Research Article
Volume 5 Issue 1 - 2024
Safety Evaluation of Overdose Intake of Fermented Barley GABA 90% – A Placebo-Controlled, Double-Blind, Randomized, Parallel-Group Comparative Trial –
1Research & Development Laboratory, Sanwa Shurui Co., Ltd., Usa, Oita, Japan
2Miura Clinic, Medical Corporation Kanonkai, Osaka, Japan
*Corresponding Author: Dr. Eriko Uehara, , Research & Development Laboratory, Sanwa Shurui Co., Ltd., 2231-1 Yamamoto, Usa, Oita 879-0495, Japan.
Abstract
Fermented barley GABA 90 % (FB-GABA, Sanwa Shurui Co., Ltd., Japan) is a powdered product of γ-aminobutyric acid (GABA) produced by Enterococcus malodoratus FC 301, which is cultured in a medium of fermented barley extract generated in the manufacture of Shochu, a Japanese distilled liquor. GABA accounts for more than 90 % of FB-GABA. The approximately 10 % remaining contains components produced by FC 301 and those derived from fermented barley extract used as a medium. Consuming 200 mg of GABA per day improves cognitive function without any health risks. In order to intake 200 mg/day of GABA, it is necessary to intake 223 mg/day of FB-GABA. Japanese Consumer Affairs Agency is requesting confirmation of the safety of overdosing (5 times the normal amount) for health foods such as tablets. We investigated the safety of intake of 1341.9 mg/day of FB-GABA for 4 weeks. Forty-eight participants were randomly divided into two groups. Each group ingested the test tablets containing 1341.9 mg of FB-GABA or the placebo tablets containing 1341.9 mg of dextrin for 4 weeks. Evaluation parameters included body measurements, physical examinations, and blood and urine tests. The principal investigator performed the medical interviews. The principal investigator judged that there were no safety issues with overdose intake of the trial food for 4 weeks based on the primary endpoints of body measurements, physical examinations, laboratory tests, and adverse events. We concluded that there were no safety issues with intake of 1341.9 mg/day of FB-GABA for 4 weeks.
Keywords: γ-aminobutyric acid; GABA; Fermented barley; Safety test; Overdose intake
Abbreviations: BP: Blood pressure; SCR: Screening; BMI: Body mass index; MCV: Mean corpuscular volume; MCHC: Mean corpuscular hemoglobin concentration; AST: Aspartate aminotransferase; ALP: Alkaline phosphatase; LDH: Lactate dehydrogenase; CK: Creatine kinase; HbA1c: Hemoglobin A1c
Introduction
Fermented barley GABA 90% (FB-GABA, Sanwa Shurui Co., Ltd., Oita, Japan) is a product that is prepared by concentrating and powdering γ-aminobutyric acid (GABA) produced by Enterococcus malodoratus FC 301. FC 301 is isolated from food and cultured in a medium of fermented barley extract generated in the manufacturing process of Shochu, a Japanese distilled liquor (Furuta et al., 2010). GABA accounts for more than 90% of FB-GABA, while components produced by FC 301 and those derived from fermented barley extract used as a medium constitute the remaining approx. 10%. It is unknown what kind of ingredients are included in that 10%.
GABA is found in plants and is an intrinsically safe amino acid that is produced in the human body,(De Bie., et al. 2023), primarily functioning as an inhibitory neurotransmitter (Bloom and Iversen, 1971). Although ingested GABA does not cross the blood-brain barrier (Kuriyama, 1979), it activates vagal afferents and modulates brain function (Nakamura et al., 2022). GABA is metabolized to succinic semialdehyde by GABA transaminase, then either reduced to gamma-hydroxybutyrate or oxidized to succinate and finally converted to CO2 and water via the citric acid cycle (Oketch-Rabah et al., 2021).
GABA is sold as a dietary supplement in the United States. A review conducted by the Environmental Protection Agency (Washington, DC, USA) indicated that the prolonged chronic administration of large doses of GABA to rats and dogs (up to 1 g/kg/day) reported no signs of toxicity or problematic effects (Oketch-Rabah et al., 2021). Clinical studies showed no serious adverse events related to GABA when 18 g/day of GABA was taken for 4 days and in a long-term trial using 120 mg/day for 12 weeks (Oketch-Rabah et al., 2021). GABA is associated with a temporary and moderate reduction in blood pressure [BP] (< 10% change). Therefore, the concomitant use of GABA and antihypertensive drugs may increase the risk of hypotension) GABA should be used with caution in pregnant and breastfeeding women as it can increase growth hormone and prolactin levels (Oketch-Rabah et al., 2021).
On the other hand, ingesting GABA brings about various physiological functions. Consuming 200 mg/day of GABA improves cognitive function (Yamatsu et al., 2020). Ingesting FB-GABA (equivalent to 100 mg of GABA) significantly increases the amount of time spent in deep non-rapid eye movement sleep in middle-aged and elderly people who experience strong mental stress and fatigue (Hokazono and Fukuda, 2018). Oral administration of FB-GABA (equivalent to 100 mg of GABA) may increase positive emotions such as vitality and energy in participants who are aware of fatigue and sleep disturbances in daily life (Hokazono and Saito, 2016). Consuming FB-GABA (equivalent to 100 mg of GABA) significantly improved total viscoelasticity (R2), net elasticity (R5), and recovery rate (R7), which are indices of skin elasticity (Hokazono and Uehara, 2016). In Japan, the Foods with Functional Claims system was implemented on April 1, 2015. Under this system, business operators can report to the Consumer Affairs Agency regarding the scientific basis of the health functionality and safety of the food they intend to sell. If the contents of the reports are found to be appropriate, it becomes the responsibility of the business operators to describe the functionality on the product packaging. Many companies have applied to have their FB-GABA products recognized as Foods with Functional Claims. Food products containing FB-GABA have ranked first in terms of the total number of GABA-containing food notifications received since the start of the system (as of February 2024).
As mentioned above, the safety and physiological functionality of GABA has been proven. FB-GABA is a fermented food that uses shochu manufacturing byproducts as a culture medium, making it an effective use of resources. However, it is necessary to investigate the safety of components other than GABA (unidentified fermentation products) contained in FB-GABA. A mutagenicity test using bacteria, a chromosome aberration test using cultured mammalian cells, and an acute oral toxicity study in mice (limit test) were conducted, and it was confirmed that FB-GABA had no safety issues in these tests (details not disclosed). FB-GABA was launched in August 2006 and approximately 200 million servings have been sold over the past 16 years. There are no reports of health hazards caused by FB-GABA. Japanese Consumer Affairs Agency is requesting confirmation of the safety of overdosing (5 times the normal amount) of health foods such as tablets (The Vice-Commissioner of Consumer Affairs Agency in Japan, 2014). However, the safety of FB-GABA overdose in humans has not been proven. Therefore, the objective of this trial was to confirm the safety of overdosing 5 times recommended daily intake of FB-GABA.
Materials and Methods
1. Trial Design
We designed a placebo-controlled, double-blind, randomized, parallel-group trial protocol that was approved by ethics review committee established at Miura Clinic, Medical Corporation Kanonkai reviewed the safety, efficacy and ethics of this trial on March 17, 2022 (approval number R2112). Written informed consent was obtained from all participants.
The trial was conducted in accordance with the spirit of the “Declaration of Helsinki (revised in October 2013)” and in compliance with the “Ethical Guidelines for Medical and Biological Research Involving Human Subjects (Ministry of Education, Culture, Sports, Science and Technology/Ministry of Health, Labour and Welfare, March 23, 2021).”
Before starting the trial, we registered with the University Hospital Medical Information Network (UMIN) Clinical Trials Registry operated by the UMIN Center (UMIN-ID: UMIN000047414).
2. Participants
After obtaining informed consent for trial participation from participants, the following participant demographics were reviewed or confirmed: sex, age, medical history, surgical history, history of present illness, allergy, drinking habit and the amount, smoking habit and the amount, status of lifestyle and exercise habit, use status for drugs, health foods, and status of participation in other clinical trials or studies. The selection was decided by the principal investigator according to the inclusion and exclusion criteria, based on reviewing the results of the screening (SCR) test and demographics data.
Inclusion Criteria
- Healthy male and female participants aged 20–64 years.
- Participants who were fully informed of the objective and details of the trial, capable of giving consent, fully understood the trial, volunteered to participate in the trial, and gave written consent to participate in the trial.
Exclusion Criteria
- Individuals with heart, liver, or kidney disease (including complications of other diseases).
- Individuals with a history of cardiovascular disease.
- Individuals with diabetes mellitus.
- Individuals currently being treated for a disease.
- Individuals with food or drug allergy.
- Individuals with anemia symptoms.
- Women who wish to become pregnant or are pregnant (including possibly pregnant) or lactating while participating in this trial.
- Individuals who regularly visit a hospital or are receiving treatment with a drug or Chinese herbal medicine for a certain disease (drugs on an as-needed basis are permitted).
- Individuals who engage in strenuous sports or diet.
- Individuals with extremely irregular diet.
- Individuals who cannot discontinue the intake of health foods (including foods for specified health uses and foods with functional claims) and designated quasi-pharmaceutical products during the trial period.
- Individuals who drink over 60 g of net alcohol amount on average per day.
- Individuals who smoke 21 or more cigarettes on average per day.
- Individuals who are participating or plan to participate in another clinical trial at the start of this trial.
- Individuals who received vaccination against influenza, novel coronavirus, or other infectious diseases within 1 month before SCR.
- Individuals who are judged by the principal investigator or subinvestigator to be inappropriate as participants of the trial.
Target Sample Size and Rationale
As the number of participants required to confirm the safety of the trial food intake, the target sample size was determined to be 24 participants per group for a total of 48 participants, taking into account changes in laboratory test values and adverse events.
3. Randomization
The allocation manager created a trial food allocation table, in which participants selected in accordance were randomly divided into the test food group and placebo food group according to allocation factors of age group, sex, and body mass index [BMI], and allocated the participants. After completing the allocation, the allocation manager sealed the trial food allocation table in an envelope and wrote the trial title ID and the date of sealing on the envelope. The allocation manager stored the sealed trial food allocation table in a strict manner until the end of the trial to ensure blinding.
4. Trial Foods
The test food was a tablet containing 134.19 mg (120.7 mg as GABA) of FB-GABA per tablet. The placebo food contained dextrin instead of FB-GABA. The recommended intake of the test food, FB-GABA, is 13.7–223 mg (equivalent to 12.3–200 mg of GABA). We set the 5-fold amount of 1115 mg in accordance with Attachment 2 “Points to consider in preparing application forms for foods for specified health uses” for the labeling permission of foods for specified health uses (Food Labeling Division Notification No. 259)11) and selected 1341.9 mg in consideration of an overage of approximately 20%.
5. Blinding
Being a double-blind trial, both participants and investigators were blinded to the treatment. The placebo food was visually indistinguishable from the test food. The package bags were numbered by the manufacturer and neither the investigators nor the participants knew what number belonged to test or placebo food.
6. Interventions
After the pre-dose Week 0 examination, participants ate their assigned trial food for 4 weeks. Two and four weeks after the start of intake, predetermined interviews and examinations were carried out.
Participants consumed 10 tablets of the trial food per day with water or warm water. If the participant was unable to consume the trial food on that day, carryover to the next day was prohibited. On the examination day (Week 2), the participants consumed the trial food after testing was complete.
From 1 week before the start of intake until the day of examination at Week 4, participants were instructed to record the intake status of the trial food, physical condition, intake of drugs, drinking status, and exercise status in the daily life diary (on the Web or paper).
The following instructions were provided to participants for management.
During the Trial Period
- Do not change the lifestyle prior to trial participation significantly such as diet, alcohol intake, exercise, bedtime, and smoking during the trial period.
- Avoid excessive exercise or temperance in eating and overeating that deviate from the range of daily life.
- In principle, ingestion of medicinal products (including over-the-counter and prescription drugs), designated quasi-pharmaceutical products, and health foods (including foods for specified health uses and foods with functional claims) is prohibited. For medicinal products, use of topical agents is also prohibited, in principle. If it is inevitable to take them because of poor physical condition or other reasons, consult with the contract research organization in advance.
- Drinking alcohol and excessive exercise are prohibited from the day before examination to the completion of the examination on the day.
- On the day before examination, eating and drinking should be completed by 22:00, and fasting should be continued until the examination is completed (intake of water or lukewarm water is permitted).
- On the day of examination, visit the trial site without taking the trial food.
- Immediately notify the person in charge of the trial if receiving vaccination. If a symptom such as heaviness of the body, headache, chills, fever, joint pain, nausea, vomiting, and diarrhea occurs, the principal investigator will determine whether to continue dosing after checking the symptoms. If dosing is continued, whether the symptom is attributable to the test article will be verified by comparing with the placebo group.
- If tested positive for novel coronavirus, promptly contact the person in charge of the trial.
Test Items and Timing
In this trial, body measurements (body weight and BMI), physical examinations (BP and pulse rate), laboratory tests (blood and urine tests) at SCR, pre-dose Week 0, Week 2, and Week 4 were conducted under direction of the principal investigator or subinvestigator. Height was measured only at the time of SCR, and was taken as the value measured at Week 0. Blood tests items were hematology (white blood cell count, red blood cell count, hemoglobin, hematocrit, mean corpuscular volume [MCV], mean corpuscular hemoglobin, mean corpuscular hemoglobin concentration [MCHC] and platelet count) and blood biochemistry (uric acid, urea nitrogen, aspartate aminotransferase/glutamic oxaloacetic transaminase [AST/GOT], alanine aminotransferase/glutamic pyruvic transaminase, gamma-glutamyl transpeptidase, alkaline phosphatase [ALP], lactate dehydrogenase [LDH], total bilirubin, total protein, albumin, Creatinine, Creatine kinase [CK], serum amylase, total cholesterol, high-density lipoprotein cholesterol, low-density lipoprotein cholesterol, triglyceride, blood glucose, hemoglobin A1c [HbA1c], sodium, chloride [Cl], potassium [K], magnesium, calcium [Ca], iron and inorganic phosphorus). Urine test items were protein qualitative, glucose qualitative, urobilinogen qualitative, bilirubin qualitative, occult blood reaction qualitative, ketone body qualitative, specific gravity and pH. In various qualitative tests, the presence of the desired component is indicated as positive (+), and the more (+) there are, the higher the content of the component. False positive cases were expressed as (±). The numbers represent the number of applicable participants. On SCR, pre-dose Week 0, Week 2, and Week 4, the principal investigator, subinvestigator or nurse under the direction of the principal investigator performed medical interviews and recorded the results (significant figures: in accordance with the reference range of each test value). When adverse events occurred during the period from the start of intake to the end of the trial, a list of symptoms, date of onset and resolution, severity of symptoms, seriousness, treatment, outcome, causal relationship with the trial food, and principal investigator’s comments was created. In principle, follow-up was to be performed as necessary until the abnormal findings of the adverse event resolved or showed a recovering trend.
Recruitment and Trial Period
Recruitment began on March 18, 2022, and the trial period was from May 9 to July 4.
Analysis Set and Statistical Analysis Methods
- Data Lock: After completion of the final examination, data lock was performed after all data and their handling were fixed and after the analysis set was confirmed.
- Opening of the trial food allocation table: The allocation manager opened the trial food allocation table under the instruction of the principal investigator after determining the handling for tabulation and analysis and confirming that the data were locked.
- Software used for tabulation and analysis: IBM SPSS Statistics (version 28, IBM Japan Ltd., Tokyo, Japan), JMP 12 (Institute Japan Ltd., Tokyo, Japan) and R (version 3, R Development Core Team, Auckland, New Zealand) were used for data tabulation and analysis.
- Participant demographics Summary statistics (mean ± standard deviation) were calculated and tabulated for the participant demographics of age, sex, height, body weight, systolic and diastolic BP, and pulse. Comparisons between groups were performed using the two-sample t-test, and between sexes were performed using the chi-square test.
- Test values Summary statistics (mean ± standard deviation) were calculated for body measurements, physical examinations, and laboratory tests (hematology and blood biochemistry, and urinalysis). Measured values at pre-dose Week 0, Week 2, and Week 4 were compared with those at pre-dose Week 0 using a Bonferroni-corrected paired t-test for evaluation. The testing was two-sided, with a significance level of 5 %. Body measurements values, physical examination values, and laboratory test values at each measurement time point were compared between the test and placebo food groups using an unpaired t-test. For qualitative parameters of urinalysis, cross-tabulation tables were prepared for evaluation.
- Adverse events, subjective symptoms/objective findings, intake status of drugs/health foods, and trial food intake rate.
Adverse events, subjective symptoms/objective findings (medical interview), intake status of drugs/health foods, and trial food intake rate were tabulated and evaluated.
The intake rate was calculated by dividing the number of intakes by the number of intakes that should be taken.
Results
1. Changes in Participants
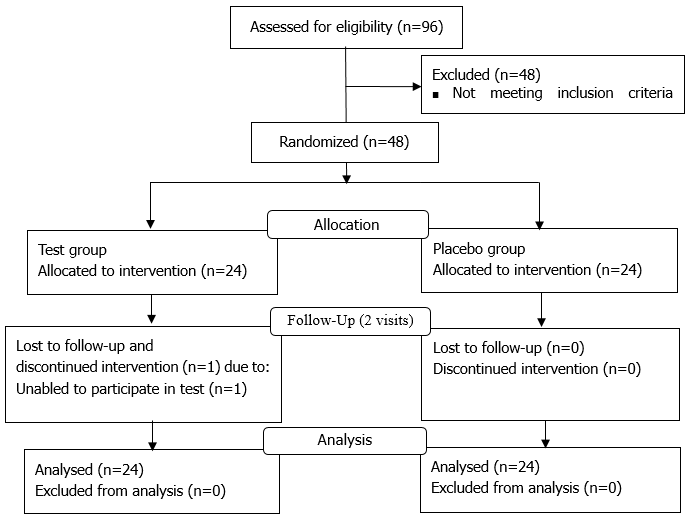
Of the 96 participants who provided written informed consent, 48 participants were excluded on SCR and 48 participants who met the inclusion criteria were selected. One participant (ID12086, test group) withdrew from the trial after randomization because the participant could not change the schedule for the examination day (Figure 1).
2. Participant Demographics
Table 1 shows the participant demographics.
No statistically significant differences were observed in participant demographics between the two groups.
| Group | n | Pretrial | ||||
| Age | (years) | Test | 24 | 43.0 | ± | 13.2 |
| Placebo | 24 | 42.8 | ± | 13.3 | ||
| Male | Test | 12 | ||||
| Placebo | 12 | |||||
| Female | Test | 12 | ||||
| Placebo | 12 | |||||
| Height | (cm) | Test | 24 | 164.98 | ± | 9.27 |
| Placebo | 24 | 165.74 | ± | 8.29 | ||
| Body weight | (kg) | Test | 24 | 59.16 | ± | 9.55 |
| Placebo | 24 | 59.54 | ± | 9.17 | ||
| Body mass index | (kg/m2) | Test | 24 | 21.61 | ± | 1.94 |
| Placebo | 24 | 21.55 | ± | 1.81 | ||
| Systolic blood pressure | (mmHg) | Test | 24 | 122.5 | ± | 10.5 |
| Placebo | 24 | 121.2 | ± | 14.1 | ||
| Diastolic blood pressure | (mmHg) | Test | 24 | 75.2 | ± | 8.2 |
| Placebo | 24 | 73.2 | ± | 10.2 | ||
| Pulse rate | (beats/min) | Test | 24 | 75.1 | ± | 14.7 |
| Placebo | 24 | 75.0 | ± | 12.3 | ||
Values are expressed as mean ± standard deviation (excluding the number of participants and sex). “N” refers to the number of applicable participants. Between-group comparisons were performed using a two-sample t-test, and between- sex comparisons were performed using the chi-square test. No significant differences between groups were found for any items.
Table 1: Participant demographics
3. Participants Analyzed
No participants met the exclusion criteria for analysis; thus, all participants were included in the analysis.
4. Trial Food Intake Rate
The mean intake rate for the trial food was 97.5 ± 4.8 % in the test food group and 98.9 ± 2.9 % in the placebo food group.
5. Body Measurements and Physical Examinations
Table 2 shows the results of body measurements and physical examinations.
In the test food group, comparison with pre-dose Week 0 showed significant differences in body weight, BMI, and diastolic BP at Week 4 and a significant trend in diastolic BP at Week 2. The other items indicated no significant differences or significant trends. In the placebo food group, a significant difference was observed in systolic BP at Week 4, and a significant trend was noted in systolic BP at Week 2 and diastolic BP at Week 4. The other items indicated no significant differences or significant trends.
Comparison between the test and placebo food groups showed no significant differences.
| P-value (intragroup) | P-value (between-group) |
||||||||||||||||||
| Group | n | Week 0 | Week 2 | Week 4 | Week 2 | Week 4 | Week 0 | Week 2 | Week 4 | ||||||||||
| Height | (cm) | TestPlacebo | 24 | 164.98 | ± | 9.27 | - | - | - | - | 0.38 | - | - | ||||||
| 24 | 165.74 | ± | 8.29 | - | - | - | - | ||||||||||||
| Body weight | (kg) | Test | 24 | 59.07 | ± | 10.01 | 59.77 | ± | 9.40 | 59.50 | ± | 9.63 | 0.99 | 0.044 | * | 0.43 | 0.88 | 0.92 | |
| Placebo | 24 | 59.55 | ± | 9.22 | 59.34 | ± | 9.36 | 59.22 | ± | 9.69 | 0.30 | 0.15 | |||||||
| Body mass index | (kg/m2) | Test | 24 | 21.56 | ± | 2.04 | 21.72 | ± | 1.83 | 21.61 | ± | 1.88 | 1.00 | 0.043 | * | 0.50 | 0.67 | 0.75 | |
| Placebo | 24 | 21.56 | ± | 1.90 | 21.48 | ± | 1.94 | 21.43 | ± | 2.04 | 0.27 | 0.10 | |||||||
| Systolic blood pressure | (mmHg) | Test | 24 | 119.5 | ± | 9.8 | 119.5 | ± | 9.4 | 117.9 | ± | 10.1 | 1.00 | 0.39 | 0.50 | 0.30 | 0.53 | ||
| Placebo | 24 | 119.5 | ± | 14.2 | 115.9 | ± | 13.7 | 115.5 | ± | 15.5 | 0.051 | † | 0.041 | * | |||||
| Diastolic blood pressure | (mmHg) | Test | 24 | 75.7 | ± | 7.7 | 72.9 | ± | 8.3 | 70.7 | ± | 7.7 | 0.083 | † | 0.006 | * | 0.058 | 0.29 | 0.40 |
| Placebo | 24 | 71.0 | ± | 10.6 | 70.0 | ± | 10.4 | 68.4 | ± | 11.2 | 0.48 | 0.078 | † | ||||||
| Pulse rate | (beats/min) | Test | 24 | 72.1 | ± | 14.2 | 71.6 | ± | 9.8 | 72.5 | ± | 11.4 | 1.00 | 1.00 | 0.46 | 0.32 | 0.64 | ||
| Placebo | 24 | 73.3 | ± | 11.4 | 74.5 | ± | 10.6 | 74.1 | ± | 12.3 | 0.88 | 1.00 | |||||||
Values are expressed as mean ± standard deviation. “N” refers to the number of applicable participants. Significant trends in a Bonferroni-corrected paired t-test of intragroup changes are expressed as *p < 0.05 and †p < 0.10.
Table 2: Results of body measurement and physical examination
6. Laboratory Tests
Tables 3-5 show the laboratory test results and reference ranges for each test item. Table 6 shows p-values between the groups.
Comparison with pre-dose Week 0 showed significant differences in MCV, MCHC, K, and Ca at Week 2 and MCV, MCHC, and ALP at Week 4, and significant trends in albumin, potassium, magnesium, and Ca at Week 4 in the test food group (Tables 3, 4). The other items indicated no significant differences or significant trends (Tables 3–5). In the placebo food group, on the other hand, significant differences were observed in LDH, albumin, HbA1c, and K at Week 2 and red blood cells, hemoglobin, MCV, MCHC, ALP, Cl, and Ca at Week 4; a significant trend was noted in Ca at Week 2 and platelet count and K at Week 4 (Tables 3, 4). The other items indicated no significant differences or significant trends (Tables 3–5).
| P-value | ||||||||||||||||||
| (intragroup) | ||||||||||||||||||
| Hematology | Reference range | n | Group | Week 0 | Week 2 | Week 4 | Week 2 | Week 4 | ||||||||||
| White blood cell count | 3300 - 9000 | /μL | 24 | Test | 5533.3 | ± | 1145.0 | 5717.4 | ± | 1654.4 | 5513.0 | ± | 1197.8 | 1.00 | 1.00 | |||
| 24 | Placebo | 5312.5 | ± | 1300.0 | 5566.7 | ± | 1261.0 | 5808.3 | ± | 1512.0 | 0.34 | 0.43 | ||||||
| Red blood cell count | Male | 430-570 | ×104/μL | 24 | Test | 472.7 | ± | 41.8 | 473.5 | ± | 40.5 | 469.9 | ± | 39.9 | 1.00 | 0.64 | ||
| cell count | Female | 380-500 | 24 | Placebo | 462.3 | ± | 50.6 | 462.0 | ± | 40.4 | 449.8 | ± | 42.9 | 1.00 | 0.03 | * | ||
| Hemoglobin | Male | 13.5-17.5 | g/dL | 24 | Test | 14.31 | ± | 1.22 | 14.33 | ± | 1.1 | 14.14 | ± | 1.15 | 1.00 | 0.12 | ||
| Female | 11.5-15.0 | 24 | Placebo | 13.83 | ± | 1.43 | 13.87 | ± | 1.2 | 13.45 | ± | 1.3 | 1.00 | 0.009 | * | |||
| Hematocrit | Male | 39.7-52.4 | % | 24 | Test | 45.02 | ± | 3.58 | 45.61 | ± | 3.22 | 45.45 | ± | 3.56 | 0.59 | 1.00 | ||
| Female | 34.8-45.0 | 24 | Placebo | 44.15 | ± | 4.33 | 44.41 | ± | 3.39 | 43.67 | ± | 3.74 | 1.00 | 0.62 | ||||
| MCV | 85 - 102 | fL | 24 | Test | 95.4 | ± | 3.0 | 96.5 | ± | 3.4 | 96.8 | ± | 3.5 | 0.01 | * | 0.0001 | * | |
| 24 | Placebo | 95.7 | ± | 3.6 | 96.3 | ± | 3.9 | 97.2 | ± | 3.5 | 0.38 | 0.001 | * | |||||
| MCH | 28.0-34.0 | pg | 24 | Test | 30.32 | ± | 1.36 | 30.31 | ± | 1.32 | 30.13 | ± | 1.44 | 1.00 | 0.12 | |||
| 24 | Placebo | 29.98 | ± | 1.28 | 30.05 | ± | 1.18 | 29.94 | ± | 1.19 | 0.75 | 1.00 | ||||||
| MCHC | 30.2-35.1 | % | 24 | Test | 31.79 | ± | 0.71 | 31.40 | ± | 0.75 | 31.11 | ± | 0.96 | 0.001 | * | 0.0001 | * | |
| 24 | Placebo | 31.33 | ± | 0.87 | 31.22 | ± | 0.92 | 30.78 | ± | 0.79 | 0.92 | 0.004 | * | |||||
| Platelet count | 14.0-34.0 | ×104/μL | 24 | Test | 25.28 | ± | 3.87 | 25.06 | ± | 3.74 | 25.23 | ± | 3.83 | 1.00 | 1.00 | |||
| 24 | Placebo | 27.04 | ± | 5.65 | 26.71 | ± | 6.05 | 25.80 | ± | 6.66 | 1.00 | 0.07 | † | |||||
Values are expressed as mean ± standard deviation. Parameters separated by sex are indicated by results for each sex only. “N” refers to the number of applicable participants. MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration. Significant differences in a Bonferroni-corrected paired t-test of intragroup changes are expressed as *p < 0.05 and †p < 0.10.
Table 3: Laboratory test results
| Blood biochemistry | Reference range | Group | n | Week 0 | Week 2 | Week 4 | (Week 2) | (Week 4) | ||||||||||
| Uric acid | Male | 3.8 - 7.0 | mg/dL | Test | 24 | 5.13 | ± | 1.14 | 5.16 | ± | 1.00 | 5.23 | ± | 1.03 | 1.00 | 1.00 | ||
| Female | 2.5 - 7.0 | Placebo | 24 | 4.83 | ± | 1.25 | 4.87 | ± | 1.23 | 4.85 | ± | 1.35 | 1.00 | 1.00 | ||||
| Urea nitrogen | 8.0 - 20.0 | mg/dL | Test | 24 | 12.53 | ± | 3.81 | 11.97 | ± | 2.44 | 12.50 | ± | 3.31 | 0.29 | 1.00 | |||
| Placebo | 24 | 12.48 | ± | 2.39 | 13.00 | ± | 3.19 | 13.1 | ± | 2.95 | 0.40 | 0.78 | ||||||
| AST | 10 - 40 | U/L | Test | 24 | 19.2 | ± | 4.3 | 24.5 | ± | 21.0 | 19.2 | ± | 4.6 | 0.48 | 1.00 | |||
| Placebo | 24 | 19.3 | ± | 5.3 | 18.8 | ± | 4.8 | 19.0 | ± | 5.1 | 0.74 | 1.00 | ||||||
| ALT | 5 - 45 | U/L | Test | 24 | 14.1 | ± | 5.5 | 17.7 | ± | 12.5 | 15.7 | ± | 9.0 | 0.16 | 0.55 | |||
| Placebo | 24 | 14.7 | ± | 7.3 | 15.3 | ± | 6.8 | 14.5 | ± | 7.0 | 1.00 | 1.00 | ||||||
| γ-GT | Male | ≤80 | U/L | Test | 24 | 18.2 | ± | 8.2 | 18.7 | ± | 8.1 | 18.3 | ± | 8.0 | 1.00 | 1.00 | ||
| Female | ≤30 | Placebo | 24 | 17.9 | ± | 7.9 | 20.2 | ± | 12.2 | 18.1 | ± | 8.3 | 0.25 | 1.00 | ||||
| ALP | 38 - 113 | U/L | Test | 24 | 71.0 | ± | 18.3 | 70.5 | ± | 18.9 | 69.1 | ± | 19.3 | 0.69 | 0.03 | * | ||
| Placebo | 24 | 64.3 | ± | 18.4 | 64.9 | ± | 19.1 | 60.6 | ± | 16.9 | 1.00 | 0.02 | * | |||||
| LDH | 124 - 222 | U/L | Test | 24 | 177.2 | ± | 24.0 | 194.9 | ± | 53.7 | 179.1 | ± | 25.1 | 0.20 | 1.00 | |||
| Placebo | 24 | 175.7 | ± | 23.6 | 181.8 | ± | 22.4 | 185.8 | ± | 49.0 | 0.02 | * | 0.62 | |||||
| Total bilirubin | 0.2 - 1.2 | mg/dL | Test | 24 | 0.89 | ± | 0.29 | 0.91 | ± | 0.28 | 0.88 | ± | 0.33 | 1.00 | 1.00 | |||
| Placebo | 24 | 0.81 | ± | 0.29 | 0.81 | ± | 0.26 | 0.90 | ± | 0.27 | 1.00 | 0.11 | ||||||
| Total protein | 6.7 - 8.3 | g/dL | Test | 24 | 7.19 | ± | 0.35 | 7.23 | ± | 0.43 | 7.27 | ± | 0.47 | 0.83 | 0.31 | |||
| Placebo | 24 | 7.08 | ± | 0.45 | 7.15 | ± | 0.43 | 7.06 | ± | 0.41 | 0.38 | 1.00 | ||||||
| Albumin | 3.8-5.2 | g/dL | Test | 24 | 4.56 | ± | 0.32 | 4.63 | ± | 0.35 | 4.65 | ± | 0.37 | 0.11 | 0.06 | † | ||
| Placebo | 24 | 4.47 | ± | 0.30 | 4.59 | ± | 0.27 | 4.52 | ± | 0.24 | 0.004 | * | 0.35 | |||||
| Cre | Male | 0.61-1.04 | mg/dL | Test | 24 | 0.785 | ± | 0.12 | 0.78 | ± | 0.10 | 0.81 | ± | 0.13 | 0.16 | 0.55 | ||
| Female | 0.47-0.79 | Placebo | 24 | 0.771 | ± | 0.13 | 0.76 | ± | 0.12 | 0.77 | ± | 0.12 | 0.79 | 1.00 | ||||
| CK | Male | 60-270 | U/L | Test | 24 | 92.6 | ± | 45.1 | 557.0 | ± | 1815.0 | 105.3 | ± | 80.3 | 0.47 | 1.00 | ||
| Female | 40-150 | Placebo | 24 | 107.8 | ± | 53.4 | 105.0 | ± | 43.6 | 97.8 | ± | 39.7 | 1.00 | 0.25 | ||||
| Serum amylase | 40-122 | U/L | Test | 24 | 76.6 | ± | 23.2 | 79.1 | ± | 21.7 | 77.6 | ± | 20.8 | 0.86 | 1.00 | |||
| Placebo | 24 | 81.9 | ± | 35.3 | 76.0 | ± | 19.6 | 74.0 | ± | 19.8 | 0.72 | 0.46 | ||||||
| Total-Cho | 120-219 | mg/dL | Test | 24 | 197.8 | ± | 24.2 | 203.1 | ± | 32.5 | 203.4 | ± | 29.3 | 0.40 | 0.33 | |||
| Placebo | 24 | 196.7 | ± | 22.0 | 200.4 | ± | 20.7 | 195.8 | ± | 24.3 | 0.58 | 1.00 | ||||||
| HDL-Cho | Male | 40-85 | mg/dL | Test | 24 | 67.5 | ± | 14.9 | 66.1 | ± | 15.6 | 65.6 | ± | 14.0 | 0.35 | 0.24 | ||
| Female | 40-95 | Placebo | 24 | 70.9 | ± | 10.5 | 72.5 | ± | 11.1 | 71.0 | ± | 11.7 | 0.30 | 1.00 | ||||
| LDL-Cho | 65-139 | mg/dL | Test | 24 | 114.5 | ± | 21.2 | 117.5 | ± | 27.1 | 118.3 | ± | 26.1 | 0.86 | 0.65 | |||
| Placebo | 24 | 111.7 | ± | 20.7 | 110.9 | ± | 18.8 | 107.5 | ± | 20.2 | 1.00 | 0.25 | ||||||
| Triglyceride | 30-149 | mg/dL | Test | 24 | 90.1 | ± | 48.4 | 97.5 | ± | 50.0 | 97.4 | ± | 56.8 | 1.00 | 0.83 | |||
| Placebo | 24 | 76.0 | ± | 26.5 | 78.0 | ± | 28.6 | 81.3 | ± | 45.4 | 1.00 | 1.00 | ||||||
| Blood glucose | 70-109 | mg/dL | Test | 24 | 84.6 | ± | 5.7 | 82.6 | ± | 6.8 | 84.3 | ± | 6.1 | 0.17 | 1.00 | |||
| Placebo | 24 | 84.6 | ± | 5.7 | 85.3 | ± | 9.0 | 85.0 | ± | 7.2 | 1.00 | 1.00 | ||||||
| HbA1c | 4.6-6.2 | % | Test | 24 | 5.28 | ± | 0.26 | 5.30 | ± | 0.25 | 5.27 | ± | 0.28 | 0.75 | 0.90 | |||
| Placebo | 24 | 5.25 | ± | 0.18 | 5.29 | ± | 0.18 | 5.23 | ± | 0.17 | 0.010 | * | 0.81 | |||||
| Na | 137-147 | mEq/L | Test | 24 | 141.4 | ± | 2.0 | 140.7 | ± | 2.3 | 141.6 | ± | 2.2 | 0.14 | 1.00 | |||
| Placebo | 24 | 141.3 | ± | 2.2 | 140.7 | ± | 1.7 | 141.2 | ± | 1.7 | 0.34 | 1.00 | ||||||
| Cl | 98-108 | mEq/L | Test | 24 | 104.5 | ± | 2.2 | 104.0 | ± | 2.1 | 103.7/td> | ± | 2.2 | 0.23 | 0.11 | |||
| Placebo | 24 | 105.2 | ± | 2.3 | 104.4 | ± | 1.7 | 103.8 | ± | 1.8 | 0.11 | 0.007 | * | |||||
| K | 3.5-5.0 | mEq/L | Test | 24 | 4.12 | ± | 0.24 | 3.99 | ± | 0.30 | 4.06 | ± | 0.29 | 0.045 | * | 0.45 | ||
| Placebo | 24 | 4.20 | ± | 0.30 | 4.00 | ± | 0.22 | 4.04 | ± | 0.23 | 0.006 | * | 0.08 | † | ||||
| Mg | 1.9-2.5 | mg/dL | Test | 24 | 2.08 | ± | 0.13 | 2.06 | ± | 0.12 | 2.13 | ± | 0.13 | 0.79 | 0.06 | † | ||
| Placebo | 24 | 2.10 | ± | 0.11 | 2.08 | ± | 0.14 | 2.10 | ± | 0.14 | 0.41 | 1.00 | ||||||
| Ca | 8.4-10.4 | mg/dL | Test | 24 | 9.33 | ± | 0.24 | 9.20 | ± | 0.30 | 9.22 | ± | 0.38 | 0.004 | * | 0.08 | † | |
| Placebo | 24 | 9.34 | ± | 0.34 | 9.25 | ± | 0.25 | 9.15 | ± | 0.31 | 0.06 | † | 0.012 | * | ||||
| Fe | Male | 50-200 | μg/dL | Test | 24 | 103.0 | ± | 34.2 | 101.2 | ± | 27.5 | 96.0 | ± | 38.2 | 1.00 | 1.00 | ||
| Female | 40-180 | Placebo | 24 | 93.0 | ± | 32.4 | 96.5 | ± | 36.3 | 110.6 | ± | 37.4 | 1.00 | 0.13 | ||||
| IP | 2.5-4.5 | mg/dL | Test | 24 | 3.69 | ± | 0.44 | 3.64 | ± | 0.56 | 3.52 | ± | 0.53 | 1.00 | 0.17 | |||
| Placebo | 24 | 3.56 | ± | 0.54 | 3.63 | ± | 0.63 | 3.63 | ± | 0.58 | 0.84 | 0.66 | ||||||
Values are expressed as mean ± standard deviation. Parameters separated by sex are indicated by results for each sex only. “N” refers to the number of applicable participants. AST/GOT, aspartate aminotransferase; ALT, alanine aminotransferase; γ-GT, gamma-glutamyl transpeptidase; ALP, alkaline phosphatase; LDH, lactate dehydrogenase; Cre, creatinine; CK, creatine kinase; Total-Cho, total cholesterol; HDL-Cho, high-density lipoprotein cholesterol; LDL-Cho, low-density lipoprotein cholesterol; HbA1c, hemoglobin A1c; Na, sodium; Cl, chloride; K, potassium; Mg, magnesium; Ca, calcium; Fe, iron; IP, inorganic phosphorus. Significant differences in a Bonferroni-corrected paired t-test of intragroup changes are expressed as *p < 0.05 and †p < 0.10.
Table 4: Laboratory test results (2)
| Urinalysis (quantitative parameters) | Reference range | Group | n | Week 0 | Week 2 | Week 4 | P-value (intragroup) | P-value (intragroup) | ||||||
| Week 2 | Week 4 | |||||||||||||
| Urine specific gravity | 1.006-1.030 | Test | 24 | 1.019 | ± | 0.010 | 1.018 | ± | 0.008 | 1.021 | ± | 0.010 | 0.27 | 1.00 |
| Placebo | 24 | 1.018 | ± | 0.008 | 1.017 | ± | 0.009 | 1.019 | ± | 0.008 | 0.86 | 0.87 | ||
| Urine pH | 5.0 - 7.5 | Test | 24 | 6.40 | ± | 0.68 | 6.48 | ± | 0.87 | 6.33 | ± | 0.73 | 1.00 | 1.00 |
| Placebo | 24 | 6.08 | ± | 0.70 | 6.19 | ± | 0.84 | 6.10 | ± | 0.81 | 1.00 | 1.00 | ||
Values are expressed as mean ± standard deviation. “N” refers to the number of applicable participants. Intragroup comparisons were analyzed using a Bonferroni-corrected paired t-test.
Table 5: Laboratory test results 3
| P-value (between-group) | ||||
| Week 0 | Week 2 | Week 4 | ||
| Hematology | White blood cell count | 0.54 | 0.73 | 0.46 |
| Red blood cell count | 0.44 | 0.34 | 0.10 | |
| Hemoglobin | 0.22 | 0.18 | 0.06 | |
| Hematocrit | 0.45 | 0.22 | 0.10 | |
| MCV | 0.76 | 0.83 | 0.74 | |
| MCH | 0.38 | 0.47 | 0.63 | |
| MCHC | 0.054 | 0.46 | 0.21 | |
| Platelet count | 0.22 | 0.27 | 0.72 | |
| Blood biochemistry | Uric acid | 0.39 | 0.38 | 0.28 |
| Urea nitrogen | 0.96 | 0.22 | 0.55 | |
| AST | 0.91 | 0.22 | 0.90 | |
| ALT | 0.74 | 0.41 | 0.64 | |
| γ-GT | 0.91 | 0.61 | 0.94 | |
| ALP | 0.21 | 0.32 | 0.11 | |
| LDH | 0.83 | 0.29 | 0.56 | |
| Total bilirubin | 0.33 | 0.23 | 0.84 | |
| Total protein | 0.34 | 0.55 | 0.12 | |
| Albumin | 0.29 | 0.68 | 0.17 | |
| Cre | 0.69 | 0.69 | 0.38 | |
| CK | 0.29 | 0.25 | 0.69 | |
| Serum amylase | 0.54 | 0.61 | 0.55 | |
| Total-Cho | 0.87 | 0.74 | 0.34 | |
| HDL-Cho | 0.36 | 0.12 | 0.16 | |
| LDL-Cho | 0.65 | 0.34 | 0.12 | |
| Triglyceride | 0.22 | 0.11 | 0.29 | |
| Blood glucose | 1.00 | 0.25 | 0.69 | |
| HbA1c | 0.57 | 0.85 | 0.51 | |
| Na | 0.89 | 0.96 | 0.49 | |
| Cl | 0.34 | 0.45 | 0.76 | |
| K | 0.31 | 0.91 | 0.85 | |
| Mg | 0.55 | 0.63 | 0.38 | |
| Ca | 0.88 | 0.61 | 0.51 | |
| Fe | 0.30 | 0.62 | 0.19 | |
| IP | 0.39 | 0.95 | 0.51 | |
| Urinalysis (quantitative parameters) | Urine specific gravity | 0.61 | 0.73 | 0.59 |
| Urine pH | 0.12 | 0.25 | 0.33 | |
The value indicates the p-value of each measurement item. MCV, mean corpuscular volume; MCH, mean corpuscular hemoglobin; MCHC, mean corpuscular hemoglobin concentration; AST/GOT, aspartate aminotransferase; ALT, alanine aminotransferase; γ-GT, gamma-glutamyl transpeptidase; ALP, alkaline phosphatase; LDH, lactate dehydrogenase; Cre, creatinine; CK, creatine kinase; Total-Cho, total cholesterol; HDL-Cho, high-density lipoprotein cholesterol; LDL-Cho, low-density lipoprotein cholesterol; HbA1c, hemoglobin A1c; Na, sodium; Cl, chloride; K, potassium; Mg, magnesium; Ca, calcium; Fe, iron; IP, inorganic phosphorus. Comparisons between groups were performed using an unpaired t-test.
Table 6: P-values for between-group comparisons of laboratory test values
Comparison between the test and placebo food groups showed no significant differences or trends (Table 6).
For urine qualitative parameters, urinary occult blood reaction was noted with (++) in 1 participant and (+++) in 1 participant at Week 2 in the test food group (Appendix 1). In the placebo food group, urobilinogen was observed with (+) in 1 participant at pre-dose Week 0 and (+) in 1 participant at Week 4 (Appendix 2). Urinary occult blood reaction was noted with (+) in 1 participant at pre-dose Week 0, (+) in 1 participant, (++) in 1 participant, and (+++) in 1 participant at Week 2, and (++) in 1 participant at Week 4 (Appendix 2). For urine ketone body, (+) was observed in 1 participant at Week 4 (Appendix 2).
| Week 2 | Week 4 | ||||||||||
| Parameter | Week 0 | (-) | (±) | (+) | (++) | (+++) | (-) | (±) | (+) | (++) | (+++) |
| Urine protein, qualitative | (-) | 17 | 1 | 0 | 0 | 0 | 18 | 0 | 0 | 0 | 0 |
| (±) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| (+) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| (++) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| (+++) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Urine glucose, qualitative | (-) | 23 | 0 | 0 | 0 | 0 | 23 | 0 | 0 | 0 | 0 |
| (±) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| (+) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| (++) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| (+++) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Urobilinogen, qualitative | (-) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| (±) | 0 | 23 | 0 | 0 | 0 | 0 | 23 | 0 | 0 | 0 | |
| (+) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| (++) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| (+++) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Urine bilirubin, qualitative | (-) | 23 | 0 | 0 | 0 | 0 | 23 | 0 | 0 | 0 | 0 |
| (±) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| (+) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| (++) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| (+++) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Urinary occult blood reaction, qualitative | (-) | 19 | 0 | 0 | 1 | 1 | 21 | 0 | 0 | 0 | 0 |
| (±) | 2 | 0 | 0 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | |
| (+) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| (++) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| (+++) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Urine ketone body, qualitative | (-) | 23 | 0 | 0 | 0 | 0 | 23 | 0 | 0 | 0 | 0 |
| (±) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| (+) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| (++) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| (+++) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
The presence of the desired component is indicated as positive (+), and the more (+) there are, the higher the content of the component. False-positive cases are expressed as (±). Numbers represent the number of applicable participants.
Appendix 1: Cross-tabulation table for urinalysis values [test food group (n = 24)]
| Week 2 | Week 4 | ||||||||||
| Parameter | Week 0 | (-) | (±) | (+) | (++) | (+++) | (-) | (±) | (+) | (++) | (+++) |
| Urine protein, qualitative | (-) | 19 | 3 | 0 | 0 | 0 | 21 | 1 | 0 | 0 | 0 |
| (±) | 1 | 1 | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | |
| (+) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| (++) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| (+++) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Urine glucose, qualitative | (-) | 24 | 0 | 0 | 0 | 0 | 24 | 0 | 0 | 0 | 0 |
| (±) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| (+) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| (++) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| (+++) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Urobilinogen, qualitative | (-) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| (±) | 0 | 23 | 0 | 0 | 0 | 0 | 23 | 0 | 0 | 0 | |
| (+) | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | |
| (++) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| (+++) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Urine bilirubin, qualitative | (-) | 24 | 0 | 0 | 0 | 0 | 24 | 0 | 0 | 0 | 0 |
| (±) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| (+) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| (++) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| (+++) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Urinary occult blood reaction, qualitative | (-) | 20 | 0 | 0 | 0 | 1 | 19 | 2 | 0 | 0 | 0 |
| (±) | 1 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | |
| (+) | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 0 | |
| (++) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| (+++) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Urine ketone body, qualitative | (-) | 24 | 0 | 0 | 0 | 0 | 23 | 0 | 1 | 0 | 0 |
| (±) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| (+) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| (++) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| (+++) | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
The presence of the desired component is indicated as positive (+), and the more (+) there are, the higher the content of the component. False-positive cases are expressed as (±). Numbers represent the number of applicable participants.
Appendix 2: Cross-tabulation table for urinalysis values [placebo food group (n = 24)]
7. Adverse Events
Table 7 lists adverse events observed during the trial period.
Before intake of the trial food, 2 adverse events were reported in the test food group and 1 in the placebo food group. During the period of intake of the trial food, 12 adverse events were reported in the test food group and 5 in the placebo food group. All 20 events were considered to be “not related” to the trial food.
There were no drugs or health foods taken during the trial period that would affect the results.
Discussion
This was a parallel-group comparative trial using the placebo food of dextrin at 1341.9 mg/day in healthy male and female adults aged 20–64 years to confirm the safety of the test food (10 tablets [1341.9 mg] per day of FB-GABA-containing food) given for 4 weeks continuously.
In the test food group, intragroup comparison in each test from the start of intake to post-intake showed significant increases in body weight and BMI, and a significant decrease in diastolic BP at Week 4. On the other hand, the placebo food group showed a significant decrease in systolic BP at Week 4.
Of the parameters showing intragroup changes in the test food group, body weight and BMI increased at Week 4. However, as the body weight increase was limited to a mean of 0.4 kg, no parameters indicated statistically significant differences between the test and placebo food groups. Therefore, the investigator determined that there would be no safety problem.
In the test food group, diastolic BP significantly decreased at Week 4. The diastolic BP of less than 60 mmHg occurred in 2 participants, but both participants had a systolic BP of 100 mmHg or over with no abnormalities in medical interviews. The principal investigator, therefore, determined that there would be no safety problem. In the placebo food group, a significant decrease in systolic BP was observed at Week 4. However, 2 of 4 participants who had a systolic BP of 100 mmHg or less at Week 4 had already shown a level of 100 mmHg or less since pre-dose Week 0. Comparison between the test and placebo food groups showed no significant differences or trends. In addition, the principal investigator considered that there would be no safety problem, since none of the participants showed abnormalities in medical interviews.
In the test food group, intragroup comparison in each test from the start of intake to post-intake showed a significant increase in MCV at Weeks 2 and 4. In addition, K and Ca levels significantly decreased at Week 2, and the ALP level was significantly decreased at Week 4. On the other hand, MCHC significantly decreased at Weeks 2 and 4. In the placebo food group, albumin, HbA1c, and LDH levels significantly increased at Week 2, and the MCV level significantly increased at Week 4. On the other hand, the K level significantly decreased at Week 2, and Cl, Ca, red blood cell, hemoglobin, MCHC, and ALP levels significantly decreased at Week 4. Comparison between the test and placebo food groups showed no significant differences or trends.
Of the parameters showing intragroup changes in the test food group, MCV significantly increased at Weeks 2 and 4. The level above the reference range at Week 2 occurred in 1 participant, but the change in the test value in this participant was minor and the value returned to the reference range at Week 4. Therefore, the change was not considered by the principal investigator to be clinically problematic. In addition, no participants had a level above the reference range at Week 4; therefore, it was judged that there would be no safety concern. In the placebo food group as well, MCV significantly increased at Week 4. The level above the reference range occurred in 2 participants at Week 4. One of them had a value above the reference range from Week 2, but no particular adverse event developed despite the high test value. The value for the other participant returned to the reference range at Week 4. Therefore, the change was not considered by the principal investigator to be clinically problematic.
MCHC significantly decreased at Weeks 2 and 4 in the test food group. The level below the lower limit of the reference range occurred in 1 participant at Week 2 and in 3 participants at Week 4. Although the values were low, no adverse events occurred. Therefore, the change was not considered by the principal investigator to be clinically problematic. MCHC significantly decreased at Week 4 in the placebo food group. The level below the lower limit of the reference range occurred in 5 participants, including 1 participant from the time of SCR and another participant from Week 2. The test values for all of the participants were low, but no particular adverse events developed. Therefore, the change was not considered by the principal investigator to be clinically problematic.
In the test food group, K and Ca levels significantly decreased at Week 2. The potassium level below the lower limit of the reference range occurred in 1 participant, but the level in this participant returned to the reference range at Week 4. The Ca level was not below the lower limit of the reference range in any participants; therefore, there appeared to be no safety problems. In the placebo food group as well, K and Ca levels significantly decreased at Week 2 and Week 4, respectively. However, these were not below the lower limit of the reference range in any participants. Therefore, the changes were not considered by the principal investigator to be clinically problematic.
ALP significantly decreased at Week 4 in both the test and placebo food groups. The level below the lower limit of the reference range occurred in 1 participant each in both groups. The ALP levels in both of the participants were below the lower limit of the reference range at SCR, pre-dose Week 0, and Week 2. However, no adverse events occurred despite the low test values. Therefore, the change was not considered by the principal investigator to be clinically problematic.
Of the parameters showing intragroup changes in the placebo food group, significant increases in albumin and HbA1c were confirmed at Week 2. However, none of the participants had a level beyond the reference range, no significant increase was detected at Week 4, no between-group difference was observed, and the changes were slight; therefore, the change was not considered by the principal investigator to be clinically problematic. LDH significantly increased at Week 2. The level above the reference range at Week 2 occurred in 1 participant. The LDH level in this participant was above the reference level at Week 4 as well, but no adverse event occurred. Therefore, the change was not considered to be clinically problematic. Cl significantly decreased at Week 4. However, the level was not below the lower limit of the reference range in any participants. Therefore, the change was not considered to be clinically problematic. A significant decrease in red blood cells was confirmed at Week 4. The level below the lower limit of the reference range at Week 4 occurred in 3 participants. The levels of red blood cells in all of these participants were below the lower limit of the reference range at pre-dose Week 0. Although the test values were low, the change was not considered by the principal investigator to be clinically problematic. Hemoglobin levels significantly decreased at Week 4. The level below the lower limit of the reference range occurred in 3 participants, including 1 participant from pre-dose Week 0. Despite the low test value, no particular adverse events were observed. Therefore, the change was not considered by the principal investigator to be clinically problematic.
Comparison between the test and placebo food groups showed no significant differences or trends.
Regarding laboratory test values in individual participants, the CK values in the participant ID12024 (test food group) were 138 at SCR, 97 at pre-dose Week 0, 2,309 at Week 2, and 423 at Week 4, which was beyond the range of physiological variation and was, therefore, judged to be an abnormal change. The interview with the participant confirmed that the participant practiced muscle training, which was not the norm, on the day before the test day for Week 2 and Week 4, and that the participant had myalgia on the test day for Week 2 and Week 4. As CK has been reported to be increased by strenuous exercise (Seifi-Skishahr et al., 2008), the causal relationship to the trial or trial food was considered by the principal investigator to be “not related.” Regarding the participant ID12030 (test food group), AST levels were 15 at SCR, 17 at pre-dose Week 0, 114 at Week 2, and 17 at Week 4; and CK levels were 74 at SCR, 81 at pre-dose Week 0, 8,607 at Week 2, and 187 at Week 4. These results were beyond the range of physiological variation and, therefore, judged to be abnormal changes. The interview with the participant confirmed that the participant had continued their usual habit light exercise such as walking during the trial but did not engage in strenuous exercise that differed from the norm during the trial. During the interview at the Week 2 test, the participant did not show any signs of poor physical condition (fever, nausea, diarrhea, abdominal pain, etc.). In the case of myocardial infarction, AST may increase along with CK (Inoue et al., 1978), so CK isozyme fractionation was performed to examine whether myocardial infarction had occurred. The results of CK isozyme fractionation separately confirmed that there was no risk of myocardial infarction, so the trial was continued. The test results at Week 4 showed that the laboratory values had decreased to the reference range; thus, the causal relationship to the trial or trial food was considered by the principal investigator to be “not related.”
Other parameters sporadically deviated from the reference range after the start of intake in multiple participants, but all of the deviations were within the range of physiological variations. Therefore, the principal investigator did not consider them to be clinically problematic.
In urine qualitative testing, the participant ID12002 (placebo food group) was positive (+) for urobilinogen at pre-dose Week 0 and Week 4, but this was a symptom peculiar to the participant and was not considered to be clinically problematic. Qualitative tests for urinary occult blood reaction showed positive results as follows: (+) for ID12097 (placebo food group) at pre-dose Week 0; (++) for ID12097 (placebo food group) and ID12061 (test food group) and (+++) for ID12035 (test food group) and ID12058 (placebo food group) at Week 2; and (++) for ID12097 (placebo food group) at Week 4. The result for ID12097 was (+) at pre-dose Week 0; therefore, it was judged to be a symptom peculiar to the participant and not clinically problematic. For the other 3 participants (ID12035, ID12058, and ID12061), the result was attributable to menstruation, and not considered to be clinically problematic. The participant ID12007 (placebo food group) was positive (+) for urine ketone body at Week 4, but this was deemed to be an influence of fasting and, therefore, was not considered to be clinically problematic.
A total of 20 adverse events occurred in this trial. All events had mild to moderate symptoms that occurred before intake of the trial food, had a definite cause, or were transient. Therefore, the causal relationship with the trial food was determined to be “not related.”
Based on the above, the principal investigator concluded that there was no problem in the safety of 4-week overdose of the trial food from the viewpoints of body measurements, physical examinations, laboratory tests, and adverse events. There has been no preceding trial on the safety of excessive intake (5 times the recommended intake) of foods that contain FB-GABA in healthy adult male and female participants. The results of this trial can be applied to all foods containing FB-GABA. Confirmation of safety in this trial will enable appropriate provision of information to consumers and is expected to be of academic significance.
This trial was a 4-week overdose intake trial, so it is necessary to examine the safety of long-term intake. We also conducted a trial in which the recommended intake was consumed over a long period and will be reported separately.
Conclusion
From the results of this trial, we concluded that consuming 1341.9 mg of FB-GABA (more than 5 times the recommended intake) daily for 4 weeks was safe from the viewpoints of body measurements, physical examinations, laboratory tests, and adverse events.
Funding
Trial funding was provided by Sanwa Shurui Co., Ltd., and Uehara and Hokazono are employees of Sanwa Shurui Co., Ltd. The other author have no conflicts of interest to disclose.
Conflict of Interest
Sanwa Shurui Co., Ltd. concluded an outsourcing contract with Oneness Support Co., Ltd. to outsource the trial. Oneness Support Co., Ltd. concluded an operating agreement with Miura Clinic, Medical Corporation Kanonkai (trial site) and LSI Medience Corporation (laboratory), and conducted the trial. The compensation based on these operating agreements was a legitimate business compensation for implementation of the trial and does not affect the trial results.
References
- Bloom EF and Iversen LL. “Localizing 3H-GABA in nerve terminals of rat cerebral cortex by electron microscopic autoradiography”. Nature 229.5287 (1971): 628-630.
- Tessa H de Bie., et al. “Effects of γ-aminobutyric acid supplementation on glucose control in adults with prediabetes: A double-blind, randomized, placebo-controlled trial”. The American Journal of Clinical Nutrition 118.3 (2023): 708-719.
- Furuta Y., et al. “Novel Conversion Processes with Lactic Acid Bacteria from Shochu Kasu into Valuable Materials”. Seibutsu-kogaku Kaishi (in Japanese) 88.3 (2010): 114–120.
- Hokazono H and Fukuda R. “Effects of oral intake of GABA on sleep in healthy adults - a randomized, double-blind, placebo-controlled, crossover study”. Japanese Pharmacology & Therapeutics 46(2018) :757-770.
- Hokazono H and Saito J. “Effects of γ-aminobutyric acid (GABA)-containing food on mood status and sleepquality in workers - A double-blind randomized controlled trial”. Japanese Pharmacology & Therapeutics 44 (2016):1445-1454.
- Hokazono H and Uehara E. “Dermal effects of oral administration of GABA in humans”. Nippon Shokuhin Kagaku Kogaku Kaishi, 63.7 (2016):306-311.
- Inoue M., et al. “Immunological determination of serum m-AST activity in patients with acute myocardial infarction”. British Heart Journal 40.11 (1978): 1251-1256.
- Kuriyama K. “Functional roles of cerebral γ-aminobutyric acid (GABA) and its implication in the action of centrally acting drugs”. The Japanese Journal of Pharmacology 29 (1979): 6.
- Utano Nakamura., et al. “Dietary Gamma-Aminobutyric Acid (GABA) Induces Satiation by Enhancing the Postprandial Activation of Vagal Afferent Nerves”. Nutrients 14.12 (2022): 2492.
- Hellen A Oketch-Rabah., et al. “United States Pharmacopeia (USP) Safety Review of Gamma-Aminobutyric Acid (GABA)”. Nutrients 13.8 (2021): 2742.
- Seifi-Skishahr F., et al. “Influence of aerobic exercise at high and moderate intensities on lipid peroxidation in untrained men”. The Journal of Sports Medicine and Physical Fitness 48.4 (2008):515-521.
- The Vice-Commissioner of Consumer Affairs Agency in Japan. Attachment 2 “Points to consider in preparing application forms for foods for specified health uses” for the labeling permission of foods for specified health uses (Food Labeling Division Notification No. 259) issued on October 30, 2014 (in Japanese).
- Yamatsu A., et al. “Intake of 200 mg/day of γ-Aminobutyric Acid (GABA) improves a wide range of cognitive functions: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial”. Japanese Pharmacology & Therapeutics 48 (2020) :461-474.
Citation:
Dr. Eriko Uehara., et al. “Safety Evaluation of Overdose Intake of Fermented Barley GABA 90% – A Placebo-Controlled, Double-Blind, Randomized, Parallel-Group Comparative Trial-”. Nutrition and Food Toxicology 5.1 (2024): 01-21.
Copyright: © 2024 Dr. Eriko Uehara., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.




































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.