Review Article
Volume 3 Issue 6 - 2019
Azadirachta indica A. Juss. (Meliaceae): it’s use Against Dental Diseases
1Department of Biotechnology & Genetic Engineering, University of Development Alternative, Lalmatia, Dhaka-1207, Bangladesh
2Department of Pharmacology, University of Cambridge, Tennis Court Road, CB2 1PD, UK
3Department of Biochemistry & Molecular Biophysics, Kansas State University, Manhattan, KS 66506-3702, USA
4Department of Pharmacy, University of Development Alternative, Lalmatia, Dhaka-1207, Bangladesh
2Department of Pharmacology, University of Cambridge, Tennis Court Road, CB2 1PD, UK
3Department of Biochemistry & Molecular Biophysics, Kansas State University, Manhattan, KS 66506-3702, USA
4Department of Pharmacy, University of Development Alternative, Lalmatia, Dhaka-1207, Bangladesh
*Corresponding Author: Mohammed Rahmatullah, Dean, Faculty of Life Sciences, University of Development Alternative, Lalmatia, Dhaka-1207, Bangladesh.
Received: July 16, 2019; Published: July 25, 2019
Abstract
Azadirachta indica A. Juss. Is a plant of the Meliaceae family and is commonly known as “neem” in both Bengali and English. Several parts of the plant are commonly used against dental diseases in India and Bangladesh. Many people in these two countries believe that regular brushing of teeth and gum with neem bark or stem or leaf or seed powder can prevent almost all dental diseases. Globally, approximately 60-90% of school children are infected by dental carries, 5-20% of adult peoples are living with edentulousness. The prevalence of orodental trauma is increased by 16-40% among 6 years old children and 4-33% among teenagers. As such, a readily available and affordable herbal substitute can be of great benefit to the particularly poorer sections of the population throughout the world. This review explores the ethnic uses and phytochemicals present in the tree along with anti-microbial and other relevant pharmacological activities, which justify more scientific research and use of the tree and its various parts against dental diseases.
Introduction
Azadirachta indica A. Juss. is a plant belonging to the Meliaceae family, commonly known as neem in both Bengali and English, and very familiar to tropical and subtropical countries. Neem is important both in various traditional medicines and as home remedies. The plant has been described in Ayurveda for its benefits against different diseases [1]. For over centuries people of India have been using neem twigs as miswak or datum (used for brushing teeth and gums), and neem leaves for skin diseases. Neem leaves are placed in bed, books, cupboard, and closets as insect repellent [2]. It is believed by the people of India and Bangladesh that if there is a neem tree in the yard of a home, there will be no diseases. They believe that the air that flows through the neem tree promotes good health.
Neem is called different names in different cultures - in Sanskrit it is known as ‘aresta’ meaning perfect and imperishable [3]; other names are margosa, Indian lilac, Persian lilac (English), limba or dhanuj hada (Gujarati), vembu (Tamil), vepa (Telegu), neem (Urdu) [4], intaran, imba, mempheuh, mimba, membha (Indonesian), nim, neem (Arabic), bowtamaka, thinboro, tamarkha, tamaka, tamabin (Burmese), margousier, azadirac de l’Inde, margosier (French), and sadu, mambu, baypay, veppam (Malay) [5].
Maintaining oral health is a big goal in developing and developed countries including the under-privileged. According to the World Health Organization (WHO), oral diseases are the most common non-transmissible diseases which may cause lifetime discomfort and even death. An evaluation done by The Global Burden of Disease Study 2016 suggested that 3.58 billion people (half of the world population) were suffering from tooth decay; severe periodontal disease was estimated as the eleventh most extensive disease [6].
Dental diseases occur all over the world. The occurence of dental carries, periodontal diseases, orodental trauma, and dental erosion are high. Globally, approximately 60-90% of school children are infected by dental carries, 5-20% of adult peoples are living with edentulousness; the prevalence of orodental trauma is increased by 16-40% among 6 years old children and 4-33% among teenagers [7].
Besides allopathic treatment other therapies have been practiced around the world to prevent and cure dental disorders. In traditional medicine various plants and plants parts have been used against tooth diseases. Neem is used to treat or prevent various dental disorders. Neem twigs, bark, leaf paste are the common parts that are used to treat tooth diseases [8, 9]. The various parts of neem have been seen in recent studies to contain phytochemicals with anti-microbial activity against tooth disorder(s) causing microorganisms. The objective of this review is to analyze the traditional uses of neem tree parts as a tooth and gum cleaning agent and for preventing dental disorders in light of modern knowledge of the phytochemicals and relevant pharmacological activities of the plant and plant parts.
Ethnomedicinal use
Ethnomedicinal uses are those longstanding practices that have been in general orally transmitted by indigenous people or a certain community of a country. In this context, neem is is known as a wonder or magic tree for dental treatment. Most of the plant parts including stems, twigs, leaves, flowers, fruits, seeds, barks and even latex are used for dental care. Among the Indian population, young twigs are used as tooth brushes [10]. Flowers of the plant are used for reducing swelling or bleeding of gums [11]. Tender twigs are used for relief of toothaches [12].
As an example, among the Bodo-Hajong community of Assam, India, young twigs and stems are popular for treating tooth infection and blood coming from clean teeth [13]. Tooth brushes made from the bark of the neem tree is helpful for maintaining optimum health of teeth. Neem powder obtained from crushed dry leaves of neem is used as a tooth powder for cleaning teeth [14]. Cleaning teeth with neem twigs gives a fresh sweet breath [15]. In the Sembiran village of India people also use this plant for dental care. In some rural areas of India, people chew neem leaves and seeds after meal as a traditional practice for dental care [16].
Toothpaste made from neem is a common tooth cleaner in India and European countries [17]. Villagers of several districts of Tamil Nadu, India, use the soft twigs as toothbrush ad leaves are used for pyorrhea [18]. Folk medicine practitioners in the Kurigram district of Bangladesh use the neem plant for various dental diseases, including bleeding from gums and gum swelling and also for fresh breath. For treatment, the roots of Areca catechu and the bark of Azadirachta indica are boiled together in water. Gargling with this water 2-3 times daily for 3 consecutive days is suggested [19].
Phytochemicals
Bioactivity of neem is presumed to be due to phytochemicals, often secondary metabolites. The various parts of the tree (leaves, twigs, seeds, flowers and roots) contain different mixtures of chemical compounds. Numerous studies of these mixtures and of individual compounds have been reported. Major constituents were isolated from aqueous and ethanolic extract of leaves of neem tree. Alkaloids, cardiac glycosides, polysaccharides, phytosterols, flavonoids, phenols, resins, saponins, tannins, terpenes and steroids have been reported to be present in aqueous, ethanolic and methanolic extracts [20-23].
Bioactivity of neem is presumed to be due to phytochemicals, often secondary metabolites. The various parts of the tree (leaves, twigs, seeds, flowers and roots) contain different mixtures of chemical compounds. Numerous studies of these mixtures and of individual compounds have been reported. Major constituents were isolated from aqueous and ethanolic extract of leaves of neem tree. Alkaloids, cardiac glycosides, polysaccharides, phytosterols, flavonoids, phenols, resins, saponins, tannins, terpenes and steroids have been reported to be present in aqueous, ethanolic and methanolic extracts [20-23].
HPLC analysis of the ethanolic extract of neem leaves confirmed the presence of b-sitosterol, lupeol, rutin, ellagic acid, quercetin, and ferulic acid [24]. Neem leaves are also known to be rich in some phytochemicals like nimbidin, nimbanene, 6-desacetylnimbinene, nimbadiol, nimbolide, ascorbic acid, n-hexacosanol and amino acids, 7-desacetyl-7-benzoylazadiradione, 7-desacetyl-7-benzoylgedunin, 17-hydroxyazadiradione and nimbiol [25]. Perhaps the best known and most studied secondary metabolite of neem is azadirachtin, a limonoid.
Siddiqui and colleagues have worked extensively on various parts parts of the neem tree and isolated several phytochemicals that appear to be at least partially responsible for therapeutic effects. From fruits they found a new triterpenoid azadirachtol, which has eight carbon side chains with an oxygenated ring system [26]. Fresh and uncrushed twigs yielded one new and three unpublished isocoumarins along with two unreported coumarins. Eight other compounds (saturated hydrocarbons) were also isolated from the petroleum ether extract of fresh neem leaves [27]. Neem bark was found to contain tricyclic diterpenes, which were named margosone and margosolone. Their structures were determined as 12, 13-dihydroxy-14-isoporpylpodocarpa-8, 11, 13-trien-7-one and 13-hydroxy-12-methoxypodocarpa-8, 11, 13-trien-7-one, respectively [28]. Naheedin, a new protolimonoid was identified from neem fruit. Seven known tetranortriterpenoids - azadirone, epoxyazadiradione, nimbin, gedunin, azadiradione, deacetylnimbin, and 17-hydroxyazadiradione were isolated along with a new limonoid named mahmoodin from neem oil [29]. Another investigation on fresh fruit skin gave three new triterpenoids named azadironolide [24,25,26,27-tetranorapoeupha-7a-acetoxy-23xi-hydroxy-21,23-epoxy-1,14, 20(22)-trien-3,21-dione], isoazadironolide [24,25,26,27-tetranorapoeupha-7a-acetoxy-21xi-hydroxy-21, 23-epoxy-1,14, 20(22)-trien-3, 23-dione], and azadiradionolide [24,25,26,27-tetranorapoeupha-7a-acetoxy-21, 23-epoxy-1,14,20(22)-trien-3, 16,21-trione] [30].
The same group reported that methanolic extract of neem leaves contain two newly found compound named meliacinol [24, 25, 26, 27-tetranorapotirucalla-(apoeupha)-1a-trimethylacryloxy-21, 23-6a, 28-diepoxy-16-oxo-17-oxa-14,20,22-trien-3a,7a-diol] and 6a-O-acetyl-7-deacetylnimocinol [24, 25, 26, 27-tetra-norapotirucalla-(apoeupha)-6a-acetoxy-7a-hydroxy-1, 14, 20, 22-tetraen-21, 23-epoxy-3-one] [31]. Among three compounds isolated from isolated from methanolic extract of leaves were newly found triterpenoids, namely 22, 23-dihydronimocinol and desfurano-6a-hydroxyazadiadione; along with meliacin7a-senecioyl-(7-deacetyl)-23-O-methylnimocinolide [32].
Another new tetracyclic triterpenoid was isolated from the methanolic extract of fresh leaves, meliatetraolenone, which has a structure of [24,25,26,27-tetranor-apotirucalla-(apoeupha)-6a-O-methyl,7a-senecioyl(7-deacetyl)-11a, 12a, 21, 23-tetrahydroxy-21, 23-epoxy-2, 14, 20 (22)-trien-1, 16-dione] along with the known compound odoratone [33]. From the pericarp of fresh fruit skin extract three aromatics 2,6-bis-(1,1-dimethylethyl)-4-methyl phenol, 2-(phenylmethylene)-octanal, 1,2,4-trimethoxy-5-(1Z-propenyl)-benzene; three benzopyranoids 3, 4-dihydro-4, 4, 5, 8-tetramethylcoumarin, 3, 4-dihydro-4, 4, 7,8-tetramethylcoumarin-6-ol, 1,3,4,6,7,8-hexahydro-4,6,6,7,8,8-hexamethyl-cyclopenta[g]-2-benzopyran; one sesquiterpene - methyl-3, 7, 11-trimethyl-2E, 6E,10-dodecatrienoate; one ester of fatty acid methyl 14-methyl-pentadecanoate, and one monoterpene 3,7-dimethyl-1-octen-7-ol were isolated for the first time. Along with these compounds sixteen n-alkanes (n-Pentadecane, n-Hexadecane, n-Heptadecane, n-Octadecane, n-Nonadecane, n-Eicosane, n-Heneicosane, n-Docosane, n-Tricosane, n-Tetracosane, n-Pentacosane, n-Hexacosane, n-Heptacosane, n-Octacosane, n-Nonacosane, n-Hentriacontane), two esters of fatty acids ethyl hexadecanoate, and ethyl 9Z-octadecenoate were identified from the fruit skin [34].
Siddiqui and his group further isolated three new tetracyclic triterpenoids from the methanolic extract of leaves; their structures were established as zafaral [24, 25, 26, 27-tetranorapotirucalla-(apoeupha)-6-methoxy-7a-acetoxy-1,14-dien-3,16-dione-21-al] and meliacinanhydride [24,25,26,27-tetranorapotirucalla-(apoeupha)-6a-hydroxy, 11a-methoxy-7a,12a-diacetoxy,1,14,20 (22)-trien-3-one] and zeeshanol [25,26,27-trinor-apotirucalla-(apoeupha)-6a-,21-dihydroxy,7a -acetoxy, 1,14,22-tri-en-3, 16-dione] [35, 36]. A new flavononeazharone (5,7,4'-trihydroxy-3'-(3''-methyl-2'',3''-epoxybutyl)flavan-4-one along with two known compound azadirone and isoazadironolide were isolated from the flowers of neem [37]. From the essential oil of fresh flowers, two newly found sesquiterpenes were isolated named germacrene B and a-himachalene. In this study Siddiqui and his group found 5 sesquiterpenes, 3 aromatics, 17 fatty acids, 5 fatty acid esters, 3 steroids and 8 hydrocarbons including two mentioned sesquiterpenes from different part of the plant [38]. Azadirachtin belongs to the C-secolimonoids group of tetranortriterpenes. The compound has several analogues, which are called “azadirachtin X”, where X is a letter from the first half of the English alphabet.
The most abundant of these in neem are azadirachtin A and azadirachtin E. Two are considered as novel compounds, namely azadirachtin M (29-oxymethylene-11-demetoxycarbonyl-11α-hydroxyazadirachtin), and azadirachtin N (22,23-dihydro-23α-hydroxy-3-tigloyl-11-deoxyazadirachtinin). Neem oil made from different parts of the tree comprises phytochemicals like meliacin, gedunin, nimbidin, nimbolides, nimbin, salanin, valassin etc. Neem seed contains tignic acid [39, 40]. Neem bark contains tannin substances - gallic acid, gallocatechin, epicatechin, catechin, epigallocatechin and catechin of which three tricyclic diterpenoids, margolone, margolonone and isomargolonone have been isolated from stem bark. Steroid compounds such as campesterol, sigmasterol have been also isolated from neem oil [41]. Neem bark contains polysaccharides GIa, polysaccharides GIIa, polysaccharides GIIIa, and NB-II peptidoglycan [42]. The structures of some important phytochemicals are shown in Figure 1.
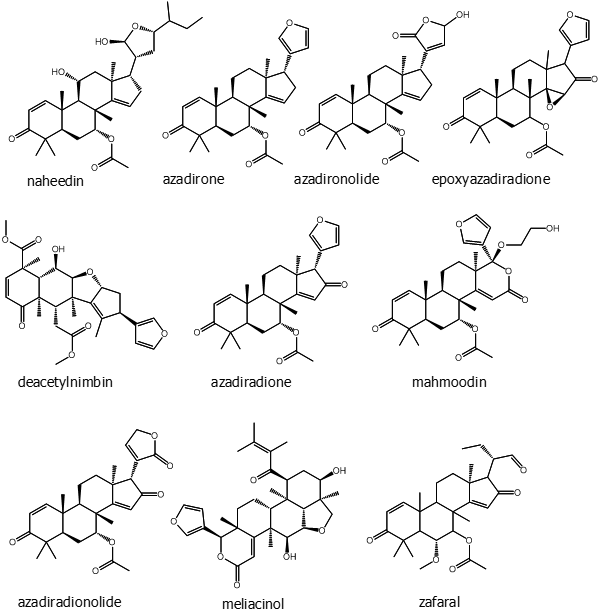
Figure 1: (Continued). Some selected phytochemicals isolated from various parts of Azadirachta indica.
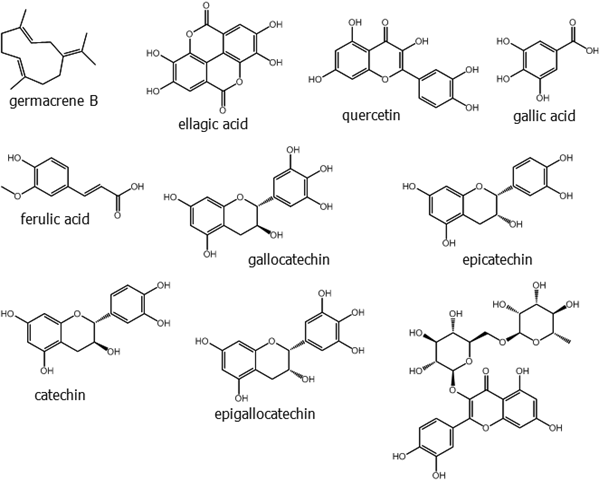
Figure 1: (Continued). Some selected phytochemicals isolated from various parts of Azadirachta indica.
Therapeutic uses against dental diseases
Neem extracts have been shown to be effective in the treatment of dental caries. A study conducted with neem gel extract showed that it significantly reduced the plaque formation over the control group where the control group used common mouthwash containing the germicide chlorhexidinegluconate. The study also showed that the growth of the bacterium responsible for tooth decay Streptococcus mutans was inhibited by the extract [15].
Neem extracts have been shown to be effective in the treatment of dental caries. A study conducted with neem gel extract showed that it significantly reduced the plaque formation over the control group where the control group used common mouthwash containing the germicide chlorhexidinegluconate. The study also showed that the growth of the bacterium responsible for tooth decay Streptococcus mutans was inhibited by the extract [15].
Microbes such as Candida albicans and Enterococcus faecalis are the common agents of infected root canals. They cause failure of treatment and thus eradication of these microbes is a major step in endodontic treatment. In a comparative study it has been shown that ethanolic extract of neem leaves significantly increased the zone of inhibition of E. faecalis compared to 2% NaOCl. On the other hand the result for C. albicans was not significant [43]. A study with neem leaf extract, grape seed and 3% sodium hypochloride was performed on a culture of E. faecalis in brain heart infusion media and broth. The result showed that the zone of inhibition obtained with neem extract was higher than the other two treatments [44]. Hedge and others assessed the antimicrobial activity of neem along with propolis, turmeric, liquorice and NaOCl against E. faecalis and C. albicans. Zone of inhibition of neem extract was 21.33 mm for E. faecalis and 15.33 mm for C. albicans. Other treatments were not as effective as neem [45].
Ethanolic extract of fresh neem leaves application to infected root canal showed significant level of inhibition of the microbe C. albicans compared to application of 2% Chlorhexidine gluconate (CHX) [46]. An evaluation for the treatment of endodontics against E. faecalis was done by Chandrappa ad others. They reported that neem along with tulsi (Ocimum sanctum) extract and chlorhexidine had significant potency of inhibiting the growth of the microbe [47]. An investigation with neem, Aloe vera extract, 3% NaOCl, and 2% CHX (chlorhexidine) against E. faecalis and C. albicans was done by Prasad and others. The study was performed by dividing the experiment into two groups comprising two different controls 3% NaOCl, and 2% CHX and both in the first and second group neem extract showed better inhibitory results than the control [48]. Damre and others in 2015 found significant antimicrobial efficacy of neem leaf extract against E. faecalis and C. albicans responsible for root canal infection [49]. It is not clear from these studies which compounds from neem contribute to the growth inhibition of the microbes.
Chewing twigs of neem plant is effective in removing dental plaque because of the fibrous nature of the twigs. Bacteria growing in the early stage of plaque formation can be reduced by twigs as they contain gallotannins [50]. Chewing twigs also increases salivary secretion which works as an antibacterial. Colony forming of periodontopathogens such as Porphyromons gingivalis can be reduced by using neem sticks, and neem gels and mouthwashes also have been found to improve clinical parameters associated with gingivitis [51].
Growth of Streptococcus mutans, a causative factor for dental carious lesion can be inhibited by using neem mouthwash and it reverses the developing of carious lesion. Neem bark extract can prevent bacterial growth [52, 53]. An investigation of in vitro plaque formation was done to evaluate the inhibiting effect of aqueous extract of neem sticks. Adhesion of Streptococcus sanguis to saliva-conditioned hydroxyapatite (a major component and an essential ingredient of normal bone and teeth, which is made of calcium phosphate, calcium carbonate, calcium fluoride, calcium hydroxide and citrate) was significantly inhibited when pre-treated with an aqueous extract of neem stick [54]. A study done by Pai and others demonstrated the anti-plaque activity of neem extract gel. Males (20-30 years old) were examined for over a period of six weeks using a commercially available dental gel chlorhexidine gluconate (0.2% w/v) as positive control. The study showed that dental gel made of neem extract significantly lessened the plaque formation [55].
Salam and others evaluated the antibacterial efficacy of neem leaf extract by collecting saliva sample from 50 patients who were suffering from dental caries and gingivitis. Saliva collected from the patients contained Streptococcus mutans, Enterococcus faecalis, Pseudomonas aeruginosa and Lactobacillus sp. Methanolic neem leaf extract showed larger area of zone of inhibition against Lactobacillus sp. (25.6 mm) followed by Streptococcus mutans (25 mm) and Pseudomonas aeruginosa (24.3 mm)at 1000 μg with Enterococcus faecalis (14.4 mm) being the lowest [56]. Three bacteria responsible for causing dental carries Streptococcus mutans, Streptococcus salivarious and Fusobacterium nucleatum were found to be sensitive to neem leaf extract. Neem leaf was extracted with petroleum ether, chloroform, ether and distilled water. Among these the petroleum ether extract and the chloroform extract exhibited highest zone of inhibition against S. mutans. The highest zone of inhibition for S. salivarious and F. nucleatum was found with chloroform extract and ethanol-aqueous extract, respectively [57].
Aspergillus flavus is one of the aspergillosis (an illness resulting from Aspergillus usually affecting respiratory system) causing species of fungus. In people treated for root canal when undergoing over extension of their root canal, the fungus finds a way to invade [58]. A study by Shrivastava and Swarnkar showed that methanolic extract of neem leaves had the highest area zone of inhibition against Aspergillus flavus. The study worked with other species of fungus and also an ethanolic extract of neem leaves was used. But the significant result came out from methanolic extract [59]. Maina and others performed an experiment to evaluate antibacterial effect of ethanol and aqueous extract of neem twigs against some bacteria that cause tooth root canal such as Enterococcus faecalis, Streptococcus mutans, Staphylococcus aureus, Fusobacterium nucleatum, Lactobacillus acidophilus, and Candida albicans. The highest inhibitory effect was found against S. aureus (100%) with 50% w/v aqueous extract, then C. albicans (84.6%) and L. acidophilus (80.9%) at 24 hour incubation. Incubation with an ethanol extract inhibited E. faecalis, S. mutans and F. nucleatum by 96.2%, 81.5% and 63.9%, respectively and aqueous extract inhibited by 85.5%, 91.7% and 62.2% for E. faecalis, S. aureus and C. albicans,respectively at 48 hour incubation [60].
Tannins are one of the major phytochemicals of neem; they work as an astringent and give a coating over the enamel which protects teeth from the adhesion and aggregation of bacteria [61]. Neem bark extract containing the active ingredient nimbidin is now frequently added to tooth paste, gel or other oral hygiene preparations. Gargling with nimbidin containing preparation can prevent from gum bleeding and pyorrhea, and it can heal the inflammation of gum as well [62]. Nimbolide was found effective in inhibiting S. aureus and S. coagulase [63]. A study conducted by Pandya and others showed that aqueous extract of neem sticks had higher antibacterial activity than babool (Acacia nilotica) against S. mutans. The highest zone of inhibition 15.6 mm was found by using neem extract at 5% concentration, which was considerably better than babool at 5.4 mm [64].
Evaluation of anti-plaque and anti-gingivitis of neem mouth rinse was done on patients with plaque induced gingivitis. The study was performed by dividing forty five patients into three groups where Group I, II and III were treated with 15 ml of neem mouth rinse, 15ml of chlorhexidine and 15ml of saline (as placebo), respectively, twice daily for 21 days. Neem mouth rinse gave result equal to that of chlorhexidine compared to placebo and also reduced gingival, bleeding and plaque formation [65].
Aqueous and acetone extract of neem leaves gave antibacterial effect against two oral bacteria Streptococcus mitis and S. viridians. Both the acetone and aqueous extracts showed inhibitory activity against Klebsiella pneumoniae [66]. An acetone extract of neem showed antibacterial activity against two pathogens Neisseria catarrhalis and S. salivarius [67]. Toothpaste produced from a neem extract was found to exhibit an anti-plaque property and it also worked against gingivitis. The study was conducted by Subraya and others [68]. Lakshmi and group also demonstrated an anti-plaque effect of neem leaf extract on fixed orthodontic appliance patients against some acedogenic bacteria. At concentrations of 250 μg/ml, 500 μg/ml, 1 mg/ml and 5 mg/ml Azadirachta indica leaf extract had bactericidal activity against S. mutans, S. mitis, S. sanguis and S. salivarius, respectively [69].
Latha and colleagues examined the effect of neem toothpaste (from bark extract) on deciduous teeth to see if it could lessen carious lesion. Twenty four deciduous teeth were taken and grouped into two; both were treated with acid resistant varnish leaving some exposed. A tooth paste base was applied to group I (control) and group II was treated with neem paste. Both groups were stored in moist environment for 24 hours followed by exposure to a pH cycling model for 14 days. The neem tooth paste caused 48.73% reduction of lesion depth. They also noted that due to less use of Azadirachta indica for teeth cleaning in recent days in India, the level of carious lesion has increased [70].
Anti-inflammatory activities of neem oil, seed, leaf and bark have also been evaluated. Macrophage and neutrophil, which have functions in inflammation can be suppressed by nimbidin. Other components possessing anti-inflammatory activity are sodium nimbidate, gallic acid, epicatehin, catechin, polysaccharide GIIa, and Nb-II peptidoglycan. They cause immunomodulation which has a crucial role in inflammatory condition [71]. Inflammation in gum can be worsened by accumulation of bacteria. The bacteria that adhere to the inflamed gum have been found to be tetracycline and penicillin resistant in one study. But the study further showed that they are sensitive to neem extract [72]. Neem extract could inhibit bacterial growth without producing allergy in the gingiva [72]. Nimbidine proved to be a vital phytochemical, which gave anti-inflammatory effect by suppressing the function of macrophages and neutrophils that are responsible for inflammation [73].
Antioxidants help in reducing inflammation by diminishing free radical or reactive oxygen species. Nowadays, antioxidants have become attractive remedies in dental medicines against gingivitis and other periodontal diseases. They are available in gel, toothpaste, and mouth rinse [74]. Polyphenols present in neem leaf extract showed strong antioxidant activity when they are used to treat bacteria, red blood cell and lysozyme and the ethanolic extract of neem leaf showed antibacterial activity against P. gingivalis as well [75]. Leaf and bark of Azadirachta indica was fractionated with methanol, hexane, ethyl acetate, water and n-butanol. Various activities were done in order to measure antioxidant potency of neem. For 1, 1-diphenyl-2-picrylhydrazyl (DPPH) free radical scavenging activity, water fraction of bark extract showed the highest inhibition (radical scavenging in percentage) with 93.11 %. It was suggested that this highest inhibiting activity of bark was due to its higher phenolic content. Also in scavenging ability on hydroxyl radical activity, the water fraction of bark extract gave the highest result (92.12%). In addition, the bark extract gave satisfactory results in a DNA damage protection assay [76]. In this context, it is to be noted that Pandey and others found free radical scavenging activity in neem leaf extract with an IC50 of 110.36 μg/ml, compared to an IC50 for ascorbic acid at 42 μg/ml [24].
Conclusion
Dental caries and other tooth and gum diseases are universal health problems and they pose a threat to a wide section of people, largely underprivileged, who have problems in maintaining oral hygiene. In this context, instead of costly toothpaste and mouth washes, which the poorer people of many countries can ill afford, it is best to provide them with a cheap and readily available substitute. The various parts of Azadirachta indica (the neem tree) but mainly the stem and bark can provide such a substitute. The stem of the tree can be used directly for brushing teeth, and bark and leaf powders can also form a substitute for toothpastes. Additionally, the tree may contain a number of phytochemicals having anti-microbial activity against various teeth and gum disease causing microorganisms. Thus various parts of the neem tree deserve continued and even expanded research in our efforts to eliminate or at least reduce the frequency and severity of diseases of teeth and gums.
References
- Verma D., et al. “Mutipurpose neem (Azadirachta indica) for beguiling cures”. Int. Arch. App. Sci. Technol 8.3 (2017): 96-101.
- Munoz- Valenzuela S., et al. “Neem Tree Morphology and Oil Content.” Issues in new crops and new uses, (2007): 126-128.
- Girish K and Shankara SB. “Neem- a green treasure”. Electron J Biol 4.3 (2008): 102-111.
- India Biodiversity Portal. https://indiabiodiversity.org/species/show/31068. Accessed on 17 May, 2019. Azadirachta indica - World agroforestry.
- http://www.worldagroforestry.org/treedb/AFTPDFS/Azadirachta_indica.PDF. Accessed on 18th May, 2019.
- Oral Health. World Health Organization. https://www.who.int/news-room/fact-sheets/detail/oral-health. Accessed on 20th May, 2019.
- Petersen EP., et al. “The global burden of oral diseases and risks to oral health”. Bulletin of the World Health Organization 83.9 (2005): 661-669.
- Subapriya R and Nagini S. “Medicinal properties of neem leaves: a review”. Curr Med Chem - Anticancer Agents 5.2 (2005): 149-146.
- Buggapati L. “Herbs in dentistry”. Int JPharm Sci Invent 5.6 (2016): 7-12.
- Kumar JV. Oral hygiene aids. In: Hiremath SS (Ed.), Textbook of preventive and community dentistry. Edn 2, Elsevier, India, 2011, p412.
- Kumar PR. “Contribution of trees to oral health in India”. Res J Pharm Biol Chem Sci 6.4 (2015): 669-673.
- Ross IA. Medicinal plants of the world: chemical constituents, traditional and modern medicinal uses. (2001): Totowa, New Jersey; vol. 2, pp: 81-85.
- Hazarika P., et al. “Traditional knowledge for using plant resources as tooth brushing stick (datun) by the indigenous communities of Assam”. India. Int J Herb Med 6.6 (2018): 22-34.
- C. Kokate, A. P. Purohit, and S. B. Gokhale. Pharmacognosy, Nirali Prakashan, Maharashtra, India (2010).
- Hashmat I., et al. “Neem (Azadirachta indca A. Juss.) - A natural drugstore: an overview”. Int Res J Biol Sci 1.6 (2012): 76-79.
- Sujarwo W., et al. “Ethnobotanical uses of neem (Azadirachta indica A. Juss.; Meliaceae) leaves in Bali (Indonesia) and the Indian subcontinent in relation with historical background and phytochemical properties”. J Ethnopharmacol 189 (2016): 186-193.
- Aneesa N and Gayathri. “Beneficial effect of neem oil- an updated review”. J Pharm Sci & Res 8.8 (2016): 756-758.
- Ganesan S. “Traditional oral care medicinal plants survey in Tamil Nadu”. Nat Prod Rad 7.2 (2008): 166-172.
- Das PR., et al. “An ethnomedicinal survey conducted among the folk medicinal practitioners of three villages in Kurigram district, Bangladesh”. Am.-Eur J Sust Agri 6.2 (2012): 85-96.
- Itelima JU., et al. “Phytochemical screening and antimicrobial activity evaluation of aqueous and ethanolic extracts of the leaf of Azadirachta indica A.Juss (neem) on some microorganism”. World J Microb 3.1 (2016): 56-60.
- Dash PS., et al. “Phytochemical and biochemical characterizations from leaf extracts from Azadirachta indica: an important medicinal plant”. Biochem Anal Biochem 6 (2017): 323.
- Ogbonna OA., et al. “Phytochemical screening quantitative estimates of bioactive compounds in Spondias mombin and Azadirachta indica”. Res J Chem Sci 6.1 (2016): 38-40.
- Ramadass N and Subramanian N. “Study of phytochemical screening of neem (Azadirachta indica)”. Int J Zoology Stud 2.1 (2018): 209-212.
- Pandey G., et al. Evaluation of phytochemical, antibacterial, and free radicle scavenging properties of Azadirachta indica (neem) leaves. Int J Pharm Pharm Sci 6.2 (2014): 444-447.
- Cheenickal M and Mendez MR. “Phytochemical screening and the antimicrobial activity of the leaves of Azadirachta indica, A.Juss”. Int J Sci Engin Res 8.5 (2017): 721-724.
- Siddiqui S., et al. “Studies in the chemical constituents of Azadirachta indica part II: isolation and structure of the new Triterpenoid Azadirachtol”. Planta Med 51.6 (1985) 478-480.
- Siddiqui S., et al. “Non-terpenoidal constituents from Azadirachta indica”. Planta Med 54.5 (1988): 457-459.
- Ara I., et al. “Tricyclic diterpenes from the stem bark of Azadirachta indica”. Planta Med 56.1 (1990): 84-86.
- Siddiqui S., et al. “Constituents of Azadirachta indica: isolation and structure elucidation of a new antibacterial tetranortriterpenoid, mahmoodin and a new protolimonoid, naheedin”. J Nat Prod 55.3 (1992): 303-310.
- Siddiqui BS., et al. “Triterpenoids of the fruit coats of Azadirachta indica”. J Nat Prod 62.7 (1999): 1006-1009.
- Siddiqui BS., et al. “Two insecticidal tetranortriterpenoids from Azadirachta indica”. Phytochem 53.3 (2000): 371-376.
- Siddiqui BS., et al. “Two new triterpenoids from Azadirachta indica and their insecticidal activity”. J Nat Prod 65.8 (2002): 1216-1218.
- Siddiqui BS., et al. “Tetracyclic triterpenoids from the leaves of Azadirachta indica and their insecticidal activities”. Chem Pharm Bull (Tokyo) 51.4 (2003): 415-417.
- Siddique BS., et al. “Analysis of Azadirachta indica A. Juss. Fractions”. Z Naturforsch C 59.1-2 (2004): 104-12.
- Siddiqui BS., et al. “Tetracyclic terpenoids from the leaves of Azadirachta indica”. Phytochem 65.16 (2004): 2363-2367.
- Siddiqui BS., et al. “A new tetracyclic terpenoids from the leaves of Azadirachta indica”. Nat Prod Res 20.12 (2006): 1036-1040.
- Siddiqui BS., et al. “A new flavonoid from the flowers of Azadirachta indica”. Nat Prod Res 20.3 (2006): 241-245.
- Siddiqui BS., et al. “GC-based analysis of insecticidal constituents of the flowers of Azadirachta indica A. Juss”. Nat Prod Res 23.3 (2009): 271-283.
- Ahmed E., et al. “A review of chemical constituents and traditional usage of Neem plant (Azadirachta indica)”. Palestinian Med Pharm J 2.2 (2017): 75‐81.
- Osman AMI. Bioactivity of neem (Azadirachta indica) callus extract. Thesis submitted to the Sudan Academy of Science in partial fulfillment for the requirements for the degree of Master of Science in Biotechnology, 2008.
- Asif M. “Antimicrobial potential of Azadirachta indica against pathogenic bacteria and fungi”. J Pharmacog Phytochem 1.4 (2012): 78-83.
- Adithya NT., et al. “A current review on Azadirachta indica (neem)”. World J Pharm Pharm Sci 6.12 (2017): 249-269.
- Bohora A., et al. “Comparison of the antibacterial efficiency of neem leaf extract and 2% sodium hypochlorite against E. faecalis, C. albicans and mixed culture - an in vitro study”. Endodontology 22 (2010): 8-12.
- Ghonmode WN., et al. “Comparison of the antibacterial efficacy of neem leaf extracts, grape seed extracts and 3% sodium hypochlorite against E. faecalis - an in vitro study”. J Int Oral Health 5.6 (2013): 61-66.
- Hegde V and Kesaria DP. “Comparative evaluation of antimicrobial activity of neem, propolis, turmeric, liquorice and sodium hypochlorite as root canal irrigant against E. faecalis and C. albicans - an in vitro study”. Endodontology 25.2 (2013): 38-45.
- Raghavendra SS and Balsaraf DK. “Antifungal efficacy of Azadirachta indica (neem) – an in vitro study”. Braz J Oral Sci 13.3 (2014): 242-245.
- Chandrappa PM., et al. “Antimicrobial activity of herbal medicines (tulsi extract, neem extract) and chlorhexidine against Enterococcus faecalis in endodontics: an in vitro study”. J Int Soc Prev Community Dent 5(suppl 2) 2015: S89-S92.
- Prasad SD., et al. “Evaluation of antimicrobial efficacy of neem and aloe vera leaf extracts in comparison with 3% sodium hypochlorite and 2% chlorhexidine against E. faecalis and C. albicans”. Journal of Dr. J NTR Univ Health Sci 5 (2016): 104-10.
- Sing H., et al. “Neem: a magical herb in endodontics”. Stomatological Dis Sci 1 (2017): 50-54.
- Ahmed J., et al. “Herbal oral care: an old concept or a new model?” Int J Res Med Sci 2.3 (2014): 818-821.
- Boloor AV., et al. “Unconventional dentistry in India-an insight into the traditional methods”. J Trad Complement Med 4.3 (2014): 153-158.
- Vanka A., et al. “The effects of indigenous neem Azadirachta indica mouth wash on Streptococcus mutans and Lactobacilli growth”. Indian J Dent Res 12 (2001): 133-44.
- Rabi RA., et al. “Antibacterial effect of neem Azadirachta indica stem bark extraction on some dental pathogens”. Dutse J Pure App Sci 4.1 (2018): 666-673.
- Wolinsky LE., et al. “The inhibiting effect of aqueous Azadirachta indica (Neem) extract upon bacterial properties influencing in vitro plaque formation”. J Dent Res 75 (1996): 816-22.
- Pai MR., et al. “Evaluation of antiplaque activity of Azadirachtaindica leaf extract gel—a 6-week clinical study”. J Ethnopharmacol 90 (2004): 99-103.
- Salam R., et al. “Effect of neem and betel leaf against oral bacteria”. Int J Nat Soc Sci 1 (2014): 52-57.
- Lekshmi PNCJ., et al. “The inhibiting effect of Azadirachta indica against dental pathogens”. Asian J Plant Sci Res 2.1 (2012): 6-10.
- Aadithya BU., et al. “Post endodontic Aspergillosis in an immunocompetent individual”. J Clin Exp Dentistry 7.4 (2015): e535–e539.
- Shrivastava DK and Swarnkar K. “Antifungal activity of leaf extract of Neem (Azadirachta indica Linn)”. Int J Curr Microbiol App Sci 3.5 (2014): 305-308.
- Maina SW., et al. “Antimicrobial efficacy of Azadirachta indica (Neem) twigs aqueous and ethanol extract on tooth root canal biofilms”. Int J Pharmacogn Phytochem Res 7.4 (2015) 735-739.
- Megalaa N., et al. “Role of herbal leaf extract in caries prevention”. Int J Cont Med Res 1.2 (2014): 71-78.
- Jadhav RS., et al. “Herbal mouth wasah: An update review”. World J Pharm Pharm Sci 7.9 (2018) 436-445.
- Chhibber S and Sharma N. Medicinal and therapeutical potential of neem (Azadirachta indica): a review. Int J Sci Res Pub 4.5 (2014): 1-5.
- Pandya SR., et al. “Antimicrobial effectiveness of Neem(Azadirachtaindica) and Babool (Acacia nilotica) on Streptococcus mutans: An in vitro study”. J Indian Assoc Public Health Dentistry 13.4 (2015): 517-520.
- Chatterjee A., et al. “To evaluate the antigingivitis and antipalque effect of an Azadirachta indica (neem) mouthrinse on plaque induced gingivitis: A double-blind, randomized, controlled trial”. J Indian Soc Periodontology 15.4 (2011): 398-401.
- Kalita C., et al. “Antibacterial and Antifungal Property of Three Plants against Oral Microbes”. J Mahatma Gandhi Institute Med Sci 23.2 (2018): 73-76.
- Chatterjee S. “Phytomedicine in dentistry”. IOSR J Pharm 4.4 (2014): 01-03.
- Devi SR., et al. “Role of herbs and their uses in dentistry”. Int J Sci Study 1.3 (2013): 112-120.
- Lakshmi T and Kumar SA. “Antibacterial evaluation of Azadirachta indica ethanolic leaf extract against selected acidogenic bacteria causing dental plaque in fixed orthodontic appliance patients- an invitro study”. Int J Botany Res 1.2 (2012): 30-40.
- Latha M., et al. “Effect of Azadirachta indica Stem Bark Extract on Carious Lesions in Deciduous teeth”. Sch J Dent Sci 3.10 (2016): 284-286.
- Gupta A., et al. “Evaluation and exploration of Azadirachta indica in dentistry: an update”. British J Med Med Res 21.8 (2017): 1-15.
- Kala BS., et al. “Treatment of periodontal disease- a herbal approach”. Int J Pharm Sci Rev Res 33.2 (2015): 126-136.
- Bhowmik D., et al. “Herbal remedies of Azadirachtaindicaand its medical application”. J Chem Pharm Res 2.1 (2010): 62-72.
- San Miquel SM., et al. “Use of antioxidants in oral healthcare”. Compend Contin Educ Dent 32.9 (2011): E156-9.
- Heyman L., et al. “Combined antioxidant effects of Neem extract, bacteria, red blood cells and Lysozyme: possible relation to periodontal disease”. BMC Complement Altern Med 17.1 (2017): 399.
- Ghimeray AK., et al. “Antioxidant activity and quantitative estimation of azadirachtin and nimbin in Azadirachta indica A. Juss grown in foothills of Nepal”. Afr J Biotech 8.13 (2009): 3084-3091.
Citation:
Mohammed Rahmatullah., et al. “Azadirachta indica A. Juss. (Meliaceae): it’s use Against Dental Diseases”. Oral Health and Dentistry 3.6 (2019): 778-788.
Copyright: © 2019 Mohammed Rahmatullah., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.



































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.