Review Article
Volume 1 Issue 2 - 2017
Common Cold of Dentistry: Dentine Hypersensitivity Revisited
1Dr. Pramod R, Associate Professor, Dentistry Program, Department of Oral Pathology, Batterjee Medical College, Jeddah, Saudi Arabia
2Dr. Mohan Kumar KP, Professor, Department of Oral Pathology and Microbiology, College of Dental Sciences, Davangere
3Dr. Suresh K V, Ast professor, Dept of Oral medicine and Radiology,SEGI University, Kualal lumpur, Malaysia.
4Dr. Fawaz Pullishery, Assistant Professor, Dentistry Program, Batterjee Medical College Jeddah, KSA
5Dr. Gautham Shetty, Private Practitioner, Mumbai
6Dr. Eby Varghese, Faculty of Dentistry Melaka Manipal Medical College Jalan Batu Hampar Bkit Baru, Melaka
2Dr. Mohan Kumar KP, Professor, Department of Oral Pathology and Microbiology, College of Dental Sciences, Davangere
3Dr. Suresh K V, Ast professor, Dept of Oral medicine and Radiology,SEGI University, Kualal lumpur, Malaysia.
4Dr. Fawaz Pullishery, Assistant Professor, Dentistry Program, Batterjee Medical College Jeddah, KSA
5Dr. Gautham Shetty, Private Practitioner, Mumbai
6Dr. Eby Varghese, Faculty of Dentistry Melaka Manipal Medical College Jalan Batu Hampar Bkit Baru, Melaka
*Corresponding Author: Dr. Pramod RC, Associate Professor, Dentistry Programme Department of Oral Pathology, Batterjee Medical
College, Jeddah, Saudi Arabia.
Received: June 05, 2017; Published: June 13, 2017
Abstract
Dentinal hypersensitivity (DH) is a common clinical condition usually associated with exposed dentinal surfaces. It can affect patients of any age group and most commonly affects the canines and premolars of both the arches. The management strategy of this dental problem requires a good understanding of the complexity of the condition and the variety of the treatment options. The objective of this review is to enhance the understanding among practitioners about DH to provide a brief overview of the epidemiology, mechanisms of pain production and aetiological factors for the condition in the hope of developing ideas for more realistic prevention and management strategies.
Keywords: Hypersensitivity; Hydrodynamic theory; Dentinal tubules
Introduction
Dentine hypersensitivity (DH) is ‘an enigma, being frequently encountered yet ill understood’. Strassler., et al. described the condition as the “common cold of dentistry ”. DH is one of the oldest recorded complaints of discomfort to humans. The pain responses induced by stimulation of exposed hypersensitive dentine will be towards the maximum level of any pain scale. [1] This condition may cause continuous discomfort to the patient during eating, drinking, brushing and sometimes even breathing throughout the day. Patients who suffer from DH expect their dentists to have an effective treatment. Indeed, if the treatment is not effective, the patients might question the professional competency of their dentists. [1,2] In spite of considerable amount of research over last fifty years, the clinical management of DH still remains empirical largely, partly due to ill-defined and poorly understood physiologic mechanism. Therapeutic intervention by desensitising agents may provide only partial pain relief and recurrence is common. [2]
Definition
According to Addy M, DH is an exaggerated response to non-noxious and noxious stimuli. [3] International Workshop on Dentine Hypersensitivity defines DH as a “short, sharp pain arising from exposed dentin in response to stimuli typically thermal, evaporative, tactile, osmotic or chemical and which cannot be ascribed to any other form of dental defect or pathology.” [4] (Holland., et al. 1997)
According to Addy M, DH is an exaggerated response to non-noxious and noxious stimuli. [3] International Workshop on Dentine Hypersensitivity defines DH as a “short, sharp pain arising from exposed dentin in response to stimuli typically thermal, evaporative, tactile, osmotic or chemical and which cannot be ascribed to any other form of dental defect or pathology.” [4] (Holland., et al. 1997)
According to the Canadian consensus document, DH has been defined as “pain derived from exposed dentin in response to chemical, thermal tactile or osmotic stimuli which cannot be explained as arising from any other dental defect or disease”. [5]
Epidemiology
Epidemiological studies have shown the wide range of prevalence of DH from 8% to 35% depending on the population studied and the methodology used to evaluate. The prevalence distribution and appearance of the disease have been reported differently in different studies, which is attributed to differences in populations, habits, dietaries, and methods of investigation. [6] The disease is prevalent in the patient with the age range of 20-50 years. However, it is more prevalent in the patient with the age range of 30-40 and more prevalent in females. Probably coinciding with their dental hygiene and dietary. [2,7] In some studies it has been reported that DH can occur at any age. [12]
Epidemiological studies have shown the wide range of prevalence of DH from 8% to 35% depending on the population studied and the methodology used to evaluate. The prevalence distribution and appearance of the disease have been reported differently in different studies, which is attributed to differences in populations, habits, dietaries, and methods of investigation. [6] The disease is prevalent in the patient with the age range of 20-50 years. However, it is more prevalent in the patient with the age range of 30-40 and more prevalent in females. Probably coinciding with their dental hygiene and dietary. [2,7] In some studies it has been reported that DH can occur at any age. [12]
Furthermore, the occurrence of DH in canines and premolars is more than other teeth. [2, 8-10] The buccal surface of the teeth has been reported to be more involved than other surfaces. [11] It has been suggested that with the lifespan of the general population increasing, and as a result of the continuing emphasis on preventive dentistry, more adults will retain their teeth into later life, which in turn could lead to increased incidence of exposed dentine surfaces and increased prevalence of DH. Also, as a result of higher frequency of acidic food and drink intake, the onset of the condition may occur earlier and in younger populations than previously reported. [2]
Etiology and Predisposing Factors
Dentine hypersensitivity has a multi-factorial etiology interaction between many factors including predisposing factors and stimuli, which can play an important role in establishing it. [13] The pain arising from dentine sensitivity is variable, ranging from mild discomfort to extreme severity. Gingival recession, abrasion, erosion and attrition are among the main predisposing factors whereas cold, as well as air stimuli, and dietary acid are considered to be important triggers. While tooth whitening, periodontal surgery and restorative treatment are some of the less common iatrogenic predisposing factors, touch and hot stimuli are regarded as occasional triggers. Gingival recession can occur in both healthy gingiva and periodontal disease–the former would be most often seen in buccal surfaces of dentine hypersensitivity patients who have overenthusiastic brushing habits. While the latter could be linked to hypersensitivity anywhere around the root in patients with periodontal disease and those who have undergone periodontal treatment. Two phases have been proposed to be involved in dentine hypersensitivity are loss of enamel or gingival recession causes dentine exposure, which should be followed up by opening of dentine tubules mainly through erosion and abrasion. [2,14]
Dentine hypersensitivity has a multi-factorial etiology interaction between many factors including predisposing factors and stimuli, which can play an important role in establishing it. [13] The pain arising from dentine sensitivity is variable, ranging from mild discomfort to extreme severity. Gingival recession, abrasion, erosion and attrition are among the main predisposing factors whereas cold, as well as air stimuli, and dietary acid are considered to be important triggers. While tooth whitening, periodontal surgery and restorative treatment are some of the less common iatrogenic predisposing factors, touch and hot stimuli are regarded as occasional triggers. Gingival recession can occur in both healthy gingiva and periodontal disease–the former would be most often seen in buccal surfaces of dentine hypersensitivity patients who have overenthusiastic brushing habits. While the latter could be linked to hypersensitivity anywhere around the root in patients with periodontal disease and those who have undergone periodontal treatment. Two phases have been proposed to be involved in dentine hypersensitivity are loss of enamel or gingival recession causes dentine exposure, which should be followed up by opening of dentine tubules mainly through erosion and abrasion. [2,14]
Biological Mechanism
The currently accepted mechanism for pain from dentine hypersensitivity is the hydrodynamic theory, proposed by Brannstorm. The theory has been proposed based on the movement of the fluid inside the dentinal tubules. The theory claims that tubules are open between dentine surface which is exposed to the environment and pulp. Whenever the exposed dentine comes into contact with a stimulus, there will be an increased fluid flow in the dentine tubules. This in turn causes an alteration in pressure across the dentine and excites a pressure-sensitive nerve receptor. Thereafter, activation of intradental nerves at the pulp-dentine border or within the dentine tubules transmits the stimulus evoking pain. This theory suggests that dentine tubules should be open at both the dentine surface and pulpal surface of the tooth to exhibit a response to the stimuli. Accordingly, the number of open tubules and their diameter are considered important factors in initiating pain from dentine hypersensitivity15. In other words, the higher the number and greater the diameter of the open dentine tubules the more intense will be the pain from dentine hypersensitivity. It has been postulated that triggers such as cold stimulate fluid to flow away from the pulp creating more rapid and rigorous neural responses than stimuli like heat, which cause somewhat sluggish fluid flow towards the pulp. This is in line with the observation that dentine hypersensitivity patients more frequently complain of pain in response to cold stimuli than heat. Cooling, drying, evaporation, and hypertonic chemical stimuli cause the dentinal fluid to flow away from the dentin-pulp complex and lead to an increase in pain. [13, 15-17]
The currently accepted mechanism for pain from dentine hypersensitivity is the hydrodynamic theory, proposed by Brannstorm. The theory has been proposed based on the movement of the fluid inside the dentinal tubules. The theory claims that tubules are open between dentine surface which is exposed to the environment and pulp. Whenever the exposed dentine comes into contact with a stimulus, there will be an increased fluid flow in the dentine tubules. This in turn causes an alteration in pressure across the dentine and excites a pressure-sensitive nerve receptor. Thereafter, activation of intradental nerves at the pulp-dentine border or within the dentine tubules transmits the stimulus evoking pain. This theory suggests that dentine tubules should be open at both the dentine surface and pulpal surface of the tooth to exhibit a response to the stimuli. Accordingly, the number of open tubules and their diameter are considered important factors in initiating pain from dentine hypersensitivity15. In other words, the higher the number and greater the diameter of the open dentine tubules the more intense will be the pain from dentine hypersensitivity. It has been postulated that triggers such as cold stimulate fluid to flow away from the pulp creating more rapid and rigorous neural responses than stimuli like heat, which cause somewhat sluggish fluid flow towards the pulp. This is in line with the observation that dentine hypersensitivity patients more frequently complain of pain in response to cold stimuli than heat. Cooling, drying, evaporation, and hypertonic chemical stimuli cause the dentinal fluid to flow away from the dentin-pulp complex and lead to an increase in pain. [13, 15-17]
Clinical Features
Most patients describe the pain arising from DH as being rapid in onset, sharp in character and of short duration. The external stimuli eliciting dentinal pain can be thermal, osmotic, chemical, physical or mechanical in nature. The thermal stimuli include hot and cold food and beverages and warm or cold blasts of air entering the oral cavity. Osmotic stimuli include sweet food and beverages. Acid stimuli include grapefruit, lemon, acid beverages and medicines. Common mechanical stimuli are toothbrushes and dental instruments. The use of cold air blasts from a dental air syringe, cold water, and suction from a dental aspirator tip may also cause discomfort. More rapid response to stimuli or the persistence of pain after removal of the stimuli has been ascribed to inflammatory changes in the pulp. It is interesting to note that a considerable variation in the intensity of the pain symptoms over time has been reported in few patients. Sometimes the pain symptoms may be more or less persistent, extremely intense and continue for years. [2, 13,16,18]
Most patients describe the pain arising from DH as being rapid in onset, sharp in character and of short duration. The external stimuli eliciting dentinal pain can be thermal, osmotic, chemical, physical or mechanical in nature. The thermal stimuli include hot and cold food and beverages and warm or cold blasts of air entering the oral cavity. Osmotic stimuli include sweet food and beverages. Acid stimuli include grapefruit, lemon, acid beverages and medicines. Common mechanical stimuli are toothbrushes and dental instruments. The use of cold air blasts from a dental air syringe, cold water, and suction from a dental aspirator tip may also cause discomfort. More rapid response to stimuli or the persistence of pain after removal of the stimuli has been ascribed to inflammatory changes in the pulp. It is interesting to note that a considerable variation in the intensity of the pain symptoms over time has been reported in few patients. Sometimes the pain symptoms may be more or less persistent, extremely intense and continue for years. [2, 13,16,18]
Diagnosis
The diagnosis of dentine hypersensitivity should be based on detailed history taking and clinical examination. The most commonly used diagnostic tools are blasting air or water using an air-water syringe and scratching the tooth surface with a sharp dental explorer. [19] Air blast, which includes both thermal and evaporative elements of stimuli, may simulate a real-life situation experienced by a dentine hypersensitivity patient rather than probing with a dental explorer. Whereas thermal stimulation would be considered more effective than tactile in detecting dentine hypersensitivity. It is very important to consider a differential diagnosis to exclude other conditions given in box 1 which would mimic hypersensitivity. It would be also relevant to take a detailed dietary history and information on oral hygiene practices including tooth brushing technique, type of brush, frequency, duration and timing of brushing as well as frequency of toothbrush change and appearance of brush at change. A comprehensive oral examination, sometimes, coupled with a radiographic investigation may seldom be necessary to confirm the diagnosis. [5, 18-19]
The diagnosis of dentine hypersensitivity should be based on detailed history taking and clinical examination. The most commonly used diagnostic tools are blasting air or water using an air-water syringe and scratching the tooth surface with a sharp dental explorer. [19] Air blast, which includes both thermal and evaporative elements of stimuli, may simulate a real-life situation experienced by a dentine hypersensitivity patient rather than probing with a dental explorer. Whereas thermal stimulation would be considered more effective than tactile in detecting dentine hypersensitivity. It is very important to consider a differential diagnosis to exclude other conditions given in box 1 which would mimic hypersensitivity. It would be also relevant to take a detailed dietary history and information on oral hygiene practices including tooth brushing technique, type of brush, frequency, duration and timing of brushing as well as frequency of toothbrush change and appearance of brush at change. A comprehensive oral examination, sometimes, coupled with a radiographic investigation may seldom be necessary to confirm the diagnosis. [5, 18-19]
| • Cracked tooth syndrome • Fractured restorations • Chipped teeth • Marginal leakage • Post-restorative sensitivity • enamel defects, •improperly insulated metallic restorations, |
• Dental caries • Gingival inflammation • Palatogingival grooves • Pulpitis • Vital bleaching •congenitally open CEJ, •occlusal traumatism. |
Box 1: Conditions to be excluded in the diagnosis of dentine hypersensitivity [3,20].
| Suggestions for Dental Professionals • Avoid over-instrumenting the root surfaces during scaling and root planing, particularly in the cervical area of the tooth • Avoid over-polishing exposed dentine during stain removal • Avoid violating the biological width during restoration placement, as this may cause recession • Avoid burning the gingival tissues during in-office bleaching, and advise patients to be careful when using home bleaching products. |
Suggestions for Patients • Avoid using large amounts of dentifrice or reapplying it during brushing • Avoid medium- or hard-bristle toothbrushes • Avoid brushing teeth immediately after ingesting acidic foods •Avoid brushing teeth with excessive pressure or for an extended period of time •Avoid excessive flossing or improper use of other interproximal cleaning devices •Avoid “picking” or scratching at the gum line or using toothpicks inappropriately |
Box 2: Recommended actions for preventing dentine hypersensitivity [2] (Adapted from Drisko, 2002).
Classification of Desensitizing Agents
In-office treatment
- Mode of administration
In-office treatment
- On the basis of mechanism of action
- Potassium nitrate
- Gluteraldehyde
- Silver nitrate
- Zinc chloride
- Strontium chloride hexahydrate
- Sodium fluoride
- Stannous fluoride
- Strontium chloride
- Potassium oxalate
- Calcium phosphate
- Calcium carbonate
- Bio active glasses (SiO2–P2O5–CaO–Na2O)
- Fluoride varnishes
- Oxalic acid and resin
- Glass ionomer cements
- Composites
- Dentin bonding agents
- Neodymium:yttrium aluminum garnet (Nd-YAG) laser
- GaAlAs (galium-aluminium-arsenide laser)
- Erbium-YAG laser
- Propolis
Management Strategy
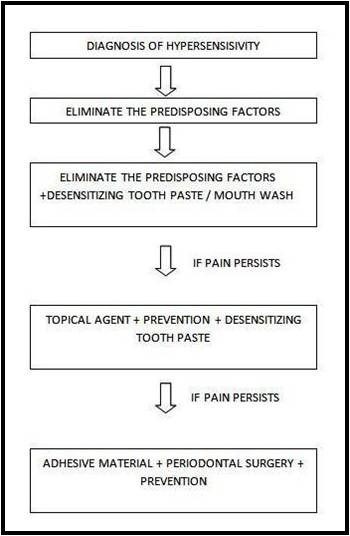
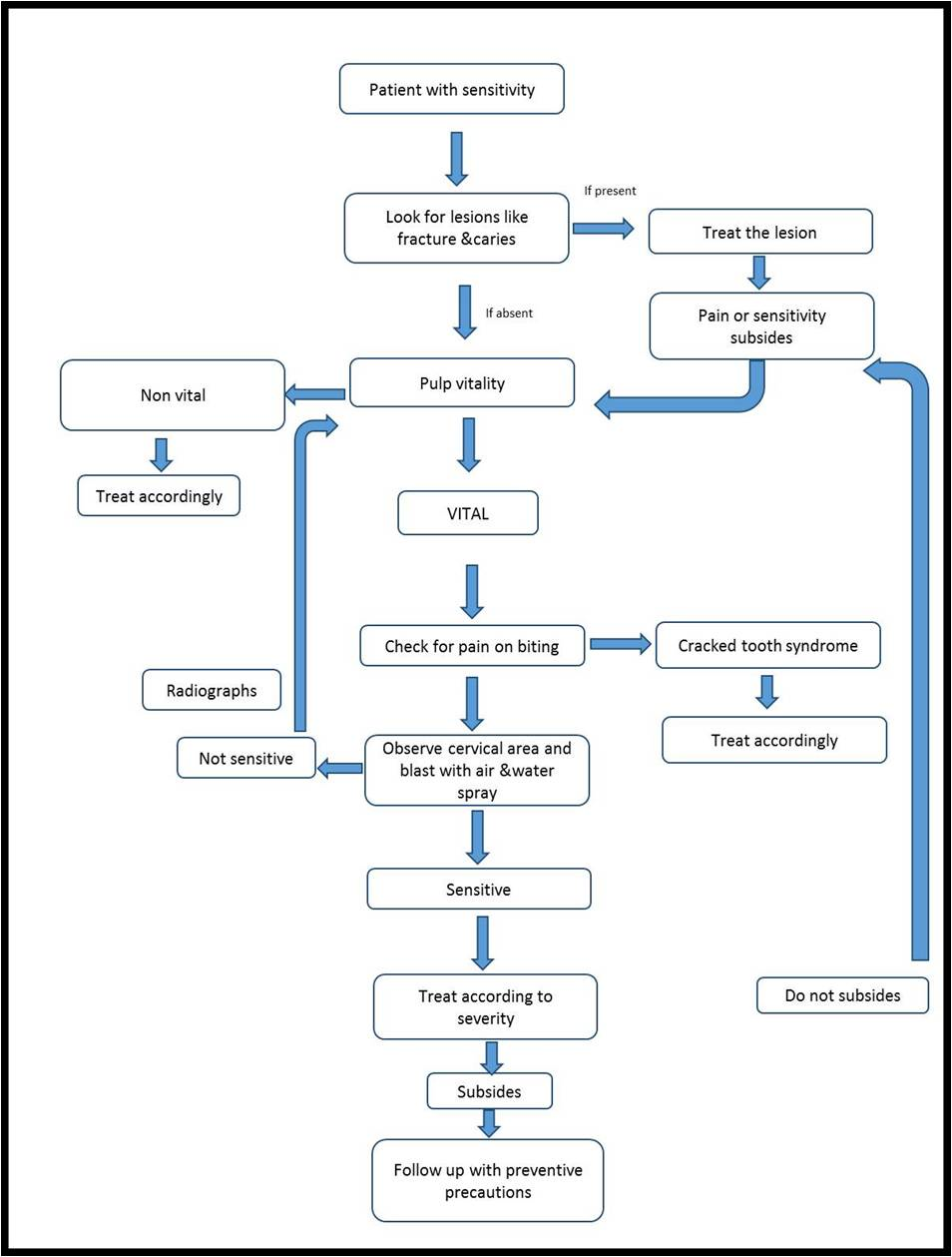
Take a detailed clinical and dietary history. Differentially diagnose the condition from other dental pain conditions should be evaluated. On confirmation etiological and predisposing factors needs to be identified and addressed. In case of mild-to-moderate sensitivity, at-home desensitizing therapy is advised. If there is no relief or in case of severe sensitivity, initiation of in-office treatment. In extreme cases, if patient does not respond to the therapy and there are individual teeth exhibiting the symptoms, then endodontic therapy can be initiated. A regular review should be made with an emphasis on prevention of the condition. [8,9,19,21] [Figure 1 and 2]
Take a detailed clinical and dietary history. Differentially diagnose the condition from other dental pain conditions should be evaluated. On confirmation etiological and predisposing factors needs to be identified and addressed. In case of mild-to-moderate sensitivity, at-home desensitizing therapy is advised. If there is no relief or in case of severe sensitivity, initiation of in-office treatment. In extreme cases, if patient does not respond to the therapy and there are individual teeth exhibiting the symptoms, then endodontic therapy can be initiated. A regular review should be made with an emphasis on prevention of the condition. [8,9,19,21] [Figure 1 and 2]
Conclusion
Dentine hypersensitivity can be a particularly uncomfortable and unpleasant sensation affecting the quality of life of patients significantly. For few, DH may represent only a minor inconvenience but for many the degree of discomfort and emotional anguish can be overwhelming. In addition, the distress caused may lead to change in diet with the condition dictating types of foods and drinks ingested. The ultimate goal in the treatment of dentin hypersensitivity is the immediate and permanent alleviation of pain with restoration of the original impermeability of the dentinal tubules.
The ideal desensitizing agent is yet to identified. Conclusive evidence of successful treatment regimen with 100% efficacy remains elusive. Despite a multitude of products available for treatment, no product represents the “gold standard” in the treatment of DH. Active management usually involves a combination of at-home and in-office therapies. Further research is needed, to permit the development of evidence-based guidelines for the treatment of DH so that clinicians may offer their patients treatments based on best evidence. Until such evidence is available, it seems prudent to employ methods of therapy that are effective, provide long-term relief and are least likely to cause harm.
References
- Strassler HE., et al. “Dentin hypersensitivity: Its inter-relationship to gingival recession and acid erosion”. Compendium of Continuing Education in Dentistry 29.5 (2008): 1-9.
- Davari A., et al. “Dentin hypersensitivity: etiology, diagnosis and treatment; a literature review”. Journal of Dentistry, Shiraz University of Medical Sciences 14.3 (2013):136-145.
- Addy M. “Dentine hypersensitivity: New perspectives on an old problem”. International Dental Journal 52 (2002):367-375.
- Holland GR., et al. “Guidelines for the design and conduct of clinical trials on dentine hypersensitivity”. Journal of Clinical Periodontology 24.11 (1997): 808-813.
- Canadian Advisory Board on Dentin Hypersensitivity. “Consensus-Based Recommendations for the Diagnosis and Management of Dentin Hypersensitivity”. Journal Canadian Dental Association 69.4 (2003): 221-226.
- American Academy of Periodontology. “Glossary of periodontal Terms”. Chicago: (2001’).
- Miglani S., et al. “Dentin hypersensitivity: Recent trends in management”. Journal of Conservative Dentistry 13.4 (2010): 218-224.
- Que K., et al. “A crosssectional study: non-carious cervical lesions, cervical dentine hypersensitivity and related risk factors”. Journal of Oral Rehabilitation 40.1 (2013): 24-32.
- Cummins D. “Recent advances in dentin hypersensitivity: clinically proven treatments for instant and lasting sensitivity relief”. American Journal of Dentistry (2010): No A: 3A- 13A.
- Cummins D. “Dentin hypersensitivity: from diagnosis to a breakthrough therapy for everyday sensitivity relief”. The Journal of Clinical Dentistry 20.1 (2009) 1-9.
- Gillam DG., et al. “Dentists' perceptions of dentine hypersensitivity and knowledge of its treatment”. Journal of Oral Rehabilitation 29.3 (2002): 219-225.
- Addy M. “Dentine hypersensitivity: Definition, prevalence, distribution and etiology. In: Addy M, Embery G, Edgar WM, Orchardson R, eds. Tooth wear and sensitivity: Clinical advances in restorative dentistry”. London: Martin Dunitz; (2000): 239-248.
- Chabanski MB and Gillam DG. “Aetiology, prevalence and clinical features of cervical dentine sensitivity”. Journal of Oral Rehabilitation 24.1 (1997): 15-19.
- Drisko CH. “Dentine hypersensitivity – dental hygiene and periodontal considerations”. International Dental Journal 52 (2002): 385-393.
- Addy M. “Etiology and clinical implications of dentine hypersensitivity”. Dental Clinics of North America 34.3 (1990): 503-514.
- Swift EJ. “Causes, prevention and treatment of dentin hypersensitivity”. Compendium of Continuing Education in Dentistry 25.2 (2004): 95-109.
- Dowell P and Addy M. “Dentine hypersensitivity – a review: aetiology, symptoms and theories of pain production”. Journal of Clinical Periodontology 10.4 (1983): 341-350.
- Walters PA. “Dentinal hypersensitivity: A review”. The Journal of Contemporary Dental Practice 6 (2005): 107-117.
- Que K., et al. “A multi-centre and cross-sectional study of dentine hypersensitivity in China”. Journal of Clinical Periodontology 37.7 (2010): 631-637.
- Bartold PM. “Dentinal hypersensitivity: a review”. Australian Dental Journal 51 (2006): 212-218.
- West NX. “The dentine hypersensitivity patient – a total management package”. International Dental Journal 57. S6 (2007): 411-419.
Citation:
Dr. Pramod RC., et al. “Common Cold of Dentistry: Dentine Hypersensitivity Revisited”. Oral Health and Dentistry 1.2 (2017):
129-135.
Copyright: © 2017 Dr. Pramod RC., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.



































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.