (P<0.0001) and basal levels (Wk 9)) with levels dropping well below basal levels by Wk 19 (P<0.0001).
While percentages of CD8 and CD4 positive cells remained relatively unchanged there was a considerable increase in both the B-cell (P<0.0001) and monocytic populations (P<0.0001) at Wk 13, consistent with the above observations of increases on Wk 13.
Interferon gamma (INF-γ) increased significantly in comparison to controls by Wk 11 (P<0.01), with an apparent, consistent escalation following increasing length of Lentinex administration. There were no significant alterations of tumor necrosis factor alpha (TNF-α), IL-1α, IL-1β or IL-6. IL-2 exhibited a transient decrease at week 11. Following an initial increase in IL-10 by Wk 9, levels subsequently normalized up to and including Wk 19. GM-CSF remained unchanged until Wk 19 when levels increased, but with a spread of data points.
Increases in platelets, erythropoiesis and white blood cells after Lentinex administration at week 13 may indicate a myeloid stimulation, in particular at the higher dose. The subsequent reductions in these values in week 19 could be due to increase body weight of the animals resulting in overall diminution in dose over time. The increase in GM-CSF may reflect the increased production of platelets, red cells and white cells.
The increase in B-cell and monocytic population are not directly reflected in cytokine profile, but the decrease in the immunosuppressive IL-10 could be related to the increase in monocytes observed. Increases observed in B-cell and monocyte populations in addition to INF-γ levels may suggest an immunostimulatory response by Lentinex.
It is suggested that the increase in B-cell and monocytic population could be partly related to the decrease in the immunosuppressive Interleukin-10 (IL-10), which was observed after Lentinex® administration. Increases observed in B-cell and monocyte populations, in addition to increased interferon-gamma (INF-γ) levels, may suggest an immunostimulatory response after Lentinex® administration.
When Lentinex was administered, an increase in B-lymphocytes was observed in the safety and efficacy studies. An almost doubling of B-cells, from 16% in controls to 25% after oral intake of Lentinex was observed in mice. The effect of increasing doses of Lentinex® resulted in a significant (p<0.0001) increase in B-cells in the rat safety study (Figure 4). It is suggested that the increase in B-cell and monocytic population could be partly related to the decrease in the immunosuppressive Interleukin-10 (IL-10), which was observed after Lentinex® administration. Increases observed in B-cell and monocyte populations, in addition to increased interferon-gamma (INF-γ) levels, may suggest an immunostimulatory response after Lentinex® administration.
Intraperitoneal (i.p.) Lentinex treatment caused a 4-fold increase in TNFa production at 12 hours by peritoneal exudate cells (PEC) and a 3-fold increase by splenocytes as well as increasing the amount of TNFa produced in response to LPS treatment in vitro by PEC (30% increase) and splenocytes (2-fold). Both oral and i.p. lentinan treatment significantly enhanced IL-1J3 production by PEC (18-fold and 6-fold, respectively). Oral Lentinex treatment had no significant effect on TNFa production by PEC or splenocytes.
The effect of Lentinex on H. pylori colonies was difficult to assess, as the bacterial loads were reduced in the Lentinex group from days 21 to day 35, but after that the bacterial loads returned to the same level as the untreated mice. As such, no definitive statement can be made about the ability of Lentinex to act as an anti-microbial for this bacterium. Animals displayed no physiological toxic side-effects to Lentinex® at the doses described and continued to thrive following 14 weeks of administration (14 cycles). There are no indications of hematological toxicity on the subset of blood cells or parts of the immune system examined following Lentinex® administration.
Conclusion
It is concluded that the animals displayed no physiological toxic side-effects related to Lentinex administration at the doses described and continued to thrive following 14 weeks of administration (14 cycles). There are no indications of hematological toxicity on the subset of blood cells or parts of the immune system examined following Lentinex administration.
Data from the studies of the effect of Lentinex on cytokine stimulation, showed that there was no effect on the health of the mice and no statistically significant effect on their growth as measured by weight gain
These studies clearly demonstrate the safety of Lentinex® when administered to rats and mice models. These data are consistent with earlier studies that showed that administration of Lentinex to humans, chickens, pigs, shrimp, and trout, showed no toxicity, and in fact caused a general increase in animal health and growth compared to controls, and an increase in Quality of Life in humans.
References
- Kristiansen B. US 7,514,085 B2; April 7, 2009; US 7,514,085 B2 and US 7,682,615
- Coligan, JE, Kruisbeek, AM, Margulies, DH, Shevach, EM, Strober, W (eds.). 1994. “In vitro assays for mouse lymphocytes” in Current Protocols in Immunology, John Wiley & Sons, Inc., Indianapolis, IN, p 3.15.4.
Citation:
Ronald J Amen and Bjorn Kristiansen. “Safety and Efficacy Studies on Administration of Undenatured Beta Glucan in Experimental Animals”. Nutrition and Food Toxicology 4.2 (2021): 24-29.
Copyright: © 2021 Ronald J Amen and Bjorn Kristiansen. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.






































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.