Research Article
Volume 1 Issue 1 - 2017
Management of Hyperlipidemia in Very High Risk and High Risk Cardiovascular Patients: a Prospective, Interventional Phase Iv Multicenter Study Focusing on The Safety and Efficacy of Ezetimibe in Combination of Atorvastatin
1Isra University Hospital, Hyderabad, Pakistan
1Ex Professor of (Cardiology) Punjab Medical College, Consultant Cardiologist at Allied Hospital & DHQ Hospital Faisalabad
1National Institute of Cardiovascular Diseases
1Sialkot Medical Complex
1Farooq Hospital Westwood
1Heart Centre Lahore
1Sadiq Hospital Cardiac Centre, Rahim Yar Khan
1Civil Hospital Karachi
1Hilton Pharma Pvt Ltd
1Ex Professor of (Cardiology) Punjab Medical College, Consultant Cardiologist at Allied Hospital & DHQ Hospital Faisalabad
1National Institute of Cardiovascular Diseases
1Sialkot Medical Complex
1Farooq Hospital Westwood
1Heart Centre Lahore
1Sadiq Hospital Cardiac Centre, Rahim Yar Khan
1Civil Hospital Karachi
1Hilton Pharma Pvt Ltd
*Corresponding Author: Feroz Memon, Isra University Hospital, Hyderabad.
Received: June 07, 2017; Published: June 22, 2017
Abstract
Objective: The purpose of this study was to observe the efficacy, safety and tolerability of Ezetimibe in combination of Atorvastatin in very high risk and high risk cardiovascular patients.
Methods: This was a prospective, interventional phase IV multicenter study with the use of purposive sampling technique, conducted from February 2016 till December 2016. After taking the ethical approval and informed consent, 240 patients between age > 18 and < 70 years with LDL-C above optimal levels (optimal levels < 100 mg/dl) and prior history of at least one cardiac event (myocardial infarction) who were stabilized clinically after acute phase were included in the study. Out of the total of 240 patients, 211 patients completed the study. Among them, 60(28 %) cases were assessed for the total lipid profile outcome, however all patients were evaluated for LDL-C level. The total lipid profile including LDL, VLDL, HDL and triglycerides were done from the Agha Khan University Hospital Laboratory, Karachi. The basic demographic variables were recorded at the base line like age, gender and weight. The history of smoking and previous cardiac events was recorded. Those who failed to follow up, had any contraindication, hypersensitivity or intolerance for Ezetimibe and Atorvastatin and pregnant women were excluded from the study.
Each patient was followed up for a period of three months. Three study visits were performed at baseline, at 4th week and at 12th week. Incidence of adverse events (AEs) and serious AEs (SAEs) were recorded. Data analysis was done through SPSS version 20.0.
Result: Out of the total 211 patients, 85 (40%) were in high risk group and 126 (60%) were in very high risk group. In high risk and very high risk patients the mean of LDL cholesterol at base line was 162.07 mg/dl and 146.92 mg/dl with standard deviation of 58.54 mg/dl and 49.49 mg/dl respectively. At 12th week of treatment it was 109.39 mg/dl and 104.93 mg/dl with a significant p value of < 0.001. The overall reduction and achievement of the goal regardless of the category projected that in 135 (67.5%) patients there was a reduction in the LDL-C level from 30 to 50%. The overall adverse events of the therapy were almost negligible with muscle cramps and myalgia in only 2% and 2% cases respectively. Around 95% of the cases did not report any adverse events. Moreover, there was no serious adverse event reported.
Conclusion: The combined use of Ezetimibe with Statin was observed to be effective in reducing the LDL-C levels and decreased the cardiovascular risk factor.
Keywords: Hyperlipidemia; High risk and very high risk cardiovascular patients; Ezetimibe; Atorvastatin
Introduction
The primary reason of death in Pakistan is Coronary heart disease (CHD), and accounted for 111.4 thousand deaths in 2012 alone [WHO statistical profile.2015]. Likewise, CHD is the important cause of death in adults in USA, which has been related with raised levels of low-density lipoprotein cholesterol (LDL-C) and decreased levels of high-density lipoprotein cholesterol (HDL-C). The major manifestation of hyperlipidemia is premature coronary atherosclerosis which is the major cause of CHD, subsequently the monitoring of plasma lipid level is important. Therefore, hyperlipidemia also results in increase in pulmonary artery disease, CHD, high blood pressure, myocardial infarction and stroke. The person with advancing age, history of smoking and positive family history of premature ischemic heart disease increases the risk for life to many folds. Scientific explorations confirmed the reduction of cardiovascular events in patients with myocardial infarction after management of LDL-C [Pedersen., et al. 2005; The Lancet. 1994]. Dyslipidemia is one of the significant risk factors for cardiovascular disease [Luo., et al. 2016]. A reduction of LDL-C decreases the risk and ameliorates the symptoms of CHD by causing a decrease in atherosclerotic lesions [Brown., et al. 1990].
There is a direct association between serum LDL cholesterol and the occurrence of CHD and other macro vascular complications [Chatterjee., et al. 2011; Murray., et al. 1997]. Likewise, a contrary association exists between HDL cholesterol and CHD. The reduction in the LDL-C and consequently CHD is observed by the management with range of drugs including statins, fibrates, bile acid resins, and niacin. The National Cholesterol Education Program (NCEP) proposes the screening for CHD with measurement of total cholesterol (TC), LDL cholesterol, HDL cholesterol, and triglyceride (TG) concentrations for all adults over the age of 20 years, while management choices primarily established on LDL cholesterol concentrations [JAMA. 2001]. In spite of its clinical importance, LDL-C elements are infrequently assessed routinely even in high risk patients [Misra., et al. 2004; Joshi. 2003]. In high risk persons, the recommendation of LDL cholesterol therapeutic goal is < 100 mg/dL, with possible objective of < 70 mg/dL or a 30-40% decrease in LDL-C levels [Grundy., et al. 2004].
The methods of risk assessment for screening and management of patients having hyperlipidemia and cardiovascular diseases can be classified into five broad risk categories: 1. Very High – Recent acute coronary syndrome, CHD or non-coronary atherosclerotic vascular disease and one of the following: - Diabetes mellitus - Metabolic Syndrome, Current smoking, chronic kidney disease 2. High – CHD or CHD risk equivalent. Patients with widespread subclinical atherosclerosis, chronic kidney disease and receivers of solid organ transplant are also at high risk. 3. Moderately High – No CHD with > 2 risk factors and 10 years CHD risk 10-20%. 4. Moderate –No CHD with > 2 risk factors and 10 years CHD risk < 10%. 5. Lower – No CHD with < 1 risk factor [Patrick., et al. 2017].
However, it is difficult to achieve these target levels with the administration of a statin alone [Weng., et al. 2010]. Lipids are closely involved in coronary artery disease due to their contribution in atherogenic process. Therefore, the beneficial mediations are intended for satisfactory modifications of lipoprotein metabolism, predominantly reducing low-density lipoprotein cholesterol (LDL-C), which is the keystone of management for primary and secondary cardiovascular disease. The statins are a group of 3-Hydroxy-3-methylglurtaryl coenzyme A inhibitors that are important medications for patients with CAD to decrease the threat of adverse cardiovascular events through their LDL-C-lowering consequence. Substantial proof exists for a strong relationship between LDL-C level and cardiovascular event rate, supporting the “lower the better” hypothesis [Flather., et al. 2010; Armitage., et al. 2010; Cannon., et al. 2004].
Recently, the National Cholesterol Education Program Adult Treatment Panel recommended an optical LDL goal of < 70 mg/dl for individuals with higher risk, including cardiovascular disease patients with additional high-risk factors: diabetes mellitus, multiple cardiovascular risk factors, multiple risk factors of metabolic syndrome, or severe or poorly controlled risk factors, especially smoking [Grundy., et al. 2004].
Ezetimibe is the medication that inhibits cholesterol absorption at brush borders of the intestine and has no effect on the absorption of triglycerides and fat soluble vitamins. Thus it impairs dietary and biliary absorption of cholesterol [Matsue., et al. 2013]. The objective of this study was to observe the efficacy, safety and tolerability of Ezitimibe in combination with Atorvastatin in very high risk and high risk patients in Pakistan.
Methods
Study Population
After taking the informed consent, 240 patients were selected for the study. Patients of either gender who gave consent to participate with the age > 18 and < 70 years with LDL-C levels above optimal levels (optimal levels < 100 mg/dl) with prior history of at least one cardiac event (myocardial infarction) but stabilized clinically after acute phase were included in the study. Those who did not give the consent, failed to follow up, having any contraindication to Ezetimibe and Atorvastatin like active liver pathology or unexplained raise of alanine aminotransferase or aspartate aminotransferase levels, with history of substantial myopathy or rhabdomyolysis with any statin or Ezetimibe, sensitivity to Ezetimibe or Atorvastatin, and pregnant women were disqualified from the study.
After taking the informed consent, 240 patients were selected for the study. Patients of either gender who gave consent to participate with the age > 18 and < 70 years with LDL-C levels above optimal levels (optimal levels < 100 mg/dl) with prior history of at least one cardiac event (myocardial infarction) but stabilized clinically after acute phase were included in the study. Those who did not give the consent, failed to follow up, having any contraindication to Ezetimibe and Atorvastatin like active liver pathology or unexplained raise of alanine aminotransferase or aspartate aminotransferase levels, with history of substantial myopathy or rhabdomyolysis with any statin or Ezetimibe, sensitivity to Ezetimibe or Atorvastatin, and pregnant women were disqualified from the study.
Study Design
This was a prospective, interventional phase IV multicenter study conducted in 30 medical centers across Pakistan. The ethical approval was taken from the Institutional Review Board of Isra University, Hyderabad and the duration of the study was from Feb 2016 till December 2016.
This was a prospective, interventional phase IV multicenter study conducted in 30 medical centers across Pakistan. The ethical approval was taken from the Institutional Review Board of Isra University, Hyderabad and the duration of the study was from Feb 2016 till December 2016.
In the total of 240 patients included using purposive sampling technique, 211 patients completed the study. Among 211, 60 cases (28%) were assessed for the total lipid profile outcome, however all patients were evaluated for LDL-C level. The total lipid profile including LDL, VLDL, HDL and triglycerides were done from the Agha Khan University Hospital Laboratory, Karachi. The basic demographic variables were recorded at the base line like age, gender and weight along with the history of smoking and previous cardiac event. The patients were classified into very high risk and high risk category on the basis of history of previous cardiac event, diabetes mellitus, dyslipidemia, smoking, advancing age. The patients who had history of any of the cardiac event like heart attack, angina, or any of the previous cardiac surgery were classified into very high risk category and the patients who were having advance age, history of smoking, and hyperlipidemia were classified as high risk patients.
All patients were treated with Ezetimibe and Atrovastatin 10 + 40 mg/day orally for 12 weeks. Patients were instructed to take each dose at the same time every day, regardless of mealtime. Every patient was monitored for a period of 03 months. 03 study visits were performed; baseline, 04 weeks and at 12 weeks. The total Lipid profile was assessed at 12th week.
Safety and Efficacy Evaluation
Serum LDL-C levels were noted at baseline and at 12th week for assessing the effectiveness of the combination. The safety valuations comprised of observing and documenting all adverse events (AEs) and serious adverse events (SAEs).
Serum LDL-C levels were noted at baseline and at 12th week for assessing the effectiveness of the combination. The safety valuations comprised of observing and documenting all adverse events (AEs) and serious adverse events (SAEs).
Statistical Analysis
Data was analyzed by SPSS version 20.0. For continuous variables, summary statistics included mean, standard deviation, median, minimum and maximum values, as well as frequencies and percentages for categorical variables are presented. The paired t-test was used to see the significance at different levels of treatment at 4th week and at 12th week. P-values of < 0.05 were considered to be significant.
Data was analyzed by SPSS version 20.0. For continuous variables, summary statistics included mean, standard deviation, median, minimum and maximum values, as well as frequencies and percentages for categorical variables are presented. The paired t-test was used to see the significance at different levels of treatment at 4th week and at 12th week. P-values of < 0.05 were considered to be significant.
Results
Out of the total number of 211 patients 85 were in high risk group and 126 were in very high risk group. The mean age of the patients in high risk group was 51.5 (± 11.2) years. The mean age of the patients in very high risk group was 52.3 (± 11.0) years. Male to female ratio was 1:2 in both the high risk and very high risk categories. (Table 1) Among 85 patients of high risk group 22 (26%) were having the history of smoking and were present smokers as well. Whereas in very high risk group 38 (30%) of the patients were having the history of smoking and were present smokers as well
| High Risk | Very High Risk | |||
| Age | Weight | Age | Weight | |
| N | 85 | 85 | 126 | 126 |
| Mean | 51.5 | 70.5 | 52.33 | 74.18 |
| Std. Deviation | 11.2 | 13.6 | 11.085 | 13.183 |
| Minimum | 22 | 45 | 24 | 40 |
| Maximum | 69 | 125 | 85 | 140 |
| Gender | High Risk | Very High Risk | ||
| Male | 33% | 32% | ||
| Female | 67% | 68% | ||
N, Number of patients; Std. Deviation, standard deviation.
Table 1: Patient demographics.
Table 1: Patient demographics.
In high risk patients the mean of heart rate at base line was 85 beats/min whereas, the mean of heart rate at 4th week of treatment was 81 beats/min with a significant p value of 0.001. In very high risk patients the mean of heart rate at base line was 81 beats/min whereas, the mean of heart rate at 4th week of treatment was 78 beats/min with a significant p value of 0.001. In high risk patients the mean of systolic blood pressure at base line was 139.7 mmHg whereas, at 4th week of treatment it was 138.7 mmHg with insignificant p value of 0.635. In very high risk patients the mean of systolic blood pressure at base line was 144.2 mmHg whereas, at 4th week of treatment it was 138 mm/Hg with a significant p value of < 0.001. In high risk patients the mean of diastolic blood pressure at base line was 86.4 mm/Hg whereas, at 4th week of treatment it was 88.5 mm/Hg with insignificant p-value of 0.11. In very high risk patients the mean of diastolic blood pressure at base line was 87.8 mm/Hg whereas, at 4th week of treatment it was 85.8 mm/Hg with a significant p value of 0.029. (Table 2).
The comparison of low density lipoprotein-cholesterol levels was done in 211 patients. Out of these 126 were in very high risk category having the history of any of the cardiac event like, heart attack, angina, or any of the previous cardiac surgery and 85 were in high risk category having advance age, history of smoking, and hyperlipidemia. In high risk patients the mean of LDL cholesterol at base line was 162.07 mg/dl with standard deviation of 58.54 mg/dl. Whereas, the mean of LDL cholesterol at 12th week of treatment was 109.39 mg/dl with standard deviation of 58.36 mg/dl with a significant p value of < 0.001. In very high risk patients the mean of LDL cholesterol at base line was 146.92 mg/dl with standard deviation of 49.49 mg/dl. Whereas, the mean of LDL cholesterol at 12th week of treatment was 104.93 mg/dl with standard deviation of 40.21 mg/dl, with a significant p value of < 0.001. (Table 2)
| Categories | Variables | Mean | Std. Dev | P-value | |
|
High Risk (85) |
Heart Rate (/min) |
At baseline | 85.1 | 11.1 | 0.001 |
| At 4th week | 81.3 | 8.9 | |||
| Systolic Blood Pressure (mm Hg) |
At baseline | 139.7 | 19 | 0.635 | |
| At 4th week | 138.7 | 16.8 | |||
| Diastolic Blood Pressure (mm Hg) |
At Baseline | 86.4 | 10.6 | 0.11 | |
| At 4th week | 88.5 | 7.8 | |||
| Serum LDL Cholesterol (mg/dl) | At baseline | 162.07 | 58.54 | < 0.001 | |
| At 12th week | 109.39 | 58.36 | |||
| Very High Risk (126) | Heart Rate (/min) |
At baseline | 81.6 | 8.6 | < 0.001 |
| At 4th week | 78.5 | 7.6 | |||
| Systolic Blood Pressure (mm Hg) |
At baseline | 144.2 | 17.9 | < 0.001 | |
| At 4th week | 138.7 | 14.4 | |||
| Diastolic Blood Pressure (mm Hg) |
At Baseline | 87.8 | 9.5 | 0.029 | |
| At 4th week | 85.8 | 7.1 | |||
| Serum LDL Cholesterol (mg/dl) | At baseline | 146.29 | 49.49 | < 0.001 | |
| At 12th week | 104.93 | 40.21 | |||
| Paired t-test is used to assess the significance | |||||
Table 2: Systolic and Diastolic Blood Pressure of High Risk and Very High Risk at Baseline and 4th Week.
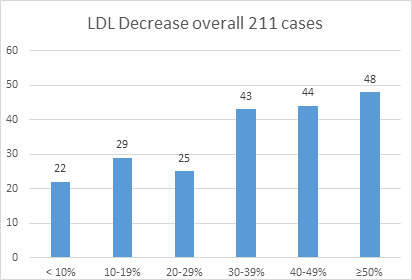
The overall reduction and achievement of the goal regardless of the category predicted that, in 135 (67.5%) patients there was a decrease in the LDL-C level from 30% to 50%. (Figure 1)
In high risk group out of 85 patients 66 (79%) achieved the goal whereas in very high risk 38 (30%) of the patients achieved the goal despite of the higher level of the baseline LDL-C in both the categories. (Table 3)
| High Risk n = 85 LDL Goal 100 mg/dl | Very High Risk n = 126 LDL Goal 70 mg/dl | ||
| Frequency | Percentage | Frequency | Percentage |
| 66 | 79 | 38 | 30 |
Table 3: LDL Cholesterol Goals according to the guideline in High Risk and Very High Risk.
In 60 patients, who were evaluated for the whole lipid profile serum cholesterol was 210.72 mg/dl with standard deviation of 47.97 mg/dl at base line and it was 151.25 with standard deviation of 38.72 at 12th week after the treatment with the significant p-value of < 0.001. The triglycerides was 205.58 mg/dl with standard deviation of 86.35 mg/dl at base line and it was 161.40 with standard deviation of 85.05 at 12th week after the treatment with the significant p-value of 0.006.Serum HDL was 41.03 mg/dl with standard deviation of 10.99 mg/dl at base line and it was 38.13 with standard deviation of 8.41 at 12th week after the treatment with the significant p-value of 0.0032. Serum LDL was 143.67 mg/dl with standard deviation of 42.26 mg/dl at base line and it was 114.57 with standard deviation of 33.38 at 12th week after the treatment with the significant p-value of < 0.001. Serum VLDL was 39.0 mg/dl with standard deviation of 20.40 mg/dl at base line and it was 32.38 with standard deviation of 15.93 at 12th week after the treatment with the significant p-value of < 0.009. (Table 4)
| Variables (n = 60) | Follow-up | Mean | Std. Dev | P-value |
| Serum Cholesterol mg/dl | Baseline | 210.72 | 47.97 | < 0.001 |
| 12th week | 151.28 | 38.72 | ||
| Serum Triglycerides mg/dl | Baseline | 205.58 | 86.35 | 0.006 |
| 12th week | 161.40 | 85.05 | ||
| Serum HDL Cholesterol mg/dl | Baseline | 41.03 | 10.99 | 0.0032 |
| 12th week | 38.13 | 8.41 | ||
| Serum LDL Cholesterol mg/dl | Baseline | 143.67 | 42.26 | < 0.001 |
| 12th week | 114.57 | 43.38 | ||
| VLDL Cholesterol mg/dl. | Baseline | 39.00 | 20.40 | 0.009 |
| 12th week | 32.38 | 15.93 | ||
| Paired t-test is used to assess the significance | ||||
Table 4: Lipid Profile in High Risk and Very High Risk at Baseline and 12th Week.
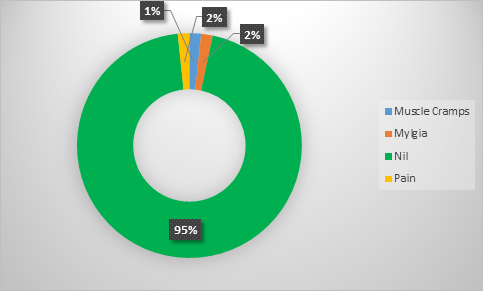
The overall adverse events of the therapy were almost negligible with muscle cramps and myalgia in only 2% and 2% cases respectively. Around 95% of the cases did not report any of the adverse events. Moreover, none of the patients reported any serious adverse event (Figure 2).
Discussion
The aim of any cholesterol lowering intervention is to decrease around 40% to 50% of LDL-C in order to decrease the threat of any cardiovascular event in both very high and high risk patients. In patients with high or very high risk of coronary heart disease (CHD) and similar critical conditions, (LDL-C) should be reduced by at least 30-50% in order to obtain a clinical benefit [Whayne., et al. 2013]. According to the guidelines formulated by American College of Cardiology (ACC) and American Heart Association (AHA), the reduction of LDL-C to 100 mg/dl in both the high risk and very high risk cardiovascular patients is greatly recommended. [ATP4. 2014]
High-risk patients need intensive lipid-modifying remedy to attain LDL-cholesterol objectives [Baigent., et al. 2010; Flather., et al. 2010]. Therefore, the first choice for reducing the LDL-cholesterol are statins, but their combination with Ezetimibe produce considerable decrease [Davis., et al. 2007; Catapano., et al. 2006]. The decrease in intestinal absorption of cholesterol with Ezetimibe at a dose of 10 mg/kg is more than 90% and for chylomicron and VLDL the reduction is 87% [Repa., et al. 2005]. Ezetimibe is metabolized mainly in the small intestine and liver through glucuronide conjugation to ezetimibe-glucuronide in humans, and is excreted through bile and kidney.
In another study in patients with metabolic disease, a comparable effect was acknowledged with combination therapy attaining a better decrease in LDL-C and non-HDL-C and greater increase in HDL-C as related to atorvastatin mono therapy [Hamilton-Craig., et al. 2010].
In one of the study there was around 21% greater reduction with combination therapy as compared to the mono therapy with Statins alone [Robinson., et al. 2009]. The study by Davis., et al. stated that the combination therapy of Ezetimibes 40 mg/d with statin resulted in 52% reduction of LDL-C level in acute coronary syndrome subjects [Davis., et al. 2001].
Ezetimibe is stated to decrease the cholesterol content in chylomicrons with apoB48, commencing a course that apparently diminishes the cholesterol content of large VLDL and subsequently decreases VLDL fragments and LDL-C [Deharo., et al. 2014].
Whereas in our study the LDL-C was reduced to 35% to 40% to the baseline both in the very high risk and very high risk categories which is in agreement to the guidelines by National Cholesterol Education Program (NCEP) through the Adult Treatment Panel ATP IV. However, the HDL-C levels did not alter substantially in our study which is inconsistent with the study by Hamilton., et al. which documented greater increase in its level [Hamilton-Craig., et al. 2010].
There is a clear evidence for safety of ezetimibe as monotherapy or in combination with other lipid-modifying drugs including statins [Pandor., et al. 2009; Robinson., et al. 2009]. Although adverse events have been labeled with all lipid-altering treatments such as statins, niacin, and fibrates, lethal toxicities are occasional and the complete safety profile of these therapies is reasonably satisfactory [Baigent., et al. 2005; Guyton., et al. 2007; Davidson., et al. 2007].
A meta-analysis of seven randomized precise trials revealed that myositis is not associated with monotherapy and combination therapy [Kashani., et al. 2008] however ezetimibe is efficient in inhibiting the rise in intestinal sterols absorption, but increases the endogenous cholesterol production [Assmann., et al. 2008].
The above findings are contrary to our study in which we had found no adverse events in 95% of the patients. The muscle cramps, myalgia and pain were reported in 2%, 2% and 1% cases respectively. However no serious adverse event was reported.
The advantages of this study are that our appropriate assortment approach has assured that we have sampled the accounts of extensive range of physician observations and patient’s perspectives and their abilities of handling the patients of dyslipidemia in very high risk and high risk category. Furthermore, it conveys extensive extractions of the practitioner’s perceptions. However, witness and recall bias were few limitations of the study. Reflecting the opinions of patients and experience and to what range they are reliable with other treatment options would be enlightening and valuable to overcome the risk of CHD and management of dyslipidemia.
Conclusion
The combined use of Ezetimibe with Statin is useful in reducing the LDL-C levels and decreases the cardiovascular risk factor. It is more useful because of its efficacy and few adverse events. The medicating of simple once regular dosage increases the compliance over many existing agents as well.
Acknowledgements
The authors acknowledge all investigators at the participating centers and all patients for their commitment to the study. All authors participated in the data collection, development and writing of the manuscript and take full responsibility for the content of the article. Authors thank Dr. Adnan Anwar, medical research consultant (The Research Professional services Ltd), for writing and editorial assistance. All authors read and delivered concluding approval of the description to be printed. All authors agree to be responsible for all characteristics of the work and ensure that uncertainty associated to the precision of any part of the work are properly explored and committed.
The authors acknowledge all investigators at the participating centers and all patients for their commitment to the study. All authors participated in the data collection, development and writing of the manuscript and take full responsibility for the content of the article. Authors thank Dr. Adnan Anwar, medical research consultant (The Research Professional services Ltd), for writing and editorial assistance. All authors read and delivered concluding approval of the description to be printed. All authors agree to be responsible for all characteristics of the work and ensure that uncertainty associated to the precision of any part of the work are properly explored and committed.
Funding
This work was supported financially by Hilton Pharma Pvt Ltd, that provided the medicine and the cost of the laboratory tests performed during the intervention.
This work was supported financially by Hilton Pharma Pvt Ltd, that provided the medicine and the cost of the laboratory tests performed during the intervention.
Conflict of interest
The designer and benefactor of the study was Hilton Pharma Pvt Ltd. Furthermore it was involved in data organization, data evaluation, drafting and appraisal of the text. NM and AS are full-time employees of Hilton Pharma Pvt ltd. The authors report no other encounters of attention. The honesty of the study was not traded for any monetary benefit. The remuneration was given to the observers for examining and entry of the data during the study duration.
The designer and benefactor of the study was Hilton Pharma Pvt Ltd. Furthermore it was involved in data organization, data evaluation, drafting and appraisal of the text. NM and AS are full-time employees of Hilton Pharma Pvt ltd. The authors report no other encounters of attention. The honesty of the study was not traded for any monetary benefit. The remuneration was given to the observers for examining and entry of the data during the study duration.
References
- Armitage J., et al.“Intensive lowering of LDL cholesterol with 80 mg versus 20 mg simvastatin daily in 12,064 survivors of myocardial infarction: a double-blind randomised trial”. Lancet (London, England) 376.9753 (2010): 1658-1669.
- Assmann G., et al. “Effects of ezetimibe, simvastatin, atorvastatin, and ezetimibe–statin therapies on non-cholesterol sterols in patients with primary hypercholesterolemia”. Current medical research and opinion 24.1 (2008): 249-259.
- Baigent C., et al. “Efficacy and safety of cholesterol-lowering treatment: prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins”. Lancet 366.9493 (2005): 1267-1278.
- Baigent C., et al. “Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170,000 participants in 26 randomized trials”. Lancet 376.9753 (2010): 1670-1681.
- Brown G., et al. “Regression of coronary artery disease as a result of intensive lipid-lowering therapy in men with high levels of apolipoprotein B”. The New England Journal of Medicine 323.19 (1990): 1289-1298.
- Cannon CP., et al. “Intensive versus moderate lipid lowering with statins after acute coronary syndromes”. The New England Journal of Medicine 350.15 (2004): 1495-1504.
- Catapano AL., et al. “Lipid-altering efficacy of the ezetimibe/simvastatin single tablet versus rosuvastatin in hypercholesterolemic patients”. Current medical research and opinion22.10 (2006): 2041-2053.
- Chatterjee S and Mendez D. “A Comparative And Correlative Study of Direct LDL Assay With Friedwald's Formula in Rural Kolar Population”. Journal of Clinical and Biomedical Sciences 1.4 (2011): 158-163.
- Danial M., et al. “Dyslipidemia ATP4”. (2014): 1-9.
- Davidson MH., et al. “Safety considerations with fibrate therapy. American Journal of Cardiology 99.6A (2007): 3C-18C.
- Davis HR Jr., et al. “The synergistic hypocholesterolemic activity of the potent cholesterol absorption inhibitor, ezetimibe, in combination with 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibitors in dogs”. Metabolism50.10 (2001): 1234-1241.
- Davis HR and Veltri EP. “Zetia: inhibition of Niemann-Pick C1 Like 1 (NPC1L1) to reduce intestinal cholesterol absorption and treat hyperlipidemia”. Journal of Atherosclerosis and Thrombosis 14.3 (2007): 99-108.
- Deharo P., et al. “Safety and effectiveness of the association ezetimibe-statin (E-S) versus high dose rosuvastatin after acute coronary syndrome: the safe-ES study”. Annales de Cardiologie et d'Angéiologie 63.4 (2014): 222-227.
- “Executive summary of the Third Report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III)”. JAMA285.19 (2001): 2486-2497.
- Flather M. “Efficacy and safety of more intensive lowering of LDL cholesterol: a meta-analysis of data from 170000 participants in 26 randomised trials”. The Lancet376.9753 (2010): 1670-1681.
- Grundy SM., et al. “Implications of recent clinical trials for the national cholesterol education program adult treatment panel III guidelines”. Circulation 110.2 (2004): 227-239.
- Guyton JR and Bays HE. “Safety considerations with niacin therapy”. American Journal of Cardiology 99.6A (2007): 22C-31C.
- Hamilton Craig I., et al. “Combination therapy of statin and ezetimibe for the treatment of familial hypercholesterolemia”. Vascular Health and Risk Management6 (2010): 1023-1037.
- Joshi SR. “Metabolic syndrome-Emerging clusters of the Indian phenotype”. The Journal of the Association of Physicians of India 51 (2003): 445-446.
- Kashani A.,et al. “Review of side-effect profile of combination ezetimibe and statin therapy in randomized clinical trials”. American Journal of Cardiology101.11 (2008): 1606-1613.
- Luo P., et al. “Impact of Atorvastatin Combined with Ezetimibe for the Treatment of Carotid Atherosclerosis in Patients with Coronary Heart Disease”. Acta Cardiologica Sinica32.5 (2016): 578-585.
- Matsue Y., et al. “Differences in action of atorvastatin and ezetimibe in lowering low-density lipoprotein cholesterol and effect on endothelial function”. Circulation Journal 77.7 (2013): 1791-1798.
- Misra A and Vikram NK. “Insulin resistance syndrome (metabolic syndrome) and obesity in Asian Indians: evidence and implications”. Nutrition 20.5 (2004): 482-491.
- Murray CJ and Lopez AD. “Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study”. The Lancet 349.9063 (1997): 1436-1442.
- Pakistan: WHO statistical profile. WHO (2015):
- Pandor A., et al. “Ezetimibe monotherapy for cholesterol lowering in 2,722 people: systematic review and meta-analysis of randomized controlled trials”. Journal of Internal Medicine 265.5 (2009): 568-580.
- Patrick McBride. Guidelines for the Diagnosis and Management of Dyslipidemia for Adults > 18 years of age (2008):
- Pedersen TR., et al. “High-dose atorvastatin vs usual-dose simvastatin for secondary prevention after myocardial infarction: the IDEAL study: a randomized controlled trial”. JAMA 294.19 (2005): 2437-2445.
- Repa JJ., et al. “Delineation of molecular changes in intrahepatic cholesterol metabolism resulting from diminished cholesterol absorption”. The Journal of Lipid Research 46.4 (2005): 779-789.
- Robinson JG., et al. “Lipid-altering efficacy and safety of ezetimibe/simvastatin versus atorvastatin in patients with hypercholesterolemia and the metabolic syndrome (from the VYMET study)”. American Journal of Cardiology 103.12 (2009): 1694-1702.
- Pedersen TR., et al. “Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). (1994)”. Atherosclerosis Supplements 5.3 (2004): 81-87.
- Weng TC., et al. “A systematic review and meta‐analysis on the therapeutic equivalence of statins”. Journal of clinical pharmacy and therapeutics 35.2 (2010): 139-151.
- Whayne TF. “Assessment of low-density lipoprotein targets”. Angiology64.6 (2012): 411-416.
Citation:
Feroz Memon., et al. “Management of Hyperlipidemia in Very High Risk and High Risk Cardiovascular Patients: a Prospective, Interventional Phase Iv Multicenter Study Focusing on The Safety and Efficacy of Ezetimibe in Combination of Atorvastatin”. Therapeutic Advances in Cardiology 1.1 (2017): 42-51.
Copyright: © 2017 Feroz Memon., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.





























 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.