Review Article
Volume 2 Issue 1 - 2018
Left Ventricular Global Longitudinal Strain Adaptation in Sport Overall and the Different Sport Modalities
Sport Cardiology Unit, HLA Inmaculada Hospital, Granada, Spain
*Corresponding Author: Antonio Luis Arrebola-Moreno, Sport Cardiology Unit, HLA Inmaculada Hospital, Granada, Spain.
Received: January 21, 2018; Published: March 29, 2018
Abstract
Introduction: There is overwhelming evidence, particularly from echocardiography, that the hearts of competitive athletes may differ from that of non-athletes, matched for age, gender, and body surface area. However, cardiac adaptation to sport may overlap with pathology in the so called “grey zone”. Thus, application of recently developed imaging technologies are required to better understand physiological cardiac adaptation to exercise in order to more clearly delineate physiology from pathology. This article will provide an overview of the evidence of structural and functional adaption of the athlete´s heart as assessed by echocardiography, with particular focus on speckle tracking technology, the calculation of global longitudinal strain (GLS), and how it may vary between different sporting disciplines.
Methods: In order to review the current data regarding GLS across different sport disciplines, an extensive literature search was conducted using the PUBMED database up until 2017. The following keywords were used: Global longitudinal strain, athletes, left ventricular, speckle tracking and strain. The reference lists of the retrieved articles and the review articles published on the subject were also screened for eligible manuscripts. The inclusion criteria for the selected studies were: 1) an athletic and control group 2) Mention of the sporting discipline, 3) Inclusion of the mean GLS.
Results: A total of 24 cohorts of athletes from 16 different studies were selected to be included in this review. In 17 groups GLS did not significantly differ from the control population. Considering both the traditional classification (endurance Vs resistance sports) and Mitchell’s classification of sport, there were discrepancies in the results obtained. Two studies reported lower levels of GLS in endurance athletes compared to resistance athletes, while another four studies demonstrated opposite or near neutral results.
Conclusions: The available evidence does not allow us to clearly conclude whether GLS is influenced by sports participation or whether it differs across sporting disciplines. Athlete factors (e.g. gender, BSA, age) or vendor differences in strain analysis may have contributed to these results. Measurement of GLS in different conditions (i.e. during submaximal exercise) may lead to a better understanding of the possible GLS adaptation to exercise.
Introduction
There is overwhelming evidence, particularly from echocardiography, that the heart of competitive athletes may differ from that of non-athletes, matched for age, gender, and body surface area. In a given population of athletes both cardiac structural and functional adaptation to exercise may differ. For example some athletes experience ventricular dilation with hypertrophy and preservation of the ventricular mass-to-volume ratio, whereas others demonstrate concentric hypertrophy with an increased mass-to-volume ratio [1]. Athletic features are induced mostly by endurance training and approximately two years of regular physical training is needed to develop characteristics of the athlete's heart [2]. However, cardiac adaptation to sport may overlap with pathology in the so called “grey zone”. Thus, application of recently developed imaging technologies are are required to better understand physiological cardiac adaptation to exercise in order to more clearly delineate physiology from pathology. This article will provide an overview of the evidence of structural and functional adaption of the athlete´s heart as assessed by echocardiography, with particular focus on speckle tracking technology, the calculation of global longitudinal strain (GLS), and how it may vary between different sporting disciplines. GLS is the most widely used strain parameter and has been shown to be the most sensitive to alterations in pathological conditions [3]. GLS shows greater reproducibility and is easier to obtain in more challenging situations (e.g. during exercise or in patients with difficult echocardiographic windows) compared to other strain parameters. These features lend GLS to use in cardiovascular assessment in athletes.
Sport categorization
Ventricular adaptation differs between sporting disciplines. Remodeling of cardiac tissue depends on the characteristic demands of a given sport, and has traditionally been studied between disciplines at polar ends of a scale, i.e., endurance versus resistance. Nevertheless, cardiac adaptation occurs as a direct result of the degree of volume and pressure challenges induced by individual sports. Therefore, there is likely to be some overlap in the adaptations seen between individual sporting disciplines that have similar static and dynamic components.
Ventricular adaptation differs between sporting disciplines. Remodeling of cardiac tissue depends on the characteristic demands of a given sport, and has traditionally been studied between disciplines at polar ends of a scale, i.e., endurance versus resistance. Nevertheless, cardiac adaptation occurs as a direct result of the degree of volume and pressure challenges induced by individual sports. Therefore, there is likely to be some overlap in the adaptations seen between individual sporting disciplines that have similar static and dynamic components.
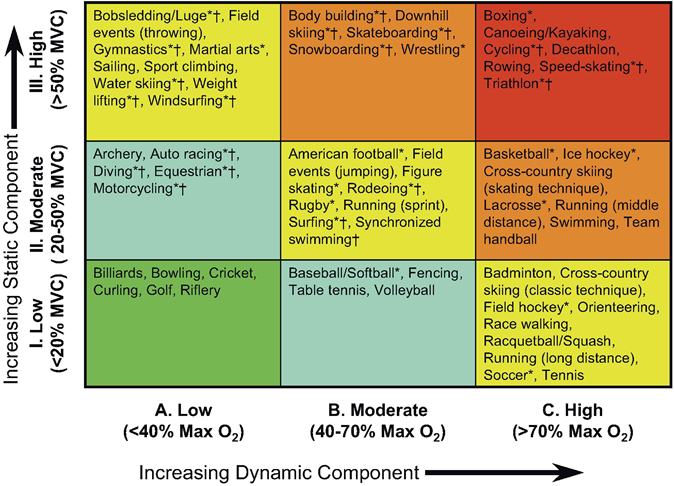
Therefore, one of the main issues when approaching cardiac adaptation in athletes should be to accurately classify the different sporting disciplines. Sports may be classified according to the type and intensity of exercise performed. Mitchell’s classification (Figure 1) [4] divides exercise into two general types: static (isometric, percentage maximum voluntary contraction) and dynamic (isotonic, percentage maximum oxygen consumption) and categorizes the intensity of exercise into low, medium or high. Finally, sports are categorized into nine groups which may ameliorate the variability seen using the traditional classification to identify sport-specific cardiac adaptations. Other studies have used other classification by subdividing Olympic athletes into four groups according to their predominant training characteristics (skill, power, mixed discipline, endurance) [5]. Another approach divides [6] sports into “predominantly endurance” and “predominantly resistance” in both “competitive” and “elite” levels.
This classification is based on peak static and dynamic components achieved during competition. It should be noted, however, that higher values may be reached during training. The increasing dynamic component is defined in terms of the estimated percentage of maximal oxygen uptake (MaxO2) achieved and results in an increasing cardiac output. The increasing static component is related to the estimated percentage of maximal voluntary contraction (MVC) reached and results in an increasing blood pressure load (From Mitchell., et al. 4)
Echocardiographic Structural changes
A larger left ventricular mass has been demonstrated in athletes performing predominantly dynamic aerobic and anaerobic sports, in athletes engaged in static training, and in players of ball sports. Predominantly dynamic (endurance) sports such as distance running, nordic skiing, and cycling require rapid and voluminous blood supply to working muscles. This is achieved via increased cardiac preload, which is typically considered to lead to eccentric ventricular hypertrophy, including chamber dilatation and proportional increases in wall thickness [9] Predominantly high-static (resistance) sports such as weightlifting, martial arts, and field throwing events induce an increase in intra vascular pressure, which enhances afterload; adaptation is suggested to cause increased wall thickness in the absence of chamber dilatation, known as “concentric hypertrophy” [7]. However, under healthy conditions, the relative wall thickness is usually preserved under 0.45. Gender also plays an important role, as women usually tend to develop more eccentric hypertrophy.
A larger left ventricular mass has been demonstrated in athletes performing predominantly dynamic aerobic and anaerobic sports, in athletes engaged in static training, and in players of ball sports. Predominantly dynamic (endurance) sports such as distance running, nordic skiing, and cycling require rapid and voluminous blood supply to working muscles. This is achieved via increased cardiac preload, which is typically considered to lead to eccentric ventricular hypertrophy, including chamber dilatation and proportional increases in wall thickness [9] Predominantly high-static (resistance) sports such as weightlifting, martial arts, and field throwing events induce an increase in intra vascular pressure, which enhances afterload; adaptation is suggested to cause increased wall thickness in the absence of chamber dilatation, known as “concentric hypertrophy” [7]. However, under healthy conditions, the relative wall thickness is usually preserved under 0.45. Gender also plays an important role, as women usually tend to develop more eccentric hypertrophy.
Nevertheless, ventricular wall thickness above 16 mm in men (13 mm in women), diastolic LV dimensions over 70 mm in men(60 mm in women) or relative wall thickness more than 0.45 mm are uncommon and should be further investigated.
Echocardiographic Cardiac function changes
Less evidence, exists regarding cardiac functional parameters. Left ventricular ejection fraction (LVEF) as assessed by 2 dimensional echocardiography (i.e. 2-dimensional biplane) is traditionally used to quantify LV systolic function. Although some studies have suggested that a minority of elite athletes might have a lower than normal ejection fraction [8], most studies show no differences between athletes and the non-athlete healthy population [9]. However, other studies suggest that LVEF might not be sensitive enough to determine minor changes in ventricular systolic function, not only in pathological conditions (i.e. ischemic or hypertrophic cardiomyopathy), but also in a healthy athletic population [10].
Less evidence, exists regarding cardiac functional parameters. Left ventricular ejection fraction (LVEF) as assessed by 2 dimensional echocardiography (i.e. 2-dimensional biplane) is traditionally used to quantify LV systolic function. Although some studies have suggested that a minority of elite athletes might have a lower than normal ejection fraction [8], most studies show no differences between athletes and the non-athlete healthy population [9]. However, other studies suggest that LVEF might not be sensitive enough to determine minor changes in ventricular systolic function, not only in pathological conditions (i.e. ischemic or hypertrophic cardiomyopathy), but also in a healthy athletic population [10].
Regarding diastolic function, the data currently available suggests the usefulness of Doppler myocardial imaging (DMI). DMI has demonstrated an interesting opportunity for: 1) the differential diagnosis of pathological left ventricular hypertrophy due to HCM; 2) the prediction of cardiac performance during physical effort; 3) the evaluation of bi-ventricular interaction; 4) the analysis of myocardial adaptations to various training protocols; and 5) the early identification of specific genotypes associated with cardiomyopathies [11]. Moreover, in several cross-sectional studies comparing untrained and endurance trained hearts a higher diastolic filling rate, a higher maximal blood flow velocity of early diastolic passive left ventricular filling and a higher early diastolic filling fraction at rest and during exercise was shown in endurance trained hearts [7]
Speckle tracking echocardiography technology
Ultrasound deformation imaging, either by Doppler myocardial imaging or speckle tracking, provides more sensitive detection of regional myocardial motion and deformation than standard echocardiography. 2D speckle tracking echocardiography (STE) is a newer technology that facilitates the measurement of cardiac deformation by tracking acoustic speckle markers frame by frame within the ultrasound image [12]. It has the advantage over Tissue Doppler imaging (TDI) of being relatively angle independent and enabling the assessment of movement within any direction of the imaging plane [13], allowing the assessment of left ventricular deformation throughout the cardiac cycle across three planes of motion: longitudinal, radial, and circumferential. Moreover, cardiac twisting and untwisting can be determined due to clockwise rotation at the base, and counter-clockwise rotation at the apex during systole and reversed directions upon diastole with the myocardium returning to its original shape and resting position [14,15].
Ultrasound deformation imaging, either by Doppler myocardial imaging or speckle tracking, provides more sensitive detection of regional myocardial motion and deformation than standard echocardiography. 2D speckle tracking echocardiography (STE) is a newer technology that facilitates the measurement of cardiac deformation by tracking acoustic speckle markers frame by frame within the ultrasound image [12]. It has the advantage over Tissue Doppler imaging (TDI) of being relatively angle independent and enabling the assessment of movement within any direction of the imaging plane [13], allowing the assessment of left ventricular deformation throughout the cardiac cycle across three planes of motion: longitudinal, radial, and circumferential. Moreover, cardiac twisting and untwisting can be determined due to clockwise rotation at the base, and counter-clockwise rotation at the apex during systole and reversed directions upon diastole with the myocardium returning to its original shape and resting position [14,15].
Therefore, several measures can be obtained, such as, 1) global longitudinal strain (GLS); 2) basal circumferential strain (BCS); 3) apical circumferential strain (ACS); 4) global circumferential strain (GCS); 5) global radial strain (GRS); and 4 measures of LV twist mechanics 6) basal rotation; 7) apical rotation; 8) twist; and 9) untwisting rate/velocity.
In this review we are going to focus on the available evidence regarding global longitudinal strain adaptation in the athletes overall and the different sport modalities as a part of the “Athlete heart” adaptation
Global longitudinal strain calculation
Longitudinal shortening is governed by fibres localized predominantly in the endocardium and epicardium. Circumferential fibers constitute most of the myocardial thickness. Longitudinal function is more sensitive to changes in disease states [16]. When longitudinal fibers are affected (i.e. hypertension or ischaemia) there is a compensatory increase in contractility in circumferential fibres.
Longitudinal shortening is governed by fibres localized predominantly in the endocardium and epicardium. Circumferential fibers constitute most of the myocardial thickness. Longitudinal function is more sensitive to changes in disease states [16]. When longitudinal fibers are affected (i.e. hypertension or ischaemia) there is a compensatory increase in contractility in circumferential fibres.
GLS is usually determined as the average segmental strain from the apical four-chamber view, a combination of apical four- and two-chamber views, or apical four-, two-, and three-chamber views [17] (Figure 2). Echocardiographic window quality may lead to one or other type of acquisition and therefore will have an influence on the results. However, GLS has shown better intra-observer reliability when compared to radial or circumferential strain [18] unfortunately, so far, the software for strain acquisition and analysis differs depending on the vendor and may lead to discrepancies. Thus, standardizing the methodology is essential for reproducibility of results.
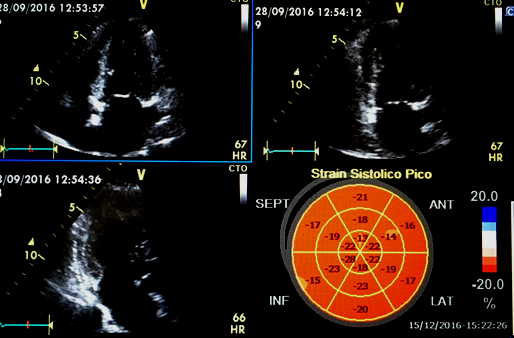
Figure 2: Example of Global longitudinal strain determination by 4 chamber, 2 chamber and 3 chamber acquisition.
Evidence of Global Longitudinal Strain Changes in Sport Overall and the Different Sport Modalities
In order to review the current data regarding the GLS value in the different sport modalities an extensive literature search was conducted using the PUBMED database up until 2017. The following keywords were used: Global longitudinal strain, athletes, left ventricular, speckle tracking and strain. The reference lists of the retrieved articles and the reviewed articles published on the subject were also screened for eligible manuscripts. The inclusion criteria for the selected studies were: 1) To include both an athletes and a control group of participants, 2) To mention the specific modality of the athletes group, 3) To specify the mean GLS of both groups and statistical significance if present. A total of 24 cohorts of athletes from 16 different studies were selected (Table 1) to be included in this review. A meta-analysis including 13 studies was also considered separately for evaluation.
In order to review the current data regarding the GLS value in the different sport modalities an extensive literature search was conducted using the PUBMED database up until 2017. The following keywords were used: Global longitudinal strain, athletes, left ventricular, speckle tracking and strain. The reference lists of the retrieved articles and the reviewed articles published on the subject were also screened for eligible manuscripts. The inclusion criteria for the selected studies were: 1) To include both an athletes and a control group of participants, 2) To mention the specific modality of the athletes group, 3) To specify the mean GLS of both groups and statistical significance if present. A total of 24 cohorts of athletes from 16 different studies were selected (Table 1) to be included in this review. A meta-analysis including 13 studies was also considered separately for evaluation.
| Study | Type of sport | Number of athletes | GLS athletes | Number of controls | GLS controls | Differences between athletes and controls | Athlete GLS value compared to controls |
| Szauder I., et al. 2015 (19) | Runners | 24 | -19.4 ± 3.4 | 15 | - 17.7 ± 3 | YES p˂0.05 |
Higher |
| Szauder I., et al. 2015 (19) | Bodybuilders | 14 | -23.3 ± 2.1 | 15 | - 24.1 ± 3 | NO | ----- |
| Capelli F., et al. 2010 (20) | Endurance athletes | 50 | -18.4 ± 3 | 24 | - 20.1 ± 3.6 | NO | ----- |
| Utomi V., et al. 2014 (21) | Endurance athletes | 18 | -18.6 ± 2.2 | 17 | - 17.7 ± 2.1 | NO | ----- |
| Utomi V., et al. 2014 (21) | Resistance athletes | 19 | -16.2 ± 1.7 | 17 | - 17.7 ± 2.1 | NO | ----- |
| Caselli., et al. 2015 (22) | Skill | 55 | -18.2 ± 2.1 | 50 | - 19.4 ± 2.3 | YES p˂0.001 |
Lower |
| Caselli., et al. 2015 (22) | Power | 30 | -18.4 ± 2.1 | 50 | - 19.4 ± 2.3 | YES p˂0.001 |
Lower |
| Caselli., et al. 2015 (22) | Mixed | 34 | -16.2 ± 1.7 | 50 | - 19.4 ± 2.3 | YES p˂0.001 |
Lower |
| Caselli., et al. 2015 (22) | Endurance | 81 | -16.2 ± 1.7 | 50 | - 19.4 ± 2.3 | YES p˂0.001 |
Lower |
| Butz T., et al. 2010 (23) | Handball players | 20 | - 15.2 ± 3.6 | 18 | - 16.0 ± 2.8 | NO | ----- |
| Nottin S., et al. 2008 (24) | Cyclists | 16 | - 19.2 ± 1.9 | 23 | - 19.5 ± 2.2 | NO | ----- |
| Galderisi M., et al. 2010 (25) | Rowers | 22 | - 22.2 ± 2.7 | 19 | - 21.1 ± 2 | NO | ----- |
| Simsek Z., et al. 2013 (26) | Runners | 24 | - 22.3 ± 2.2 | 20 | N/A | N/A | ----- |
| Simsek Z., et al. 2013 (26) | Wrestlers | 15 | - 21.8 ± 1.7 | 20 | N/A | N/A | ----- |
| Santoro., et al. 2014 (27) | Cyclists | 33 | –16.5 ± 1.7 | 17 | - 17.7 ± 2.8 | NO | ----- |
| Santoro., et al. 2014 (27) | Weightlifters | 36 | –16.6 ± 2.1 | 17 | - 17.7 ± 2.8 | NO | ----- |
| Santoro., et al. 2014 (28) | Waterpolo players | 45 | - 19.2 ± 5.0 | 17 | - 20.1 ± 2.3 | NO | ----- |
| Santoro., et al. (2015)(29) | Swimmers | 125 | - 20.4 ± 2.5 | 95 | - 19.3 ± 2.8 | YES p˂0.05 |
Higher |
| Vitarelli., et al. (2013)(30) | Marathones | 35 | - 21.7 ± 2.6 | 35 | - 20.3 ± 2.6 | NO | ----- |
| Vitarelli., et al. (2013)(30) | Power lifters | 35 | - 22.5 ± 2.4 | 35 | - 20.3 ± 2.6 | YES p˂0.05 |
Higher |
| Vitarelli., et al. (2013)(30) | Martial artists | 35 | - 21.6 ± 2.2 | 35 | - 20.3 ± 2.6 | NO | ----- |
| Stefani., et al. (2008)(31) | Soccer players | 25 | - 18.6 ± 3.3 | 25 | - 19.4 ± 5.2 | NO | ----- |
| Cote., et al. (2013)(32) | Cyclists | 11 | - 18.5 ± 2.1 | 10 | - 19.0 ± 2.9 | NO | ----- |
| Donal., et al. (2011)(33) | Cyclists | 18 | - 17.0 ± 1.3 | 27 | - 17.7 ± 1.6 | NO | ----- |
Table 1: Summary of the selected studies assessing global longitudinal strain in the different sport modalities.
Out of the 24 cohorts of athletes, 17 GLS did not significantly differ from the control population. The mean number of included athletes in the significant studies was 54.85 while in the non-significant ones was 25.64.
Out of the 7 groups of athletes in which differences from the control group were significant, 4 of them were included in the same study [22] including skill, endurance, power and mixed sports, showing lower GLS values than the controls. While in the other 3 studies [19,29,30] including runners, swimmers and power-lifters, GLS was lower in the athletes group.
The range of mean GLS values in athletes was from - 15.2 (found in handball players) to -23.3 (found in bodybuilders). In the control groups we also found a big range of GLS from -16.0 to -24.1, which corresponded to the control groups matched to the handball players and bodybuilders previously mentioned.
Only 6 studies [19,21,22,26,27,30] and one meta-analysis (6) demonstrated GLS changes between sporting disciplines. Using the traditional classification (endurance vs resistance sports) results varied, 2 studies showed lower levels of GLS in endurance athletes compared to resistance, and another 4 studies showing opposite or near neutral results. Moreover, the only reviewed meta-analysis showed that heterogeneity was significant and inconsistency was moderate in all groups. In fact, the same meta-analysis also showed similar inconsistency and heterogeneity when considering the different Michell´s sporting classification.
Discussion of the Results, Limitation of the Studies and Further Directions
Many different studies can be found in the literature regarding GLS in the different sport modalities, however, only a small number compared an athletic with a control population and a specific sporting discipline was addressed (Table 1).
Many different studies can be found in the literature regarding GLS in the different sport modalities, however, only a small number compared an athletic with a control population and a specific sporting discipline was addressed (Table 1).
In general the included studies (Table 1) show that GLS reduction due to training is very uncommon. Levels below 12% should be considered abnormal and further testing must be performed. Levels of GLS above 18% are considered normal, and values between 12% and 18% constitute a “grey zone” which has been demonstrated not only in athletes but also in the non-athlete population.
Although it has been suggested [34] that if GLS is altered in athletes, it is likely to be increased since a reduction is not a common feature of the athlete’s heart, the available data is inconsistent. Among our selected studies only 3 groups of athletes showed higher levels of GLS than the control while in 4 groups GLS was lower. Thus, this data cannot allow us to conclude whether the athlete condition might have a clear impact on GLS.
The discrepancy between study results can be attributed to various items. First of all, many of the available studies include a small number of individuals leading to non-significant results as samples lacked power to detect subtle differences in GLS changes. Significance was only reached in larger groups of athletes.
The lack of homogeneity in the strain acquisition among the different manufacturers may also play a role in the given results; it is possible that vendor differences in the algorithms and thus analysis of speckle-tracking measurements may account for some heterogeneity observed [21]; Demographic factors such as…. ethnicity, age or gender might also have modified the results,. Moreover, body surface area and gender [35] as well as cardiac chamber volume [36], have shown to modify GLS. This could explain why the highest or lowest values of GLS were found not only in a certain sport modality population of athletes but also in their control-matched individuals. Cardiac adaptation to sports may also vary among athletes depending on intrinsic characteristics (i.e. predominant CV adaptation or muscle adaptation) unlike cardiac adaptation to diseases like hypertension where structural and functional changes are usually more homogeneous.
When sporting discipline was taken into account similar inconsistencies were found. In fact, a previous meta-analysis [6] has shown that with the available data no athlete–control differences existed for GLS overall, following sporting categorization or training level. Therefore the quality of the available data does not allow us to conclude whether GLS is influenced by sport practice overall or whether it varies between sporting disciplines sport modalities.
Measuring strain at rest might not be the optimal method to assess the adaptation of the heart to exercise. Athletes have greater functional reserve leading to greater efficiency. Therefore “strain reserve, that is, the difference between strain at rest and during exercise, may better demonstrate… A study [37] has already demonstrated that GLS may change after a high intensity training when it is measured under standardized exercise challenge but not at rest. Other studies [38] have shown that incremental load in healthy individuals does not seem to increase GLS, suggesting that while GLS may have limited augmentation during exercise, other myocardial strain parameters (i.e., circumferential strain, LV twist mechanics) play a more pivotal role in augmenting myocardial function during effort. However, this testing was carried out under very light conditions (20 to 40% of maximum), which a gross underestimate of an athlete’s effort. Therefore GLS behavior or reserve should be tested under these conditions (i.e. 85% of maximal capacity).
Finally, another strain derived parameters such as circumferential and radial strain or twisting mechanics, although less implemented in clinical practice, should be considered for further evaluation together with GLS. In fact, promising results have been shown regarding LV twisting [6,39], as athletes exposed to differing cardiac loading, associated with the dynamic and static components of sports possess divergent twisting mechanical profiles, with low-dynamic high-static sports presenting a potential compensated increase in twist. However, these parameter may be challenging to obtain due to limitations of frame rate.
Conclusions
The available evidence does not allow us to clearly conclude whether GLS is affected by athletic status or across sporting disciplines Sample size, demographic factors (i.e. gender, body surface area, age…) or vendor differences for strain analysis may have contributed to these results. Measurement of GLS under different conditions (iexercise.g. during exercise) may lead to better understanding GLS adaptation during sport practice.
References
- Colan SD. “Mechanics of left ventricular systolic and diastolic function in physiologic hypertrophy of the athlete's heart”. Cardiology Clinics 15.3 (1997): 355-357.
- Pavlik G., et al. “The athlete's heart. Part II: influencing factors on the athlete's heart: types of sports and age (review)”. Acta Physiologica Hungarica 100.1 (2013): 1-27.
- Galderisi M., et al. “Improved cardiovascular diagnostic accuracy by pocket size imaging device in non-cardiologic outpatients: the NaUSiCa (Naples Ultrasound Stethoscope in Cardiology) study”. Cardiovascular Ultrasound 8 (2010): 51.
- Mitchell JH., et al. “Task Force 8: classification of sports”.Journal of the American College of Cardiology 45.8 (2005): 1364-1367.
- Caselli S., et al. “Patterns of left ventricular longitudinal strain and strain rate in olympic athletes”. Journal of the American Society of Echocardiography 28.2 (2015): 245-253.
- Beaumont A., et al. “Left ventricular speckle tracking-derived cardiac strin and cardiac twist mechanics in athletes: A systematic review and meta-analysis of controlled studies”.Sports Medicine 47.6 (2017): 1145-1170.
- Barbier J., et al. “Sports-specific features of athlete’s heart and their relation to echocardiographic parameters”. Herz 31.6 (2006): 531-543.
- Engel DJ., et al. “Athletic Cardiac Remodeling in US Professional Basketball Players”. JAMA Cardiology 1.1 (2016): 80-87.
- Fazel P., et al. “Echocardiographic findings in professional hockey players”. Proceedings (Baylor University. Medical Center) 22.3 (2009): 218-220.
- Lin J., et al. “Blood Pressure and LV Remodeling Among American-Style Football Players”.JACC: Cardiovascular Imaging 9.12 (2016): 1367-1376.
- Caso P., et al. “The athlete's heart and hypertrophic cardiomyopathy: two conditions which may be misdiagnosed and coexistent. Which parameters should be analysed to distinguish one disease from the other?” Journal of Cardiovascular Medicine 7.4 (2006): 257-266.
- Perk G., et al.“Non-Doppler two-dimensional strain imaging by echocardiography–from technical considerations to clinical applications”. Journal of the American Society of Echocardiography 20.3 (2007): 234-243.
- Mor-Avi V., et al.“Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography”. Journal of the American Society of Echocardiography 24.3 (2011): 277-313.
- Buckberg G., et al. “Ventricular torsion and untwisting: further insights into mechanics and timing interdependence: a viewpoint”. Echocardiography 28.7 (2011): 782-804.
- Notomi Y., et al.“Measurement of ventricular torsion by two-dimensional ultrasound speckle tracking imaging”. Journal of the American College of Cardiology 45.12 (2005): 2034-2041.
- Nesto RW and Kowalchuk GJ. “The ischemic cascade: temporal sequence of hemodynamic, electrocardiographic and symptomatic expressions of ischemia”. American Journal of Cardiology 59.7 (1987): 23C-30C.
- Delgado V., et al. “Acute effects of right ventricular apical pacing on left ventricular synchrony and mechanics”. Circulation: Arrhythmia and Electrophysiology 2.2 (2009): 135-145.
- Oxborough D., et al.“Intraobserver reliability of two-dimensional ultrasound derived strain imaging in the assessment of the left ventricle, right ventricle, and left atrium of healthy human hearts”. Echocardiography 29.7 (2012): 793-802.
- Szauder I., et al. “Comparison of left ventricular mechanics in runners versus bodybuilders using speckle tracking echocardiography”. Cardiovascular Ultrasound 13 (2015): 7.
- Cappelli F., et al. “Adaptative or maladaptative hypertrophy, different spatial distribution of myocardial contraction”. Clinical Physiology and Functional Imaging 30.1 (2010): 6-12.
- Utomi V., et al. “Predominance of normal left ventricular geometry in the male 'athlete's heart'”. Heart 100.16 (2014): 1264-1271.
- Caselli S., et al. “Patterns of left ventricular longitudinal strain and strain rate in Olympic athletes”. Journal of the American Society of Echocardiography 28.2 (2015): 245-253.
- Butz T., et al. “Two-dimensional strain analysis of the global and regional myocardial function for the differentiation of pathologic and physiologic left ventricular hypertrophy: a study in athletes and in patients with hypertrophic cardiomyopathy”. The International Journal of Cardiovascular Imaging 27.1 (2011): 91-100.
- Nottin S., et al. “Alteration in left ventricular normal and shear strains evaluated by 2D-strain echocardiography in the athlete's heart”. The Journal of Physiology586.19 (2008): 4721-4733.
- Galderisi M., et al. “Differences of myocardial systolic deformation and correlates of diastolic function in competitive rowers and young hypertensives: a speckle-tracking echocardiography study”. Journal of the American Society of Echocardiography 23.11 (2010): 1190-1198.
- Simsek Z., et al. “Speckle tracking echocardiographic analysis of left ventricular systolic and diastolic functions of young elite athletes with eccentric and concentric type of cardiac remodeling”. Echocardiography 30.10 (2013): 1202-1208.
- Santoro A., et al. “Endurance and strength athlete’s heart: analysis of myocardial deformation by speckle tracking echocardiography”. Journal of Cardiovascular Ultrasound 22.4 (2014): 196-204.
- Santoro A., et al. “Left ventricular twisting modifications in patients with left ventricular concentric hypertrophy at increasing after-load conditions”. Echocardiography 31.10 (2014): 1265-1273.
- Santoro A., et al. “Age related diastolic function in amateur athletes”. The International Journal of Cardiovascular Imaging 31.3 (2015): 567-573.
- Vitarelli A., et al. “Comprehensive assessment of biventricular function and aortic stiffness in athletes with different forms of training by three-dimensional echocardiography and strain imaging”. European Heart Journal - Cardiovascular Imaging 14.10 (2013): 1010-1020.
- Stefani L., et al. “Supernormal functional reserve of apical segments in elite soccer players: an ultrasound speckle tracking handgrip stress study”. Cardiovascular Ultrasound 6 (2008): 14.
- Cote AT., et al. “Left ventricular mechanics and arterial-ventricular coupling following high-intensity interval exercise”. Journal of Applied Physiology 115.11 (2013): 1705-1713.
- Donal E., et al. “Comparison of the heart function adaptation in trained and sedentary men after 50 and before 35 years of age”. American Journal of Cardiology 108.7 (2011): 1029-1037.
- D’Ascenzi F., et al. “Novel echocardiographic techniques for the evaluation of athletes’ heart: a focus on speckle-tracking echocardiography”. European Journal of Preventive Cardiology23.4 (2015): 437-446.
- Giraldeau G., et al. “Gender differences in ventricular remodeling and function in college athletes, insights from lean body mass scaling and deformation imaging”. American Journal of Cardiology 116.10 (2015): 1610-1616.
- Oxborough D., et al. “Left and right ventricular longitudinal strain-volume/area relationships in elite athletes”. The International Journal of Cardiovascular Imaging 32.8 (2016): 1199-1121.
- Stewart GM., et al. “Altered ventricular mechanics after 60 min of high-intensity endurance exercise: insights from exercise speckle-tracking echocardiography”. American Journal of Physiology-Heart and Circulatory Physiology 308.8 (2015): H875-883.
- Doucende G., et al. “Kinetics of left ventricular strains and torsion during incremental exercise in healthy subjects: the key role of torsional mechanics for systolic-diastolic coupling”. Circulation: Cardiovascular Imaging 3.5 (2010): 586-594.
- Maufrais C., et al. “Left ventricles of aging athletes: better untwisters but not more relaxed during exercise”. Clinical Research in Cardiology 106.11 (2017): 884-892.
Citation:
Antonio Luis Arrebola-Moreno. “Left Ventricular Global Longitudinal Strain Adaptation in Sport Overall and the Different
Sport Modalities”. Therapeutic Advances in Cardiology 2.1 (2018): 202-210.
Copyright: © 2018 Antonio Luis Arrebola-Moreno. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.





























 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.