Research Article
Volume 1 Issue 1 - 2017
Genetic Variation in the Retinal Vasculature of the Collaborative Cross
1Centre for Diabetes Research, Harry Perkins Institute of Medical Research
2Centre for Medical Research, The University of Western Australia, Perth
3School of Medical and Health Sciences, Edith Cowen University, Joondalup, Perth, Western Australia
2Centre for Medical Research, The University of Western Australia, Perth
3School of Medical and Health Sciences, Edith Cowen University, Joondalup, Perth, Western Australia
*Corresponding Author: Grant Morahan, Centre for Diabetes Research Harry Perkins Institute of Medical Research, Nedlands, West Australia.
Received: March 10, 2017; Published: April 17, 2017
Abstract
Purpose: Examination of the vessels of the retina provides an insight into the health of the eye. Variation in the number of major retinal blood vessels may indicate development of various diseases. Identification of mouse strains with a varied number of vessels will be useful to understand the genetic basis for, and the physiological consequences of, such variation. We therefore analyzed variation in retinal vasculature among strains of the next-generation genetic resource, The Collaborative Cross (CC).
Methods: The retinal vasculature from both flat mounted whole-retina and histology sections were examined in our panel of CC and common inbred mouse strains, to characterize the vascular pattern. In the course of this work, we optimized the trypsin digest method for examination of fragile retinal samples. We describe our optimized method, providing a step-by-step protocol and list of specific tools to isolate the vascular network.
Results:The CC strains showed wide variation in numbers of the major retinal arteries and veins. One strain had only a single capillary network. Our modified method was able to isolate the retinal vasculature successfully and was particularly suited to strains with minimal neural structure.
Conclusions: Different retinal anatomy, with wide variation in the numbers of retinal arteries and veins was observed and could be explained by genetic variation. Our optimized trypsin digest method can be applied to study ocular vasculature pathology in any retinal disease in humans or animal models.
Keywords: Trypsin Digest Method; Retinal Vasculature; Mouse Retina; Collaborative Cross
Funding
This study was funded by the Diabetes Research Foundation of Western Australia, by Discovery Project 11010206 from the Australian Research Council and Program Grant 1037321 from the National Health and Medical Research Council of Australia.
This study was funded by the Diabetes Research Foundation of Western Australia, by Discovery Project 11010206 from the Australian Research Council and Program Grant 1037321 from the National Health and Medical Research Council of Australia.
Introduction
The retina is composed of multiple layers. The retinal and choroidal vascular systems provide oxygen and nutrients [1]. The inner retina is sustained via the retinal blood supply whereas the outer retina is nourished and sustained by the choriocapillary network [2]. The retinal pigment epithelium transports ions, water, and metabolic end-products from the sub-retinal space into the blood stream. The uptake of nutrients such as glucose, retinol and fatty acids from the retinal blood supply is then delivered to the photoreceptor cells [3]. A disruption to either of these blood supplies can lead to the degeneration of the retina, loss of visual function and blindness [3]. Examining specific vascular cells of the retina can provide vital information for studying mechanisms of retinal pathology.
Different methods have been employed to study the retinal vasculature. Among these, the trypsin digest method is well established; it was first described in 1960 by Kawabara and colleagues to study the structure of blood vessels in the human retina [4] and has since been used extensively to study the retinal vasculature in many species, under normal and disease conditions [5]. The method involves digestion of the retinal neural structure, so the vasculature collapses onto a single plane. The structure is then stained to visualize the blood vessels. In addition, histochemical staining techniques can be implemented to detect specific elements of the vasculature.
The complexity of the retinal vascular tree is indicated by the number of retinal arteries and veins. An abnormal vessel count is an important symptom in diabetic retinopathy and other diseases such as high blood pressure or diseases of the pancreas [6,7] .
The genetic control of the number of arteries and veins has been studied, but the only component identified to be under genetic influence was twisted blood vessels [8]. Tortuosity was suggested to be linked to several diseases (arterial hypertension, retinopathy, cerebral vessel disease and stroke) [9]. The vessel length, numbers of crossings and regional branch points were not found to have a genetic component [8].
Here, we examined the major blood vessels that form the retinal vasculature in strains of the novel mouse resource “The Collaborative Cross” (CC) [10,11]. The CC is a genetic reference population established from eight diverse founder strains [10,11]. It captures over 90% of common genetic diversity of the mouse species, with polymorphisms occurring on average every ~200 base pairs [12]. Each CC strain is unique, having a distinct mosaic genome pattern of alleles inherited from the eight founders[12]. Therefore, the CC strains should harbor phenotypic diversity in any trait of interest. This resource provides an ideal opportunity to study the metrics of vessel length, numbers of crossings, regional branch points, and tortuosity for a genetic basis using the CC strains.
In this paper, we studied the variation in number of vessels in the retinas of CC mice and of some common inbred mouse strains. In the course of this work, we optimized the trypsin digest method which enables the observation of the overall retinal vasculature and specific morphological characteristics of retinal blood vessels such as pericytes and endothelial cells.
Materials and Methods
Animals: All experimental and animal handing activities were performed in accordance with the guidelines of institutional Animal Ethics Committee and ARVO statement for the Use of Animals in Ophthalmic and Vision Research. The principles, development and initial characterization of the CC have been described [10,11]. Mice were bred at the Animal Resources Center (Perth WA) and were generously provided by Geniad Pty Ltd. [11]. Common inbred strains A/J, C57BL/6J (B6), 129S1/SvImJ, NOD/LtJ, FVB/NJ (FVB) and DBA/2J (D2) were purchased from the Animal Resource Centre. Male and female mice were used at 8-10 weeks of age, housed under a 12-h light/dark cycle and given a standard diet with free access to food and water. All experiments were performed in accordance with The University of Western Australia institutional animal care and use committee guidelines and ethics. Experimental mice were housed and maintained in The University of Western Australia animal care services facility.
Specimen collection: At experimental end-point, animals were euthanased; eyes were enucleated, cleaned and weighed. The right eye was collected into 10% buffered formalin for histology or trypsin digest. The left eye was collected into phosphate buffered saline (PBS) for retinal whole mount preparation.
Enucleation and fixation: After the mouse was euthanised the eyelid was opened widely and the eye socket gently pressed down with the thumb and forefinger to achieve proptosis of the eye. Critical: Do not press hard against the socket, excess pressure will damage the eye. With the use of 45° angle forceps (World Precision Instruments, Inc., USA. Cat No 14101) the muscles were loosened around the eye socked. Critical: This avoids unnecessary muscle and tissue damage. While pressing on the eye socket, the eye was gently pulled to obtain the eye together with about 5mm of the optic nerve. Critical: The eye specimen must be obtained with the optic nerve to ensure the integrity of the posterior portion of the eye. Any excess tissue was removed and the eye then transferred to cold 1x PBS. The eye was then washed gently and the PBS removed, tubes were filled with buffered formalin and stored at room temperature. Critical: For eyes kept in fixative for longer than a week, ensure lids are tightened well to avoid evaporation of fixative.
Retinal Dissection: The formalin-fixed eye was remove from the tube and washed in cold 1x PBS three times for 5 minutes per wash on a shaker at room temperature. Using a cut plastic pipette, the eye was transferred onto a sterile petri dish containing distilled water. Critical: Add adequate amount of distilled water to fully immerse the eye. With a dissection microscope the eye ball was held using the 45° Dumont Tweezers where a small incision was made limbus using a No.12 scalpel. Critical: The eye ball must be held gently with the Dumont medical biology tweezers (World Precision Instruments, Inc., USA. Cat No 500341 1) to avoid damage to the retina. The anterior segment of the eye (cornea) was gently pinched with the use of capsulotomy scissors (VANNAS, Germany. Cat. No. OP3300), followed by cutting at the point of incision, along the limbus. Once the anterior and posterior segments of the eye are separated, the cornea was discarded.
Whilst holding the eyecup with the virtuous side facing down, carefully move the angled forceps closer to the optic nerve head. Using the capsulotomy scissors, optic nerve was clasped from the base. Critical: The clasping of the optic nerve head must take place in the correct position to release the retina from the eyecup with ease. Cutting too deep will damage the retina whereas insufficient cutting can hinder the retina coming off the eyecup. The retina was gently rolled away from the retinal pigment epithelium/choroid. Critical: Extra care must be taken rolling the retina as long-term fixed retinae can be brittle. The dissected retina holds a cup-like shape. The retina was gently cleaned to remove any choroid particles and gently washed with distilled water.
Whole Mount Preparation: Freshly dissected retina was fixed immediately in 4% paraformaldehyde (pH 7.3) for 5 minutes at room temperature followed by washing in PBS. Retina were incubated overnight at 48C with endothelial cell marker biotinylated Griffonia (Bandeiraea) simplicifolia Lectin 1 Isolectin-B4 (Isolectin-B4, 1:100 in PBS; Vector Laboratories, Burlingame, CA, USA), followed by 3 hours at 48C in Cy3-Streptavidin (1:500 in PBS; GE Healthcare, Amersham, UK). Following incubation, samples were washed in PBS, mounted, and cover-slipped with Vectashield hard-set mounting medium (Vector Laboratories). The overall retinal vasculature of labeled whole mounts was evaluated from the vitreous side of all diabetic and control retina using fluorescent microscopy. The detailed structure of the vascular beds and lesions was analyzed by capturing representative areas in the Z-plane at 1- to 2-lm steps.
Retinal Digestion: Retina was transferred to a well of a 96 well plate with 500 µl of trypsin. Critical: The retina must be fully immersed in the trypsin solution to avoid drying of tissues. The plate was then placed in a 37°C humidified chamber for ~12 hrs. When the retinal neural structure appeared too disintegrated, the plate was removed from the incubator.
Retinal vascular isolation: Trypsin was replaced in each well with distilled water and washed for 5 minutes on a shaker at room temperature. Using a wide bore plastic transfer pipette, the retina was moved to a sterile petri dish filled with distilled water. Using forceps (World Precision Instruments, Inc., USA. Cat No 500341 1) and a single-hair paintbrush, initial portions of neural tissue were removed with gentle movements. With the use of a pipette, place 500 µl of distilled water one drop at a time on to the retina, to remove majority of the neural tissue. When most of the neural tissue is removed, transfer the retina to a super frost slide using a transfer pipette. Critical: the exposed retinal vessels can easily adhere to the edges of the transfer pipette, care must be taken to avoid direct contact with the plastic surface. Using a higher magnification on the dissection microscope, identify any areas with small amounts of remaining neural tissue and gently brush these away using only the single-hair paintbrush. Critical: Do not use any metal-based forceps during this process. The isolated vasculature has a great affinity to metal, so will adhere to the metal surface turning the vasculature into a tangled, unusable structure. Gradually remove excess water around the isolated vasculature and gently spread it using only the single-hair paintbrush. Samples were left to dry at room temperature for 20-30 minutes.
PAS Staining: Approximately 150 µl of 0.5% Periodic Acid-Schiff’s stain was added to each sample and incubated at room temperature for 15 minutes. The samples were then washed with filtered running tap water for 15 minutes. Then add 100-200 µl of Schiff’s reagent onto each slide and incubate for 15 minutes. Critical: Schiff’s reagent must be colorless when used. The samples were the wash with filtered running tap water for 15-20 minutes. Critical: Wash until the water runs clear. Inadequate washing will not intensify the pink colour. Counterstain was done by adding 100-200 µl of Haemtoxylin for approximately 5 minutes at room temperature. Critical: Haemtoxylin must be filtered prior to use to avoid any partial sediment, which can mask the visualization of tissue of interest. Wash with filtered running tap water for 15 minutes. Dehydrate and coverslip using DPX.
Microscopy: Using a bright field microscope (Nikon Eclipse TiE, Japan) samples were screened for overall retinal vascular architecture, followed by screening for acellular capillaries, pericyte ghost and microaneurysms.
Results
Mice from a total of 29 strains from our CC colony [10] were analyzed. All mice were screened for retinal vasculature and specific morphological characteristics of retinal blood vessels such as pericytes and endothelial cells. During the course of this work, we found retinas for some strains could not be examined due to inadequate digestion of the retinal neural structure. This prompted us to develop an optimized trypsin digest method as described below. The original trypsin digest method enabled successful isolation of the retinal vasculature from neural structures from the B6, D2 and several CC strains. However, this protocol was not satisfactory for isolation of the retinal vascular structure in many strains, especially FVB and ZOE (Figure 2A). Excellent results were obtained using the modified method for preparation of retinal samples from all strains. This method also improved the visualization in the B6, D2 and CC strain digest preparations.
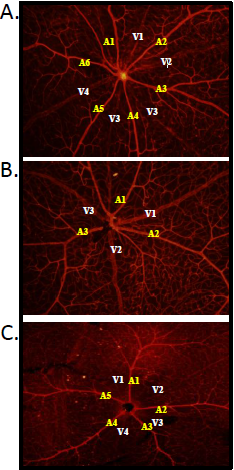
Figure 1: Variation in the number of retinal arteries and veins originating from the optic disc.
Freshly dissected retina were fixed immediately then stained with Isolectin-B4 and Cy3-Streptavidin an endothelial cell marker and visualized. More arteries and veins were observed in the GEK2 strain (A) in comparison to the DAVIS (B) and ZOE (C) strains.
Freshly dissected retina were fixed immediately then stained with Isolectin-B4 and Cy3-Streptavidin an endothelial cell marker and visualized. More arteries and veins were observed in the GEK2 strain (A) in comparison to the DAVIS (B) and ZOE (C) strains.
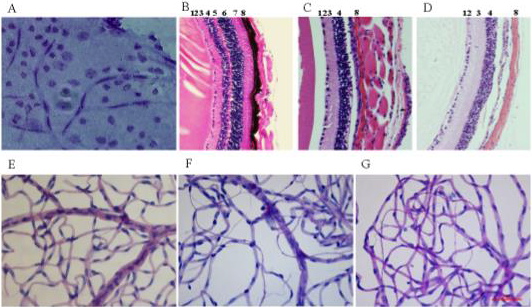
Figure 2: Retinal architecture of B6, FVB and ZOE mice.
Enucleated eyes were fixed then sectioned or stained with and without our optimized trypsin method. Current trypsin digestion with FVB retina (A), haematoxylin and eosin staining of B6 (B), FVB (C) and ZOE (D) retinal sections. The retinal layers are; 1-Nerve Fibre Layer, 2-Ganglion Cell Layer, 3-Inner Plexiform Layer, 4-Inner Nuclear Layer, 5-Outer Plexiform Layer, 6-Outer Nuclear Layer, 7-Photoreceptor Layer and 8-Choroid. The lower figures are the result of our improved trypsin digest method on B6 (E), FVB (F) and ZOE (G) retina displaying clear vascular architecture. Magnification; B, C, D - 40x and A, E, F, G - 20X.
Enucleated eyes were fixed then sectioned or stained with and without our optimized trypsin method. Current trypsin digestion with FVB retina (A), haematoxylin and eosin staining of B6 (B), FVB (C) and ZOE (D) retinal sections. The retinal layers are; 1-Nerve Fibre Layer, 2-Ganglion Cell Layer, 3-Inner Plexiform Layer, 4-Inner Nuclear Layer, 5-Outer Plexiform Layer, 6-Outer Nuclear Layer, 7-Photoreceptor Layer and 8-Choroid. The lower figures are the result of our improved trypsin digest method on B6 (E), FVB (F) and ZOE (G) retina displaying clear vascular architecture. Magnification; B, C, D - 40x and A, E, F, G - 20X.
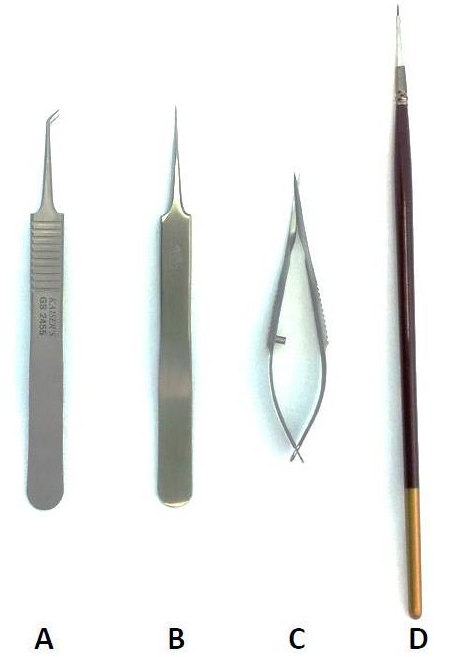
Tools for fine retinal dissection: To successfully dissect the retina and to isolate the vasculature, we used several specific tools (Figure 3). For enucleating the eye, we preferred number 5, 11cm, 45° angle forceps (Figure 3A). It is often difficult to hold the whole eye when dissecting the retina so it is critical to avoid damage to the retina during this process. By using the number 5, 11cm Dumont tweezers (Figure 3B) the whole eye was suitably secured, preventing damage. Fine capsulotomy scissors (Figure 3C) were used for cutting around the limbus to expose the retina. Lastly, to remove the neural tissue without damage to the retinal vasculature, a single hair paintbrush was used, made from natural material (Figure 3D).
Figure 3: Tools used for retinal dissection and vasculature isolation.
For the isolation of the retina with no tearing and perfect placement these tools are recommended. (A) Angled forceps, (B) Dumont tweezers, (C) Capsulotomy scissors and (D) Single hair paint brush.
For the isolation of the retina with no tearing and perfect placement these tools are recommended. (A) Angled forceps, (B) Dumont tweezers, (C) Capsulotomy scissors and (D) Single hair paint brush.
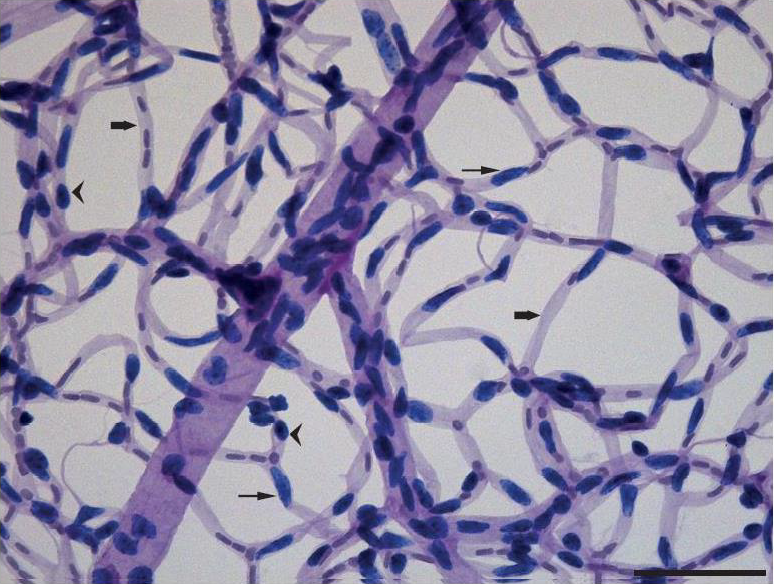
Trypsin Digest Optimization: For the application of the optimized trypsin digest method, retinal tissue was cleaned using the fine-hair paintbrush, leaving the clear thread-like network of retinal capillaries. A representative field from a retinal digest from the FVB strain is shown in (Figure 4).
Figure 4: Trypsin digest preparation of the retinal vasculature strained with Periodic Acid Schiff’s stain and
Haematoxylin of the FVB mouse using our modified trypsin method.
Whole eyes were processed to isolate the intact retinal microvasculature using trypsin. The retinal vasculature was then mounted on a slide, stained with Periodic Acid Schiff’s and analysed using bright field microscopy. Round-shaped pericytes outside the capillary wall are stained dark blue (Arrowheads), Elongated endothelial cells within the capillaries are stained light blue (Thin Arrows) and Basement membrane of retinal blood vessels are stained light purple (Thick Arrows). Scale Bar 50 mm.
Whole eyes were processed to isolate the intact retinal microvasculature using trypsin. The retinal vasculature was then mounted on a slide, stained with Periodic Acid Schiff’s and analysed using bright field microscopy. Round-shaped pericytes outside the capillary wall are stained dark blue (Arrowheads), Elongated endothelial cells within the capillaries are stained light blue (Thin Arrows) and Basement membrane of retinal blood vessels are stained light purple (Thick Arrows). Scale Bar 50 mm.
The intensity of staining in the trypsin digest preparation will depend on the staining method and the condition of reagents used. As stated in the methods, it is of utmost importance to use freshly prepared Schiff’s reagent. Schiff’s reagent is stable at low pH (Acidic pH of 2) at 4°C and must be refrigerated in the dark. Failing to do so may result in improper staining, which will impact severely on the visualisation of the desired retinal vasculature. Endothelial cells were identified within the vessel wall as large oval cell nuclei whereas pericyte nuclei appeared small, round in shape and were observed prominently protruding from the vessel wall. The basement membranes appeared as clear tube-like structures devoid of endothelial or pericyte cells (Figure 4). Subpopulations of migrating pericytes and their implication in retinopathy have been reported, indicating the importance of detecting these early vascular changes in ocular complications [15]
Variation in numbers of retinal arteries and veins originating from the optic disc: For further analysis, the trypsin digest method was used on all CC and inbred mouse strains. Fluorescent microscopy studies captured in the Z-plane showed retinal vascular architecture of all tested CC strains had the classical structure of retinal capillary distribution, where the extensive capillary network spanned across the inner two thirds of the retina, forming two distinguishable capillary networks: the superficial capillary network and the deep capillary network.
Variation in the number of retinal arteries and veins originating from the optic disc was noted within different CC strains (Table 1). For example, the GEK2 strain had on average 6 arteries and 4 veins (Figure 1A) while the DAVIS strain had only 3 arteries and 4 veins (Figure 1B). The most extreme example was noted in the ZOE strain, which had only a single capillary network and 4 arteries and 5 veins. Further analysis of this strain was conducted in order to establish the localisation of the capillary network.
| Strain | Number of Arties | Number of Veins | |
| 1 | BOM | 4 ± 0.3 | 5 ± 0.6 |
| 2 | CIS | 4 ± 0 | 4 ± 0.2 |
| 3 | DAVIS | 3 ± 0.3 | 4 ± 0.7 |
| 4 | FIM | 4 ± 0.3 | 5 ± 0.3 |
| 5 | GALASUPREME | 4 ± 0.3 | 5 ± 0.3 |
| 6 | GEK2 | 6 ± 0.5 | 5 ± 0.5 |
| 7 | HAX2 | 5 ± 0.5 | 5 ± 0.4 |
| 8 | NUK | 3 ± 0 | 3 ± 0 |
| 9 | POH | 6 ± 0.5 | 4 ± 0 |
| 10 | ROGAN | 5 ± 0.5 | 4 ± 0.5 |
| 11 | SHE | 4 ± 0.5 | 4 ± 0 |
| 12 | WAD | 4 ± 0.5 | 5 ± 0.5 |
| 13 | XAP | 5 ± 0 | 4 ± 0 |
| 14 | LUZ | 4 ± 0.3 | 4 ± 0.3 |
| 15 | LAX | 5 ± 0.3 | 5 ± 0.3 |
| 16 | LEL | 5 ± 0 | 5 ± 0.5 |
| 17 | YID | 5 ± 0.3 | 4 ± 0.3 |
| 18 | PIPING | 4 ± 0.3 | 4 ± 0.3 |
| 19 | TOFU | 5 ± 0.2 | 5 ± 0.2 |
| 20 | GIG | 5 ± 0.3 | 4 ± 0 |
| 21 | PEF | 5 ± 0.2 | 5 ± 0.2 |
| 22 | PUB | 4 ± 0.3 | 4 ± 0 |
| 23 | STUCKY | 5 ± 0.3 | 5 ± 0.3 |
| 24 | TAS | 4 ± 0 | 5 ± 0.5 |
| 25 | LAM | 5 ± 0.3 | 4 ± 0.3 |
| 26 | VUX2 | 4 ± 0 | 5 ± 0.5 |
| 27 | FUF | 4 ± 0.5 | 4 ± 0 |
| 28 | BEM | 5 ± 0.4 | 5 ± 0.4 |
| 29 | ZOE | 5 ± 0.3 | 4 ± 0.3 |
Table 1: Overall number of retinal arteries and veins in CC strains.
Retinas were enucleated and strained with Isolectin-B4 and Cy3-Streptavidin followed counting the number of arteries and veins.
Retinas were enucleated and strained with Isolectin-B4 and Cy3-Streptavidin followed counting the number of arteries and veins.
Retinal Architecture: The B6 strain (Figure 2B) showed a normal retinal structure where all eight retinal neural layers were visible and two capillary nets were noted spanning to the external margin of the inner nuclear layer. In contrast, the ZOE strain had a thinner retina with only 5 retinal layers and a single capillary net that was visible towards the external margin of the inner nuclear layer. The superficial capillary network usually seen in the ganglion cell layer was absent from the ZOE strain. The outer plexiform, nuclear and photoreceptor layers were not observed in the ZOE retina. The FVB and ZOE strains (Figure 2C, 2D) showed similar retinal layer distributions. The FVB strain’s retinal architecture showed markedly different retinal layer depth (Figure 2C) in comparison to B6 (Figure 2B) and other CC strains. The defect in the FVB retina was reported to be due to a mutation in Pde6b, the gene encoding Phosphodiesterase 6B, CGMP-Specific, Rod, Beta [13,14]. This mutation resulted in the absence of several neural layers, which was evident in the reduced depth of the retinal vasculature in whole mounts and with the absence of retinal layers (beyond the inner nuclear layer) (Figure 2C). Interestingly, the ZOE retinas were found to be missing the same retinal layers as the FVB strain (Figure 2D). In comparison to the B6 (Figure 2E), the FVB (Figure 2F) and ZOE (Figure 2G) retinas had long clusters of acellular capillaries distributed across the retina.
Differential numbers of lesions: Retinal lesions such as acellular capillaries were visible in four outbred strains B6, D2, FVB and ZOE and many CC strains retinas (Table 2). However, the degree of lesions varied among these strains. For example, the FVB and ZOE strains showed a large number of acellular capillaries and migrating pericytes in comparison to the B6 and D2 strains.
| Mouse Strain | Acellular Capillaries | Pericyte Ghost | Migrating Pericytes |
| C57BL/6 | + | - | + |
| DBA/2 | + | - | - |
| FVB/N | +++++ | - | ++++ |
| ZOE_AH | + | - | - |
Table 2: Quantified retinal lesions number.
Retinas were enucleated, subjected to optimised trypsin digested method to identify the number of retinal lesions. Numbers of acellular capillaries, pericyte ghosts and migrating pericytes were determined. The ‘+’ sign indicates the presence of lesions and the total number as a comparison between strains.
Retinas were enucleated, subjected to optimised trypsin digested method to identify the number of retinal lesions. Numbers of acellular capillaries, pericyte ghosts and migrating pericytes were determined. The ‘+’ sign indicates the presence of lesions and the total number as a comparison between strains.
Discussion
Marked differences are exhibited in the retinal vascularization between species. Primates show a complex arrangement with four quadrants and an avascular zone at the fovea; rabbits and hares have a rather simple narrow band of superficial vessels; rodents have a wagon-wheel spoke-like arrangement; guinea pigs have no inner retinal vessels [16]. There are many diseases with an abnormal size and number of arteries and veins in the retina [7]. For example, in diabetic subjects the veins are often abnormally wide [17]. To detect diseases of the eye, the retina is routinely examined for the count of arteries and veins, and blood circulation parameters are determined [7].
Changes in collateral circulation can extend to variation in other tissues in the same individual, at least in mice [18,19] . A recent mouse study identified differences in arterial branch-patterning in the retina defined by genetic background as a prediction for variation in collateral extent and stroke severity [20]. Our findings of varying number of retinal arteries and veins in a genetically diverse and unique population of mice can be taken further using retinal patterning metrics to predict similar outcomes. This could further lead to identification of novel genes that mediate the variations seen in the retinal vascular tree.
In addition, understanding the vascular distribution and neural structure of the retina plays an important role in identifying the most suitable species that can be used in a particular line of research. For instance, the ZOE strain can be an ideal candidate for regenerative studies of the retinal photoreceptor layer yet would be unsuitable for tests that depend on visual cues.
The mouse retina is small and fragile, making its analysis particularly difficult and challenging to obtain good preparations [4,21]. Retinas of different species and different individuals within the same species do vary in size and thickness [22,23]. Hence, careful monitoring during the digestion process is essential for the optimization of the digestion period. For retinas that do not have a thick neural structure, such as those of the FVB and ZOE strains, it is imperative to use the optimized trypsin digest method we described. A longer digestion period with a lower trypsin concentration at a lower pH provided excellent results for samples having a variety of retinal thicknesses and lesions.
Lesions such as pericyte ghosts and pericyte position, acellular capillaries and micro- aneurysms could be reliably visualized by our optimized trypsin method, allowing detection of retinal vascular changes early in a disease process. In addition, the number of endothelial cells and pericytes could be counted to determine the endothelial to pericyte ratio, which is a useful measurement in defining the degeneration of the retinal vasculature. However, since the vasculature is collapsed on to a single plane, this method restricts the visualization of the two retinal capillary networks, limiting the ability to identify the precise location of vascular lesions such as microaneurysms.
The duration of fixation had an impact on the digestion process. Kawabara (1989) used eyes fixed for several years [4]. However, we used eyes fixed for no longer than 3 months; these required a lower trypsin concentration, with a longer digestion period to completely release the vasculature from the neural structure. Shorter incubation periods gave only partial digestion of the neural retina, resulting in damage to the vasculature during the cleaning process.
This optimized trypsin method can be easily adapted to retinal tissue from other animal species, with suitable optimization of digestion duration and staining according to the specimens and laboratory conditions. The procedure outlined here, with critical steps identified, can be used to isolate the vasculature for the study of any retinal vascular lesions.
Acknowledgments
The authors thank Andrew Wallace and Glynn Manship for providing animal care services. The authors acknowledge the scientific and technical assistance of the Centre for Microscopy, Characterization and Analysis of the University of Western Australia. This work was supported by grants from the Diabetes Research Foundation of Western Australia (Perth, WA, Australia), Discovery Project 11010206 from the Australian Research Council (Canberra, ACT, and Australia), Program Grant 1037321 from the National Health and Medical Research Council of Australia.
The authors thank Andrew Wallace and Glynn Manship for providing animal care services. The authors acknowledge the scientific and technical assistance of the Centre for Microscopy, Characterization and Analysis of the University of Western Australia. This work was supported by grants from the Diabetes Research Foundation of Western Australia (Perth, WA, Australia), Discovery Project 11010206 from the Australian Research Council (Canberra, ACT, and Australia), Program Grant 1037321 from the National Health and Medical Research Council of Australia.
Disclosure: L.Y. Weerasekera, None; L.A. Balmer, None; G. Morahan, None
References
- Kuwabara T. “Blood vessels in the normal retina”. UCLA Forum in Medical Sciences 8 (1969): 163-176.
- Ahmed J., et al. “Oxygen distribution in the macaque retina”. Investigative Ophthalmology and Visual Science 34.3 (1993): 516-521.
- Strauss O. “The Retinal Pigment Epithelium in Visual Function”. Physiological Reviews 85.3 (2005): 845-881.
- Kuwabara T., et al. “Studies of Retinal Vascular Patterns: Part I. Normal Architecture”. Archives of Ophthalmology 64 (1960): 904-911.
- Dietrich N and HP Hammes. “Retinal Digest Preparation: A Method to Study Diabetic Retinopathy, in Animal Models in Diabetes Research”. Animal Models in Diabetes Research 933 (2012): 291-302.
- Yau J W., et al. “Retinal fractal dimension is increased in persons with diabetes but not impaired glucose metabolism: the Australian Diabetes, Obesity and Lifestyle (AusDiab) study”. Diabetologia 53.9 (2010): 2042-2045.
- Huang K.,et al. “A region based algorithm for vessel detection in retinal images”. Medical Image Computing and Computer-Assisted Intervention 9 (2006): 645-653.
- Glenny R., et al. “Quantifying the genetic influence on mammalian vascular tree structure”. Proceedings of the National Academy of Sciences of the United States of America 104.16 (2006): 6858-6863.
- Han HC. “Twisted blood vessels: symptoms, etiology and biomechanical mechanisms”. Journal of Vascular Research 49.3 (2012): 185-197.
- Churchill GA., et al. “The Collaborative Cross, a community resource for the genetic analysis of complex traits”. Nature Genetics36.11 (2004): 1133-1137.
- Morahan G., et al. “Establishment of "The Gene Mine": a resource for rapid identification of complex trait genes”. Mammalian Genome 19.6 (2008): 390-393.
- Consortium CC., et al. “The Genome Architecture of the Collaborative Cross Mouse Genetic Reference Population”. Genetics 190.2 (2012): 389-401.
- Bowes C., et al. “Localization of a retroviral element within the rd gene coding for the beta subunit of cGMP phosphodiesterase”. Proceedings of the National Academy of Sciences of the United States of America90.7 (1993): 2955-2959.
- Bowes C., et al. “Retinal degeneration in the rd mouse is caused by a defect in the beta subunit of rod cGMP-phosphodiesterase”. Nature 347.6294 (1990): 677-680.
- Pfister F., et al. “Pericyte Migration”. Diabetes 57.9 (2008): 2495-2502.
- Kiel, J.W., 2010.
- Kondermann C.,et al. “Blood vessel classification into arteries and veins in retinal images”. 2007.
- Chalothorn D., et al. “Collateral density, remodeling, and VEGF-A expression differ widely between mouse strains”. Physiological Genomics30.2 (2007): 179-191.
- Clayton JA., et al. “Vascular endothelial growth factor-A specifies formation of native collaterals and regulates collateral growth in ischemia”. Circulation Research 103.9 (2008): 1027-1036.
- Prabhakar P., et al. “Genetic variation in retinal vascular patterning predicts variation in pial collateral extent and stroke severity”. Angiogenesis18.1 (2015): 97-114.
- Fischer MW and DH Slatter. “Preparation and Orientation of Canine Retinal Vasculature A Modified Trypsin Digestion Technique”. Clinical & Experimental Opthalmology 6.1 (1978): 46-50.
- Buttery RG., et al. “How thick should a retina be? A comparative study of mammalian species with and without intraretinal vasculature”. Vision Research31.2 (1991): 169-187.
- Radius RL. “Thickness of the retinal nerve fiber layer in primate eyes”. Archives of Ophthalmology 98.9 (1980): 1625-1629.
Citation:
Grant Morahan., et al. “Genetic Variation in the Retinal Vasculature of the Collaborative Cross”. Ophthalmology and Vision Science
1.1 (2017): 36-45.
Copyright: © 2017 Grant Morahan., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.



































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.