Case Report
Volume 1 Issue 3 - 2017
Short-Term Contralateral Recurrence of an Aldosterone-Producing Adenoma: A Possible Case of Familial Hyperaldosteronism Type II
1Endocrinology and Nutrition Dpt, University Hospital of Gran Canaria Doctor Negrin, Las Palmas de Gran Canaria, Canary Islands, Spain
2Endocrinology and Nutrition Dpt, Hospitales San Roque, Las Palmas de Gran Canaria, Canary Islands, Spain
3Outpatient Hypertension Clinic, University Hospital of Gran Canaria Doctor Negrin, Las Palmas de Gran Canaria, Canary Islands, Spain
2Endocrinology and Nutrition Dpt, Hospitales San Roque, Las Palmas de Gran Canaria, Canary Islands, Spain
3Outpatient Hypertension Clinic, University Hospital of Gran Canaria Doctor Negrin, Las Palmas de Gran Canaria, Canary Islands, Spain
*Corresponding Author: Francisco Javier Martinez-Martin, Outpatient Hypertension Clinic, University Hospital of Gran Canaria Doctor Negrin, Las Palmas de Gran Canaria, Canary Islands, Spain.
Received: October 29, 2017; Published: November 03, 2017
Abstract
A hypertensive 59-year-old woman with chronic renal failure (eGFR 23.44 ml/min/1.73 m2) was referred to our Hypertension Clinic after the finding of a left adrenal mass during hypertension workup, with a normal right adrenal. Physical exam was unremarkable except for blood pressure 167/98 mmHg. Plasma aldosterone was 35.3 ng/dL, renin activity 1.3 ng/ml/h, ratio 90.2, K+ 3.1 mEq/l, and metanephrines were normal. Primary aldosteronism was confirmed by a standard captopril test. Adrenal vein sampling showed full left lateralization of aldosterone secretion. The patient underwent laparoscopic left adrenalectomy, and a 21 g left adrenal -including a 4 cm adenoma with clean margins- was removed. One month after surgery, K+, aldosterone and renin activity were normal, and blood pressure was controlled with manidipine 10 mg q.d.
One year later, blood pressure and plasma K+ were still controlled but the patient showed again primary aldosteronism: aldosterone 48.8 ng/dL, renin activity 0.4 ng/mL/h, ratio 122, K+ 4.6 mEq/L. A new CT showed absent left adrenal, and a 17 x 11 mm adenoma in the right adrenal. Eplerenone was added to the treatment, but no new surgery was performed.
We diagnosed: Primary aldosteronism caused by aldosterone-producing adenoma with contralateral recurrence after one year; Secondary hypertension with stage IV chronic renal failure.
Contralateral recurrence of an aldosterone-producing adenoma in a previously normal adrenal after a complete surgical removal is exceedingly rare, particularly after such a short follow-up. As several family members have also presented with bilateral adrenal adenomas, the patient’s differential diagnosis would include familial type II aldosteronism.
Keywords: Primary Aldosteronism; Aldosterone-Producing Adenoma; Contralateral Recurrence; Familial Hyperaldosteronism type II; Secondary Hypertension
Abbreviations: APCC: aldosterone-producing cell clusters; AVS: adrenal venous sampling; BP: blood pressure; BMI: body mass index; CACNA1: calcium voltage-gated channel subunit alpha-1: CKD-EPI: Chronic Kidney Disease Epidemiology Collaboration; CT: computed tomography; CYP11B1, 2: cytochrome P450 family 11 subfamily B member 1, 2; eGFR: estimated glomerular filtration rate; FH-I, II, III, IV: familial hyperaldosteronism type I,II, III, IV; HDL: high-density lipoprotein; HR: heart rate; KCNJ5: potassium voltage-gated channel subfamily J member 5; LDL: low-density lipoprotein; NSAID: non-steroid anti-inflammatory drug; PA: primary aldosteronism; PRA: plasma renin activity; SPARTACUS: subtyping primary aldosteronism: a randomized trial comparing adrenal vein sampling and computed tomography scan; SPECT-MIBI: single-photon emission computed tomography-methoxy-isobutyl-isonitrile [99mTc-label]
Introduction
It is commonly accepted that the prevalence of primary aldosteronism (PA) in hypertensive patients is about 10%, much higher than previously thought [1], although this might still be a major underestimation [2]. PA is known to have adverse consequences above and beyond hypertension development [1], with a greatly increased incidence of major cardiovascular events than in essential hypertensives well matched for age, gender and blood pressure (BP) elevation [1, 3-5]. Thus, PA has become a public health issue, with less than 1% of the patients appropriately treated and diagnosed [2].
The current guidelines for the management of PA [6] stress the importance of a correct diagnosis. The distinction between unilateral and bilateral disease is best made with adrenal venous sampling (AVS) [1, 6-8]. When AVS clearly shows lateralization, surgical resolution of the PA is the norm, including patients with non-single adenoma [9]. In fact the recent French consensus on PA [10] go as far as to state that lateralized PA “never shows recurrence in the contralateral adrenal” (high blood pressure, notwithstanding, may persist in about 40% of the patients, with wide variation among series [7, 11-12]).
The PASO study [13] (Primary Aldosteronism Surgery Outcome, a recent international analysis of remission rates in PA) shows that complete biochemical cure of unilateral PA is achieved in 94% of the patients after surgery. The few patients who are not cured are almost invariably not recurrences but primary failures with misdiagnosis of lateralization in AVS [11] or incomplete surgery [14-15]. Thus, contralateral recurrence of a lateralized PA is exceedingly rare.
Hereby we present the case of a woman who had a short-term contralateral recurrence of an aldosterone-producing adenoma after showing lateralization in AVS and undergoing successful complete surgery. We hypothesize that the underlying cause may be a variant of familial hyperaldosteronism type II (FH-II).
Clinical Case
A 59-year-old female patient with personal history of hypertension and nephroangiosclerotic stage IV chronic renal failure (CKD-EPI estimated GFR 23 ml/min/1.73 m2) was referred to our Hypertension Clinic due to the finding of a left adrenal nodule measuring 1.9 x 1.7 mm in an abdominal ultrasonography performed as a part of hypertension workup (April 2010). An abdominal CT requested for confirmation showed a 26 mm mass with adenoma density in the left adrenal with normal right adrenal gland.
Her family history was notorious: She had a son with hypertension and end-stage renal failure who had undergone renal transplant at the age of 35 yr. after a protracted history of primary aldosteronism with bilateral adrenal adenomas (Figure 1). She had also two hypertensive first-grade cousins who underwent surgery of adrenal-producing adenomas and renal transplant. Her mother suffered from hypertension as well although no diagnostic workup had been performed. The patient’s father died at the age of 61 of acute myocardial infarction.
The patient had been on antihypertensive treatment for the last 20 years (bisoprolol 2.5 mg and irbesartan 150 mg q.d. at the moment of consultation) but no episodes of hypokalemia had been reported. She was also treated for combined dyslipidemia with atorvastatin 40 mg plus fenofibrate 145 mg q.d., and for hyperuricemia with 100 mg allopurinol q.d. She had complained of occasional chest pain but an ergometry and a heart SPECT-MIBI gammagraphy were negative. She had been diagnosed of allergy to penicillin and methamizole, and had neither smoking nor drinking habits. She had received prolonged courses of NSAIDs for chronic osteoarthritis. Her only previous surgery was a lumpectomy of a benign left breast fibroadenoma (2004).
On examination the BP was high: 167/98 mmHg, with heart rate (HR) 57 bpm. Height was 162 cm, weight 72 kg, body mass index (BMI) 27.4 Kg/m2, waist circumference 99 cm. The rest of physical exam was unremarkable.
Lab tests were requested, with the following results: Glucose 85 mg/dL, creatinine 1.92 mg/dL, Na+ 140 mEq/L, K+ 4.4 mEq/L, total cholesterol 172 mg/dL, HDL-cholesterol 41 mg/dL, LDL-cholesterol (estimated) 105 mg/dL, triglycerides 132 mg/dL, liver enzymes were normal, TSH, plasma cortisol and ACTH were normal, 24h. free urinary cortisol was normal. Plasma aldosterone was 35.3 ng/dL, plasma renin activity (PRA) 1.3 ng/mL/h, ratio 27.2, K+ 3.1 mEq/L, albuminuria was negative and metanephrines were normal. Primary aldosteronism was confirmed by a standard captopril test.
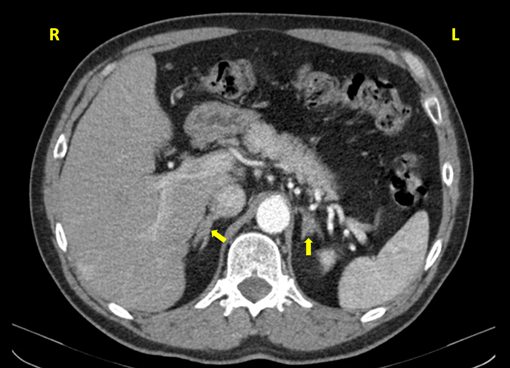
The abdominal CT scan, performed in January 2012 (Figure 2) confirmed the presence of the left adrenal nodule, 2.6 cm in diameter, somewhat heterogeneous but mostly with adenoma density (< 10 Hounsfield units). The right adrenal was normal, with both kidneys of normal size and slight hyperdensity and thinning of the cortical layer, without cysts or nodules.
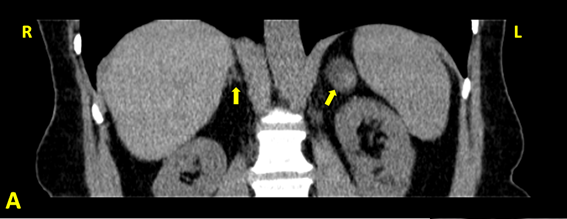
Figure 2A: Abdominal CT of the patient (coronal view): a normal right
adrenal and a left adrenal adenoma.
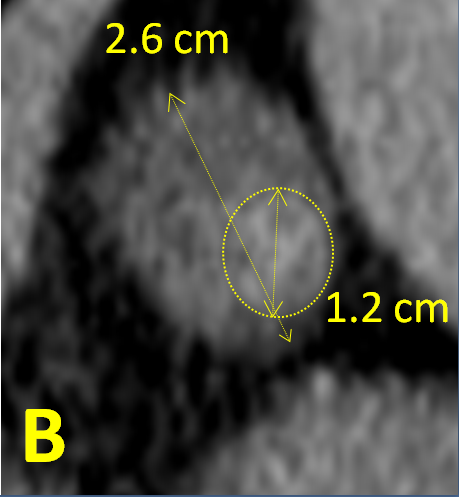
Figure 2B: Closer view of the left adrenal adenoma (major diameter 2.6 cm)
heterogeneous in density (predominantly < 10 Hounsfield units but with a denser
region, major diameter 1.2 cm, averaging 36 Hounsfield units).
AVS (November 2011) revealed full left lateralization of aldosterone secretion (peripheral aldosterone/cortisol ratio 18.77, right adrenal vein 19.54, left adrenal vein > 100).
Surgery was scheduled and in the meanwhile, eplerenone 50 mg daily was added to the treatment. On March 2012 the patient underwent unilateral laparoscopic adrenalectomy, and a 21g left adrenal - including a 4.0 cm adenoma with clean margins - was removed. The adrenal cytology was clearly benign but the adenoma included a 1.2 cm oncocytic nodule.
One month later, K+, aldosterone and PRA were normal, and blood pressure was controlled in the successive months (135/75 mmHg maximum) with manidipine 10 mg q.d. only. Eplerenone, irbesartan and bisoprolol had been withdrawn immediately after surgery.
One year after the surgery (March 2013), during the control check-out, blood pressure and plasma K+ were still controlled but the patient showed again a pattern of primary aldosteronism with plasma aldosterone 48.8 ng/dL, PRA 0.4 ng/mL/h, ratio 122, K+ 4.6 mEq/L. These findings were confirmed in August 2013, with plasma aldosterone 38.7 ng/dL, PRA 0.2 ng/mL/h, ratio 193.5. A new CT was requested and performed on February 2014 (Figure 3): the left adrenal was absent, and the right adrenal showed presence of a 1.7 x 1.1 cm mass with adenoma density (< 10 Hounsfield units).
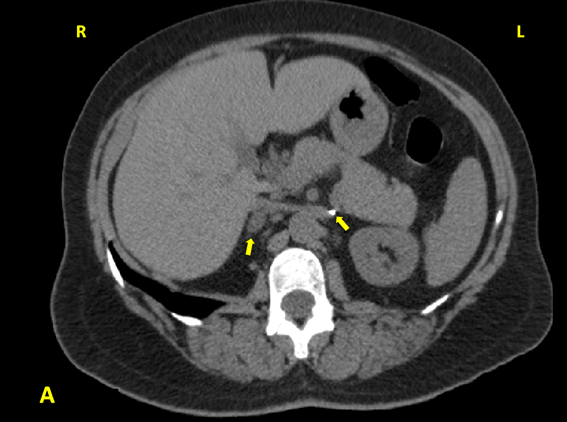
Figure 3A: Abdominal CT of the patient: surgical clip marking the location of the absent left
adrenal; right adrenal adenoma which was not apparent in the previous abdominal CT scan.
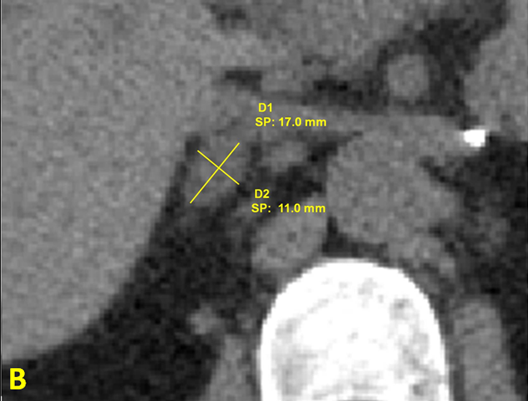
Figure 3B: Closer view of the right adrenal adenoma
(diameter 1.7 x 1.1 cm), density < 10 Hounsfield units.
Low-dose (12.5 mg q.d.) eplerenone was added to the treatment. On follow up the dose was increased to 25 mg b.d. Surgery of the right adrenal vs. mineralocorticoid receptor blockade was considered, but the additional benefit was considered uncertain while the harm of causing permanent adrenal insufficiency seemed clearly larger.
At present, 4 years after the second adenoma was diagnosed, the patient’s BP is well controlled and her K+ levels are normal, but her renal function is slowly deteriorating and she is in predialysis nephrologic care. A recent blood test (September 2017) shows normal glucose, K+, Na+, Ca++, phosphate, lipids, liver enzymes, hemogram, free T4, TSH and metanephrines, bur her creatinine is 2.65 mg/dL, CKD-EPI estimated GFR 19 ml/min/1.73 m2, PTH 240 pg/mL, with normal vitamin 25-OH-D3. Aldosterone is 82.5 ng/dL with PRA 1.1 ng/mL/h, ratio 75.
The diagnosis was: Primary aldosteronism caused by aldosterone-producing adenoma (Litynski-Conn Syndrome), with contralateral recurrence one year after surgery; secondary hypertension with nephroangiosclerotic stage IV chronic renal failure; secondary normocalcemic hyperparathyroidism due to renal failure.
Contralateral recurrence of an aldosterone-producing adenoma in a previously normal adrenal after a complete surgical removal is exceedingly rare, particularly after such a short follow-up (only one year). The apparent rapid growth of a clearly benign lesion (1.9 cm in the April 2010 ultrasonography, 2.6 cm in the January 2012 CT scan, 4.0 cm at surgical exeresis (March 2012) and the presence of several family members with aldosterone-producing adenomas and renal failure was also remarkable. With the explicit consent of the patient a genetic test for familial hyperaldosteronism was performed, with negative result. However, this test cannot exclude at present type II familial hyperaldosteronism (FH-II), which may cause aldosterone-producing adenomas in adult patients, because there is no known genetic marker for this condition. Therefore we still suspect FH-II in our patient, although no confirmatory test is yet available.
Discussion
PA is defined by autonomous aldosterone hypersecretion; it is the most frequent cause of secondary hypertension and also of resistant hypertension [16], and often begets hypokalemia, but not in the majority of the patients [6,17]. The exposure to supraphysiological concentrations of aldosterone leads to cardiovascular and kidney damage, partly independent from increased blood pressure [1,5,12]. In most patients with clinical suspicion of PA, a confirmatory test is warranted [6,18,19], and after the confirmation, a subtype diagnosis is required [6,18]. The main purpose of the subtype diagnosis is to distinguish unilateral from bilateral PA. In about 60% of the cases PA is bilateral [20], predominantly idiopathic but sometimes with bilateral aldosterone-producing adenomas or bilateral nodular hyperplasia. Unilateral PA can be resolved by adrenal surgery in the vast majority of the cases [6,10,11], while patients with bilateral PA are candidates for medical therapy including mineralocorticoid receptor antagonists [6,21].
The main cause for unilateral PA is a single aldosterone-producing adenoma, present in about 40% of the patients with PA [20]. These lesions are usually named Conn’s adenomas, after the well-known description by Jerome W. Conn in 1955 [22], but should be also named after Michal Litynski, a Polish military surgeon who described them first in 1953 [23,24]. Other causes for unilateral PA are unilateral adrenal hyperplasia, multiple aldosterone-producing adenomas, and a combination of both [9]. Functioning adrenal carcinomas with predominant mineralocorticoid secretion are extremely rare [20,25,26] but occasionally can masquerade as benign lesions and cause unexpected recurrences [27].
The algorithm for detection, confirmation, subtype testing and treatment of PA from the present Endocrine Society guidelines for the management of PA [6] is presented in Figure 4. Workup for PA is recommended in patients with hypertension which is severe or resistant, or associated with hypokalemia, sleep apnea, adrenal incidentaloma, early onset, early cerebrovascular accident; and also in patients with family history of early onset hypertension, early cerebrovascular accidents or PA [6,17]. The preferred screening test is the plasma aldosterone/renin activity ratio. Confirmation by a suppression test is recommended except in cases where a clear pattern of PA (suppressed renin activity, plasma aldosterone > 20 ng/mL and hypokalemia) is present [6,28]. The saline infusion test is the most widely validated test for PA confirmation, but in subjects with advanced renal disease (which was the case of our patient) or heart failure, the captopril test may be preferable in order to avoid sodium overload [6,28]. After confirmatory testing, the preferred imaging technique for subtype testing is non-contrast CT [6,8]: it has better spatial resolution than magnetic resonance imaging, and better discrimination of benign vs. malignant masses: homogeneous, low-density masses are almost invariably benign adenomas or angiomyolipomas.

Figure 4: Algorithm for detection, confirmation, subtype testing
and treatment of PA. Adapted from [6].
After the fourth decade, non-functioning adrenal adenomas are a very common CT finding and may coexist with idiopathic bilateral PA [6,8]. Therefore, the present guidelines [6,28] recommend AVS to confirm lateralization of aldosterone secretion, except in young patients (< 35 years old in the present French consensus [8]) with a clear biochemical pattern of PA and unilateral adenoma in the CT scan.
The present consensus favor AVS vs. adrenal imaging [6-8] as the accuracy of imaging test for predicting PA tends to be much lower (< 60% in a recent large series, although close to 100% in patients younger than 35 years [7]). However, AVS is complex and expensive, and hardly available for the vast majority of the patients with PA. SPARTACUS, a new randomized study of AVS vs. simple adrenal CT has challenged the current recommendations, not showing significant differences between both diagnostic strategies [29].
Adrenal surgery achieves about 95% of biochemical remission in PA [6,10-11,13] and in most cases lack of remission is due to misdiagnosis of lateralization in AVS [11]. Patients with larger adenomas, longer history of hypertension, more antihypertensive medication for presurgical BP management and higher BMI had significantly lower probability of achieving a normal BP without medication after surgery [30].
Recurrence after previous biochemical cure is very uncommon, associated with a very long follow-up (15 years on average in a recent series [31]), and in most cases it is ipsilateral. There is an ongoing debate between complete unilateral adrenalectomy vs. adrenal-sparing adenomectomy, and in most cases partial adrenalectomy may be an adequate option, with success rates comparable to full unilateral adrenalectomy. However, a significant minority (25-30%) of the patients preoperatively diagnosed of single adenomas have more complex lesions, including multiple adenomas or nodular hyperplasia [9,14], and their risk of recurrence has been significantly higher in some series [14,15] and case reports [32]. Alternatives to surgery such as CT-guided radiofrequency ablation [33] or percutaneous chemical ablation with ethanol or acetic acid can also lead to recurrence, albeit rarely [34]. In the last few years it has been described that in PA patients there is an increased remodeling of the zona glomerulosa adrenal cells that leads to hyperplasia, and nodulation, which may account for early failures after partial adrenalectomy in unilateral PA [35]. Even in patients with CT-negative unilateral PA, diffuse micronodules and hyperplasia with renin-independent aldosterone production (APCC, or aldosterone-producing cell clusters) driven by somatic mutations can be demonstrated in the histopathological study of the removed adrenals [36]. Therefore the current guidelines do not favor partial adrenalectomy, stating that it may result in persistence of the disease [6].
However, contralateral recurrence of unilateral AP is an exceedingly rare occurrence [10]. It has been reported in the context of familial primary hyperaldosteronism [37,38]. There are several known types of familial hyperaldosteronism (FH), but in most of them PA is present since childhood and there are no aldosterone-producing adenomas, with adrenals of normal appearance or occasional evidence of adrenal hyperplasia [38]. This is true in FH-I (caused by a chimeric CYP11B1/CYP11B2 gene), in FH-III (caused by KCNJ5 mutations), FH-IV (caused by CACNA1H mutations) and FH associated with complex neurologic disorders in children (caused by CACNA1D mutations) [37]. The exception is FH-II, which has no known genetic cause (although the inheritance is autosomal dominant and maps to chromosome 7p22 in some families [39]). The presentation can be indistinguishable from sporadic PA, with unilateral or bilateral aldosterone-producing adenomas presenting usually in adulthood, and marked phenotypic variability that may imply that there is a constellation of genetic causes instead of a single one [40, 41]. In absence of a simple cause the diagnosis is based in the clinical profile plus the presence of two or more affected family members [37]; other types of FH can be excluded by genetic testing if the clinical profile is compatible [38].
Our patient had a history of unilateral PA caused by a Litynski-Conn adenoma, with severe hypertension and advanced renal disease (which probably contributed to the absence of hypokalemia). The abdominal CT showed a left adrenal adenoma but the right adrenal was normal. PA was confirmed with a standard captopril test, and left lateralization of aldosterone production was clearly verified by AVS. A total left adrenalectomy was performed, with complete removal of a single adenoma; malignancy was excluded by the histopathologic examination. A few weeks after the surgery, the biochemical remission of PA was complete and the BP control was improved, requiring only monotherapy with a calcium channel blocker. Up to this point, the patient’s history is unremarkable. However, her PA relapsed and she developed a contralateral aldosterone-producing adenoma in an astonishingly short time (only one year at most). Contralateral recurrence after a clearly diagnosed unilateral PA is by itself a rarity, but in our knowledge it has never been reported in so short a time (typically a recurrence after a resolved unilateral PA takes over a decade [30]). In some cases of FH-II contralateral recurrence tends to be shorter than in sporadic PA, and the existence of three family members who required adrenal surgery because of a similar condition strongly suggests that our patient can have this condition. Unfortunately no genetic test is available for FH-II, as the causal gene is still unidentified. The patient’s history, with middle age debut of hypertension and development of adrenal adenomas, does not suggest other types of FH.
The therapeutic approach for an adenoma on the remaining adrenal gland following unilateral adrenalectomy is not well established, as this is a very uncommon situation and the current guidelines do not address it. We have decided against surgery of the recurrent adenoma as it would surely cause permanent adrenal deficiency, while medical treatment with the addition of a mineralocorticoid receptor antagonist seems a more reasonable option, at least while the patient’s blood pressure is well controlled and her kalemia remains normal.
Conclusions
PA is highly prevalent and largely underdiagnosed, which poses a public health problem, as the correct diagnosis and treatment can prevent major cardiovascular and renal events much more efficiently than standard treatment for essential hypertension in these patients.
When PA is shown to be unilateral by AVS, surgical treatment can achieve biochemical resolution of PA in about 95% of the cases. Hypertension is only cured in about 40% of the patients, but usually improves significantly in the rest, with reduced need for antihypertensive medication.
Recurrence after resolution of unilateral PA is uncommon (< 5%) and usually presents at least a decade after surgery, while contralateral recurrence is exceedingly rare. Our patient had a contralateral recurrence in a uniquely short time, which along with the presence of several family members who underwent adrenalectomy strongly suggests a variant form of FH-II as a diagnostic hypothesis.
References
- Stowasser M. “Update in primary aldosteronism”. The Journal of Clinical Endocrinology & Metabolism 100.1 (2015): 1-10.
- Funder JW. “Primary aldosteronism as a public health issue”. The Lancet Diabetes & Endocrinology 4.12 (2016): 972-973.
- Milliez P., et al. “Evidence for an increased rate of cardiovascular events in patients with primary aldosteronism”. Journal of the American College of Cardiology 45.8 (2005): 1243-1248.
- Catena C., et al. “Cardiovascular outcomes in patients with primary aldosteronism after treatment”. Archives of Internal Medicine 168.1 (2008): 80-85.
- Savard S., et al. “Cardiovascular complications associated with primary aldosteronism: a controlled cross-sectional study”. Hypertension 62.2 (2013):331-336.
- Funder JW., et al. “The Management of Primary Aldosteronism: Case Detection, Diagnosis, and Treatment: An Endocrine Society Clinical Practice Guideline”. The Journal of Clinical Endocrinology & Metabolism 101.5 (2016): 1889-1916.
- Lim V1., et al. “Accuracy of adrenal imaging and adrenal venous sampling in predicting surgical cure of primary aldosteronism”. The Journal of Clinical Endocrinology & Metabolism 99.8 (2014): 2712-2719.
- Bardet S., et al. “SFE/SFHTA/AFCE consensus on primary aldosteronism, part 4: Subtype diagnosis”. Annales d'Endocrinologie 77.3 (2016): 208-213.
- Quillo AR., et al. “Primary aldosteronism: results of adrenalectomy for nonsingle adenoma”. Journal of the American College of Surgeons 213.1 (2011): 106-112.
- Steichen O., et al. “SFE/SFHTA/AFCE consensus on primary aldosteronism, part 6: Adrenal surgery”. Annales d'Endocrinologie 77.3 (2016): 220-225.
- Steichen O., et al. “Outcomes of adrenalectomy in patients with unilateral primary aldosteronism: a review”. Hormone and Metabolic Research 44.3 (2012): 221-227.
- Steichen O. “Rating the blood pressure outcome after adrenalectomy for unilateral primary aldosteronism”. The Lancet Diabetes & Endocrinology 5.9 (2017): 670-671.
- Williams TA., et al.“Primary Aldosteronism Surgery Outcome (PASO) investigators. Outcomes after adrenalectomy for unilateral primary aldosteronism: an international consensus on outcome measures and analysis of remission rates in an international cohort”. The Lancet Diabetes & Endocrinology 5.9 (2017): 689-699.
- Ishidoya S., et al. “Laparoscopic partial versus total adrenalectomy for aldosterone producing adenoma”. Journal of Urology 174.1 (2005): 40-43.
- Muth A., et al. “Systematic review of surgery and outcomes in patients with primary aldosteronism”. The British Journal of Surgery 102.4 (2015): 307-317.
- Kline GA., et al. “Primary aldosteronism: a common cause of resistant hypertension”. Canadian Medical Association Journal 189.22 (2017): E773-E778.
- Baguet JP., et al.“SFE/SFHTA/AFCE consensus on primary aldosteronism, part 1: Epidemiology of PA, who should be screened for sporadic PA?” Annales d'Endocrinologie 77.3 (2016): 187-191.
- Douillard C., et al.“SFE/SFHTA/AFCE Consensus on Primary Aldosteronism, part 2: First diagnostic steps”. Annales d'Endocrinologie 77.3 (2016): 192-201.
- Reznik Y., et al. “SFE/SFHTA/AFCE consensus on primary aldosteronism, part 3: Confirmatory testing”. Annales d'Endocrinologie 77.3 (2016): 202-207.
- Vilela LAP and Almeida MQ. “Diagnosis and management of primary aldosteronism”. Archives of Endocrinology and Metabolism 61.3 (2017): 305-312.
- Pechère-Bertschi A., et al.“SFE/SFHTA/AFCE consensus on primary aldosteronism, part 7: Medical treatment of primary aldosteronism”. Annales d'Endocrinologie 77.3 (2016): 226-234.
- Conn JW. “Primary aldosteronism”. Journal of Laboratory and Clinical Medicine 45.4 (1955): 661-664.
- Lityński M. “Hypertension caused by tumors of the adrenal cortex”. Polski Tygodnik Lekarski8.6 (1953): 204-208.
- Kucharz EJ. “Michał Lityński, a forgotten author of the first description on primary hyperaldosteronism”. Polish Archives of Internal Medicine 117.1-2 (2007): 57-58.
- Veron Esquivel D., et al. “Adrenocortical carcinoma, an unusual cause of secondary hypertension”. BMJ Case Reports (2016): bcr2016217918.
- Hussain S., et al. “Pure aldosterone-secreting adrenocortical carcinoma in a patient with refractory primary hyperaldosteronism”. Endocrinology, Diabetes & Metabolism Case Reports 2015 (2015): 150064.
- Gundara JS., et al.“Recurrent hyperaldosteronism after adrenalectomy: an indication for careful radiologic and histologic re-evaluation”. ANZ Journal of Surgery 85.4 (2015): 289-290.
- Reznik Y., et al.“SFE/SFHTA/AFCE consensus on primary aldosteronism, part 3: Confirmatory testing”. Annales d'Endocrinologie 77.3 (2016): 202-207.
- Dekkers T., et al.“Adrenal vein sampling versus CT scan to determine treatment in primary aldosteronism: an outcome-based randomised diagnostic trial”. The Lancet Diabetes & Endocrinology 4.9 (2016): 739-746.
- Carter Y., et al. “Persistent hypertension after adrenalectomy for an aldosterone-producing adenoma: weight as a critical prognostic factor for aldosterone's lasting effect on the cardiac and vascular systems”. Journal of Surgical Research 177.2 (2012): 241-247.
- Citton M., et al. “Outcome of surgical treatment of primary aldosteronism”. Langenbeck's Archives of Surgery 400.3 (2015): 325-331.
- Fendrich V., et al. “Persistenz eines primären Hyperaldosteronismus nach subtotaler Adrenalektomie”. Der Chirurg 74.5 (2003): 473-477.
- Yang MH., et al.“Comparison of radiofrequency ablation versus laparoscopic adrenalectomy for benign aldosterone-producing adenoma”. La Radiologia Medica 121.10 (2016): 811-819.
- Chang FC., et al. “Recurrence of primary aldosteronism after percutaneous ethanol injection”. Journal of the Formosan Medical Association 111.3 (2012): 176-178.
- Boulkroun S., et al. “Adrenal cortex remodeling and functional zona glomerulosa hyperplasia in primary aldosteronism”. Hypertension 56.5 (2010): 885-892.
- Yamazaki Y., et al. “Histopathological Classification of Cross-Sectional Image-Negative Hyperaldosteronism”. The Journal of Clinical Endocrinology & Metabolism 102.4 (2017): 1182-1192.
- Zennaro MC and Jeunemaitre X. “SFE/SFHTA/AFCE consensus on primary aldosteronism, part 5: Genetic diagnosis of primary aldosteronism”. Annales d'Endocrinologie 77.3 (2016): 214-219.
- Dutta RK., et al. “Genetics of primary hyperaldosteronism”. Endocrine-Related Cancer 23.10 (2016): R437-454.
- Lafferty AR., et al. “A novel genetic locus for low renin hypertension: familial hyperaldosteronism type II maps to chromosome 7 (7p22)”. Journal of Medical Genetics 37.11 (2000): 831-835.
- Médeau V., et al. “Familial aspect of primary hyperaldosteronism: analysis of families compatible with primary hyperaldosteronism type 2”. Annales d'Endocrinologie 66.3 (2005): 240-246.
- Mulatero P., et al.“Prevalence and characteristics of familial hyperaldosteronism: the PATOGEN study (Primary Aldosteronism in TOrino-GENetic forms)”. Hypertension 58.5 (2011): 797-803.
Citation:
Agnieszka Kuzior., et al. “Short-Term Contralateral Recurrence of an Aldosterone-Producing Adenoma: A
Possible Case of Familial Hyperaldosteronism Type II”. Therapeutic Advances in Cardiology 1.3 (2017): 121-130.
Copyright: © 2017 Agnieszka Kuzior., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.





























 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.