Research Article
Volume 1 Issue 2 - 2017
Efficacy and Safety of Combined Trans-Scleral Cyclophotocoagulation (Tscp) and Phacoemulsification Surgery in Advanced Glaucoma
Eye Ear and Mouth Unit, Maidstone & Tunbridge Wells NHS Trust, Maidstone Hospital, Hermitage Lane, Maidstone ME16 9QQ, Kent, UK
*Corresponding Author: Ejaz Ansari, FRCOphth MD, Consultant Ophthalmic Surgeon, Eye Ear and Mouth Unit, Maidstone & Tunbridge Wells NHS Trust, Maidstone Hospital, Hermitage Lane, Maidstone ME16 9QQ, Kent, UK.
Received: October 01, 2016; Published: July 28, 2017
Abstract
Introduction: Previous studies have shown trans-scleral cyclophotocoagulation (TSCP) to be an effective method for controlling intraocular pressure (IOP) in glaucoma. Phacoemulsification (Phaco) has also been shown in some studies to decrease IOP in patients with glaucoma. To our knowledge there are no previous studies evaluating vision and IOP control following combined TSCP and Phaco.
Purpose: To assess the effect of combined TSCP and phacoemulsification surgery with respect to a) IOP control, b) visual acuity (VA) c) change in topical therapy and d) side effect profile, in cases of advanced glaucoma.
Methods: A retrospective review of 35 eyes of 34 patients undergoing combined TSCPC and phacoemulsification were studied at 12 months.
Results: 30 eyes had open angle glaucoma, 4 had mixed mechanism glaucoma and 1 with neovascular glaucoma. The average total energy used was 87.51 J, (Range: 33.75-346 J). Mean pre-treatment IOP was 22.7 mmHg (Range: 16-47 mmHg) and mean IOP at 12 months post treatment was 14.7 mmHg (Range: 0-20 mmHg). This difference was statistically significant, p = 0.00013 (p < 0.05). Mean total medications were reduced from 2.1 to 1.9 (p = 0.55). All patients were able to stop treatment with oral Diamox.
At 12 months, visual acuity was better or unchanged in 80% and worse in 20%. Chronic hypotony occurred in only 1 eye which had neovascular glaucoma (NVG). There were no cases of phthisis.
Conclusion: Combined TSCP and phacoemulsification is a safe and effective method to control IOP in cases of advanced glaucoma and cataract.
Introduction
Purpose
The purpose of this study was to assess the effect of combined TSCPC and cataract surgery in patients with advanced glaucoma, with respect to; a) IOP control; b) VA; c) change in topical therapy and d) complications at 12 months post-surgery.
The purpose of this study was to assess the effect of combined TSCPC and cataract surgery in patients with advanced glaucoma, with respect to; a) IOP control; b) VA; c) change in topical therapy and d) complications at 12 months post-surgery.
Tran-scleral diode laser cyclophotocoagulation (TSCP) has been established as a relatively safe and effective intervention for glaucoma resistant to conventional management [1-4]. The procedure has become increasingly popular in recent years as an alternative to the surgical options for refractory glaucoma, such as antimetabolite augmented trabeculectomy and tube shunt surgery. It has been shown to be safer than other cyclodestructive procedures, such as Nd: YAG laser cyclophotocoagulation and cyclocryotherapy, which present a significant risk of hypotony and phthisis because of excessive ciliary body ablation [5-6]. However, the outcome of cyclodiode therapy is unpredictable and multiple treatments may be required to achieve the desired result [7-8]. Furthermore, no consensus exists for an optimum treatment protocol.
Methods
A single centre, retrospective case note study was conducted for all patients that underwent combined TSCP and phacoemulsification (TSCP + phaco) between January 2010 and July 2013 at Maidstone Hospital, Kent, UK. Cases were identified using ‘Theatreman,’ electronic operating theatre logbook. Data collected included age, gender, IOP, visual acuity, type of glaucoma, mean deviation on Humphrey 24-2 visual field testing, side effect profile and intraocular pressure therapy. The primary outcomes for this study were intraocular pressure and visual acuity at 12 months following combined TSCP + phaco. The secondary outcomes were topical therapy changes before and after treatment and side effect profile. Patients that underwent both TSCP + phaco on separate occasions were excluded. Treatment success was defined as IOP reduction greater than 30%, irrespective of topical anti-glaucoma medications.
The criteria for treatment were [1] patients with advanced glaucoma with inadequate control of IOP despite maximum medical therapy, [2] and presence of cataract.
All procedures were performed by the same surgeon (EA) under peribulbar local anaesthesia. The iris medical OcuLight SLx laser semiconductor diode 810nm laser and contact G probe (IRIDEX, Mountain view, CA, USA) was used for TSCPC. TSCPC was applied across 270 degrees using the Gaasterland criteria immediately after completion of the phaco + IOL procedure. The 3 and 9 o’clock areas were spared to avoid the anterior ciliary vessels and nerves. Subtenons triamcinolone acetonide 40 mg and subconjunctival dexamethasone and cefuroxime were administered immediately afterwards. Guttae prednisolone 1% x6 daily was used post-operatively for 6 weeks with reducing frequency regimen. The patients were reviewed at weeks 1, 4, 12, 24 and at 12 months, and the post-operative steroid and glaucoma drops were adjusted according to clinical progress. At every visit VA, IOP (measured by applanation tonometry), and any complications were noted.
For statistical analysis, 2-tailed Student ttests were used, the data being normally distributed, to determine the statistical significance of changes in IOP pre and post-treatment.
Results
The results are presented according to the primary and secondary outcomes at 12 months following combined TSCPC and cataract surgery.
A total of 35 eyes were treated. There were 15 males and 20 females. The mean age of patients in this study was 78 (range 49-94).
Open angle glaucoma (OAG) accounted for 86% (n = 30/35) of patients, mixed mechanism glaucoma (MMG) included patients with pseudoexfoliation glaucoma and secondary glaucoma accounted for 11% (n = 4/35). One patient had neovascular glaucoma (3%). All patients had advanced glaucoma and the mean deviation on Humphrey visual field testing 24-2 was -17.48 dB. The average Cyclodiode energy used was 87.51J, (Range: 33.75-346J) the mean laser power was 1350 mW (range 1250-1500 mW). The mean laser burns were 21 (range 10-37).
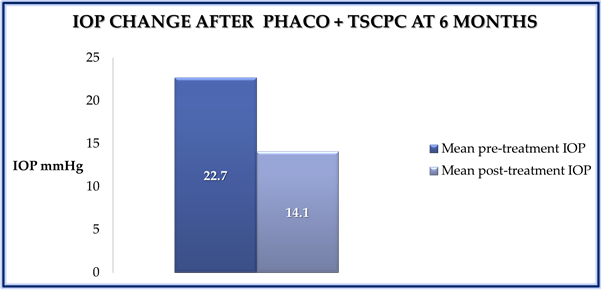
The IOP lowering effect of combined TSCP and phaco was statistically significant at 12 months post treatment (P = 0.0001, paired t-test) (Figure 1). Overall there was a 35% mean reduction in IOP. The overall success rate, defined as IOP reduction of 30% was 61% at 12 months follow up.
Figure 1: Bar chart showing the mean change in IOP before and 12 months after
combined TSCPC and phaco. (P = 0.0001).
The majority of patients had an improvement in visual acuity. At 12 months follow up, visual acuity was better or unchanged in 80% and worse in 20%. Of those 20% (n = 7/35) of patients who had a deterioration of visual acuity at 12 months, the causes were chronic hypotony (< 5 mmHg) (n = 1), chronic cystoid macular oedema (n = 2), worsening glaucoma (n = 3) and corneal oedema (n = 1). The overall complication rate of this procedure was 20% (7/35) [Table 1]. There was no significant change in mean total number of glaucoma medications required at 6 months (P = 0.55). Oral Acetazolamide was discontinued in all 7 cases who were taking this pre-operatively.
| Chronic hypotony | 1 |
| Chronic cystoid macular oedema | 2 |
| Corneal oedema | 1 |
| Worsening of glaucoma | 3 |
Table 1: Complications of TSCP+phaco (n = 35).
Discussion
Advanced glaucoma is defined using the Hodapp Classification [9] and includes mean deviation (MD) of > -12 dB, absolute deficit (0 dB) in the central 5 degrees, sensitivity of < 15 dB in the central 5 degrees of both hemifields. All of our patients satisfied at least one of these criteria. All of them also had visually significant cataract. Cataract surgery alone may be of limited benefit in lowering IOP in OAG [10,11], and, therefore, TSCP was performed concurrently with the aim of effecting a greater reduction of IOP, which was particularly required in this group of patients. Although 22% of respondents to the UK national audit on Cyclodiode laser [12] stated that they performed combined phaco + TSCP, there is no study to date evaluating this treatment combination. Therefore, in this discussion we will quote previous studies on TSCP alone.
As with previous studies, TSCP was very effective in lowering IOP [1-4, 8]. The success rate in this study was 61% as defined by a reduction of IOP of more than 30%, and this was with one session of TSCP. It compares favourably with a previous study with a mean of 10 months duration, although that looked at TSCP alone and had a mean of 1.75 treatments in 10 months [8]. For our group as a whole, the mean reduction in IOP was 35% at 12 months with one session of TSCP.
The most serious adverse effects of TSCP are hypotony and phthisis. There were no cases of phthisis and one case of hypotony (2.9%), which occurred in NVG. The rate of hypotony after TSCP is particularly high in NVG and was 15.6% in one study [13]. Hypotony rates after TSCP vary between 0-18% in the literature and could be related to higher pre-treatment IOP and greater energy levels used per treatment session [8, 13,14].
In our series, 80% had better or no change in vision, whereas 20% had a reduction of vision. This included one case of hypotony, one case of corneal decompensation, 2 cases of CMO and 3 cases where the central visual field deteriorated (8.6%). The data on vision confirm the findings of a previous study on TSCP alone [8].
The rates of vision loss following other surgical treatments for advanced glaucoma are comparable. In one study following augmented trabeculectomy the rate was 27% defined as a loss of >/= 2 Snellen lines [15]. In another study on tube shunt surgery in advanced glaucoma, at 1 year the vision remained at 20/200 or worse in 50% of individuals [16]. On comparing glaucoma tube implants with Cyclo-YAG and Cyclodiode alone in cases of refractory glaucoma, reduced vision occurred in 16% of tube cases compared to 9% of Cyclodiode cases [17].
Patients with advanced glaucoma tend to have a worse visual and overall prognosis. They are at imminent danger of losing remaining vision, and may also have various socioeconomic and health challenges. This makes advanced glaucoma a particularly challenging condition to deal with, not only clinically but also in terms of patients’ apprehensions of losing vision completely and the increased risks of surgical intervention [18].
The only proven treatment in preserving the visual function in glaucoma is by control of IOP. Patients with advanced glaucoma can do reasonably well in terms of protecting remaining visual field and quality of life if their IOP is very low. In the advanced glaucoma intervention study (AGIS), patients that did not progress had a mean IOP of 12 mm Hg [19]. This has to be balanced with the risks of further vision loss or even “wipe-out” of the remaining visual field (0-7%) [20,21] following surgical intervention. However, the risk of doing nothing is also very high in patients with advanced glaucoma and uncontrolled IOP. The risk of severe complications therefore needs to be balanced with the risk of inaction or under treatment. Generally speaking, combined procedures allow for greater IOP reduction and reduced IOP spikes than phaco alone [22-24].
The weaknesses of this study are those of a retrospective study. It is also non-comparative- a study comparing TSCP + phaco with TSCP alone or with phaco + IOL alone would be useful. In the current study in which all cases had advanced glaucoma, TSCP + phaco led to a mean reduction of IOP by 35% at 12 months. For a comparison with TSCP alone, in a previous study by the same group, TSCP alone effected a 43% reduction of IOP at a mean of 12.5 months, in a cohort of glaucoma patients with mixed aetiology, some having advanced glaucoma, but other cases being non-refractory [25]. This could explain the greater success in IOP reduction in the previous TSCP alone study.
The IOP reducing effect of phaco + IOL in open angle glaucoma is unclear and the mechanism is unknown [26]. Most studies suggest a drop in IOP after cataract surgery is only transient. There is a large range of IOP change following phaco + IOL in the published literature, from a reduction of 8.5 mm Hg in eyes with a high starting pressure (24-29 mm Hg) to a 1.7 mm Hg rise in IOP in low-pressure eyes (14 mm Hg or less) [27]. Duration of effect is also variable, with effects lasting 12, 18 or 24 months [26,28>] but continued response beyond this is rare. A meta-analysis showed a 2-4 mm Hg pressure drop from cataract surgery with effect diminishing after 18-24 months, [27] but more recent papers with longer follow-up revealed IOP reduction of approximately 3 mm Hg with 75%-85% of patients maintaining this IOP reduction at 5 years [29-33]. In summary, there is a mild and variable IOP drop following lens extraction in open angle glaucoma; there is a marginal benefit in only the milder forms of open angle glaucoma and there is a lack of robust evidence for lens extraction to be considered a clinically useful treatment for open angle glaucoma [26].
In summary, TSCP combined with phaco + IOL proved to be effective in controlling IOP in this group of patients with advanced glaucoma. Although this stage of glaucoma is associated with poorer prognosis and greater risk of vision loss, the vision in the vast majority of individuals either improved or remained constant. Caution must be exercized in cases of NVG where lower energy settings should be used.
Therefore, TSCP + phaco + IOL can be considered as a suitable combined procedure for the treatment of advanced glaucoma and cataract, but also, attention should be directed to other combined procedures that are emerging [33].
References
- Hennis HL and Stewart WC. “Semiconductor diode laser transscleral cyclophotocoagulation in patients with glaucoma”. American Journal of Ophthalmology 113.1 (1992): 81-85.
- Kosoko O., et al. “Long-term outcome of initial ciliary ablation with contact diode laser transscleral cyclophotocoagulation for severe glaucoma. The diode laser ciliary ablation study group”. Ophthalmology 103.8 (1996): 1294-1302.
- Brancato R., et al. “Contact transscleral cyclophotocoagulation with diode laser in refractory glaucoma”. European Journal of Ophthalmology 5.1 (1995): 32-39.
- Mistlberger A., et al. “Diode laser transscleral cyclophotocoagulation for refractory glaucoma”. Journal of Glaucoma 10.4 (2001): 288-293.
- Benson MT and Nelson ME. “Cyclocryotherapy: a review of cases over a 10-year period”. British Journal of Ophthalmology 74.2 (1990): 103-105.
- Ulbig MW., et al. “Clinical comparison of semiconductor diode versus neodymium: YAG non-contact cyclophotocoagulation”. British Journal of Ophthalmology 79.6 (1995): 569-574.
- Schlote T., et al. “Efficacy and safety of contact transcleral diode laser cyclophotocoagulation for advanced glaucoma”. Journal of Glaucoma 10.4 (2001): 294-301.
- Bloom PA., et al. “Cyclodiode: trans-scleral diode laser cyclophotocoagulation in the treatment of advanced refractory glaucoma”. Ophthalmology104.9 (1997): 1508-1519.
- Hodapp E., et al. “Clinical decisions in glaucoma”. St Louis: The CV Mosby Co, (1993):
- Vizzeri G and Weinreb RN. “Cataract surgery and glaucoma”. Current Opinion in Ophthalmology 21.1 (2010): 20-24.
- Slabaugh MA and Chen PP. “The effect of cataract extraction on intraocular pressure. Current Opinion in Ophthalmology 25.2 (2014): 122-126.
- Agrawal P1., et al. “The UK National Cyclodiode Laser Survey”. Eye (Lond) 25.2 (2011): 168-173.
- Murphy CC., et al. “A two centre study of the dose-response relation for transscleral diode laser cyclophotocoagulation in refractory Glaucoma”. British Journal of Ophthalmology 87.10 (2003): 1252-1257.
- Walland MJ. “Diode laser cyclophotocoagulation: longer term follow up of a standardized treatment protocol”. Clinical & Experimental Ophthalmology 28.4 (2000): 263-267.
- Stead RE and King AJ. “Outcome of trabeculectomy with Mitomycin C in patients with advanced glaucoma”. British Journal of Ophthalmology 95.7 (2011): 960-965.
- Gandhi A., et al. “Analysis of long-term outcomes for combined pars plana vitrectomy (PPV) and glaucoma tube shunt surgery in eyes with advanced glaucoma”. Eye (Lond) 28.3 (2014): 290-295.
- Bloom PA., et al. “A comparison between tube surgery, ND: YAG laser and diode laser cyclophotocoagulation in the management of refractory glaucoma”. BioMed Research International 2013 (2013): 371951.
- Girum W Gessesse and Karim F Damji. “Advanced glaucoma: Management pearls”. Middle East African Journal of Ophthalmology 20.2 (2013): 131-141.
- The AGIS Investigators. “The Advanced Glaucoma Intervention Study (AGIS): 7. The relationship between control of intraocular pressure and visual field deterioration”. American Journal of Ophthalmology 130.4 (2000): 429-440.
- Martinez JA., et al. “Risk of postoperative visual loss in advanced glaucoma”. American Journal of Ophthalmology 115.3 (1993): 332-337.
- Topouzis F., et al. “Risk of sudden visual loss following filtration surgery in end-stage glaucoma”. American Journal of Ophthalmology 140.4 (2005): 661-666.
- Shingleton BJ., et al. “Combined cataract and trabeculectomy surgery in eyes with pseudoexfoliation glaucoma”. Journal of Cataract & Refractive Surgery 37. 11 (2011): 1961-1970.
- Marchini G., et al. “Management of concomitant cataract and glaucoma”. Developments in Ophthalmology 59 (2012): 146-156.
- Liaska A., et al. “Phaco-Trabeculectomy in Controlled, Advanced Open-angle Glaucoma and Cataract: parallel, randomized clinical study of efficacy and safety”. Seminars in Ophthalmology 29.4 (2014): 226-235.
- Ansari E and Gandhewar J. “Long-term efficacy and visual acuity following transscleral diode laser photocoagulation in cases of refractory and non-refractory glaucoma”. Eye 21 (2006): 936-940.
- Walland MJ., et al. “There is insufficient evidence to recommend lens extraction as a treatment for primary open angle glaucoma: an evidence based perspective”. Clinical & Experimental Ophthalmology40.4 (2012): 400-407.
- Liu DT., et al. “Long-term intraocular pressure control after clear corneal phacoemulsification in glaucoma patients”. Journal of Cataract & Refractive Surgery 32.2 (2006): 183.
- Shrivastava A and Singh K. “The effect of cataract extraction on intraocular pressure”. Current Opinion in Ophthalmology 21.2 (2010): 118-122.
- Friedman DS., et al. “Surgical strategies for coexisting glaucoma and cataract: an evidence-based update”. Ophthalmology 109.10 (2002): 1902-1913.
- Kim DD., et al. “Intraocular pressure reduction following phacoemulsification cataract extraction with posterior chamber lens implantation in glaucoma patients”. Ophthalmic Surgery, Lasers and Imaging Retina 30.1 (1999): 37-40.
- Shingleton BJ., et al. “Long-term changes in intraocular pressure after clear corneal phacoemulsification: normal patients versus glaucoma suspect and glaucoma patients”. Journal of Cataract & Refractive Surgery 25.7 (1999): 885-890.
- Shingleton BJ., et al. “Three and five year changes in intraocular pressures after clear corneal phacoemulsification in open angle glaucoma patients, glaucoma suspects, and normal patients”. Journal of Glaucoma 15.6 (2006): 494-498.
- Budenz DL and Gedde SJ. “New options for combined cataract and glaucoma surgery”. Current Opinion in Ophthalmology 25.2 (2014): 141-147.
Citation:
Ejaz Ansari and Elizabeth D Hawkes. “Efficacy and Safety of Combined Trans-Scleral Cyclophotocoagulation (Tscp) and
Phacoemulsification Surgery in Advanced Glaucoma”. Ophthalmology and Vision Science 1.2 (2017): 93-98.
Copyright: © 2017 Ejaz Ansari and Elizabeth D Hawkes. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.



































 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.