Case Report
Volume 2 Issue 2 - 2018
Cardiac Amyloidosis: Case Report and Short Literature Review
1Department of Medicine, Melaka Manipal Medical College, Melaka 75150, Malaysia
2Oncohematologist, Cancer Center of Guam, Tamuning, GU 96913, USA
3Jasin General Hospital, Jasin 77000, Malaysia
2Oncohematologist, Cancer Center of Guam, Tamuning, GU 96913, USA
3Jasin General Hospital, Jasin 77000, Malaysia
*Corresponding Author: Mra Aye, Department of Medicine, Melaka Manipal Medical College, Melaka 75150, Malaysia.
Received: August 24, 2018; Published: November 13, 2018
Abstract
Cardiac amyloidosis is a rare disorder, and in early stages, the diagnosis is often missed due to lack of specific criteria and lack of recognition. While overall prognosis is poor, outcome depends upon early diagnosis, potential therapy and type of amyloidosis. We report a case of cardiac amyloidosis.
Keywords: Cardiac Amyloidosis; Diagnosis and Prognosis
Introduction
The amyloidoses are a group of systemic diseases characterized by organ deposition of misfolded protein fragments of diverse origins. Manifestations, disease course, organ involvement, and treatment options vary significantly based upon the protein of origin. In light chain amyloidosis (AL), amyloid protein is derived from immunoglobulin light chains, and most often involves the kidneys and heart. Transthyretin amyloidosis (ATTR) is categorized as mutant or wild-type depending on the genetic sequence of the transthyretin (TTR) protein produced by the liver. Mutant TTR is marked by cardiac and/or peripheral nervous system involvement while wild-type ATTR mainly involves the heart. Cardiac involvement is associated with symptoms of heart failure which usually dictates the clinical course of the disease.[1] Cardiac biomarkers are often elevated during amyloidosis due to diastolic dysfunction leading to high left ventricular (LV) filling pressure and wall stress. Cardiac-specific troponin serum concentrations may rise because of myocyte necrosis from amyloid deposits and ischemia related to intramural vessel obstruction. Both NT pro BNP and Troponin levels are valuable predictors of overall survival in patients with AL despite treatment with standard chemotherapy or stem cell transplantation [2] and are useful to prognosticate and monitor response to the treatment. [3-5]
Cardiac amyloidosis can be detected noninvasively by echocardiography, cardiac MRI, or nuclear scintigraphy. Endomyocardial biopsy may be needed in the case of equivocal imaging or discordant data. [1] Increased echogenicity of myocardium particularly with a granular or sparkling appearance has been reported in several studies and considered a hall mark of LV amyloidosis involvement in transthoracic and two-dimensional, M-mode and Doppler echocardiography. [6-7] Cardiac magnetic resonance (CMR) with gadolinium (Gd) enhancement show promise for diagnosing cardiac amyloidosis if ECG and echocardiographic features are inconclusive. As an extracellular fluid tracer, Gd accumulates in expanded interstitial space without crossing intact sarcolemma membranes. When planar Serum Amyloid P component (SAP) scintigraphy is unable to image amyloid, technetium 99mTc-3,3, -diphospho-1,2-propropanodicarboxylic acid may be useful in detecting cardiac amyloid depots and is capable of differentiating ATTR from AL. [8] Hence, while there are many standard and very modern forms of detecting cardiac amyloidosis, this usually remains a tissue diagnosis. Treatment is aimed at relieving congestive symptoms and targeting the underlying amyloidogenic process. This includes anti-plasma cell therapy in AL, and stabilization of the TTR tetramer or inhibition of TTR protein production in ATTR. Cardiac transplantation can be considered in highly selected patients in tandem with therapy aimed at suppressing the amyloidogenic process, and is associated with durable long-term survival. [1]
Despite advances in treatment, the prognosis of amyloidosis is still poor, depending on type of amyloidosis and degree of dysfunction of involved organs. Thus, early diagnosis is important as patients with advanced disease are usually too ill for treatment. [9]
Case Presentation
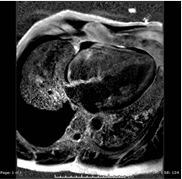
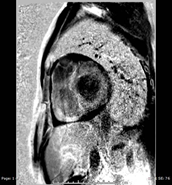
A 59-year-old man, diagnosed with and treated for hypertension and dyslipidemia two years previously, presented in Oct 2016 with “unstable angina” for which coronary angiogram revealed only mild distal occlusion of left descending artery. CMR Imaging suggested cardiac amyloidosis due to sparkling pattern of left ventricle and diffuse sub-endocardial infiltrations of left ventricle, right ventricle and atrial walls, concentric hypertrophy of left ventricle with maximal wall thickness (18mm) without systolic anterior movement or left ventricular outflow tract obstruction, bi-atrial dilatation, thickened atrial wall and interatrial septum diffuse infiltration of the left and right ventricles and atrial walls. Associated findings were mild dilated pulmonary arteries, mild global pericardial effusion, large right pleural effusion, left ejection fraction of 67% (normal:55-70), and right ventricle ejection fraction 69% (normal: 52-80),moderate mitral regurgitation, mild tricuspid and aortic regurgitation, and moderate right ventricular hypertrophy.
He was readmitted in November 2016 for symptoms of congestive heart failure. He was thin, pale and breathless on admission. His tongue was mildly enlarged, JVP was raised. An enlarged left submandibular node with multiple small bilateral cervical lymphadenopathy was noted. There were findings of a moderate right sided pleural effusion, bilateral basal crackles and bilateral peripheral pitting edema, hepatomegaly with mild ascites, but no splenomegaly. CNS examination was normal.
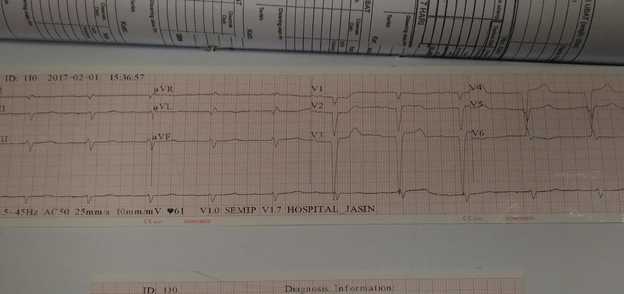
ECG revealed bradycardia, atrial fibrillation, first degree heart block, Mobitz’s type 2 block, and intraventricular blocks, ST segment elevation in V1, AVL, V5 and V6 and no R wave progression in chest leads. (Figure 3)
Cardiac biomarkers, lipid profile, TSH and free T4, renal function, complete blood count and electrolytes were all normal other than a slight hyponatremia, 131 meq. (135-146). Urinalysis 1+ protein, 1+glucose, 1-3 RBC and 1-3 WBC/hpf, with no casts. Liver functions showed normal total protein, albumin, globulin, total bilirubin, with alkaline phosphatase 173.0U/L (30-120), alanine transferase (ALT) 55.04 (<50). C-reactive protein was normal and ESR was 22mm/hour. Pleural fluid showed a sero-sanguinous exudate with elevated LDH. AFB stain and polymerized chain reaction (PCR) were negative for tuberculosis and no malignant cells were seen. High Resolution Computerized Tomogram of lung revealed moderate pleural effusion with some right atelectasis and mild pericardial effusion. Fine needle aspirate of neck gland showed normal salivary gland tissue.
Blood culture revealed no growth. Coronary angiogram revealed mild single vessel disease coronary artery, mild occlusion of distal left descending artery, normal left main stem, normal small left circumflex, normal and dominant right coronary artery.
Cardiovascular 2D – Echocardiography in Nov: 2016 suggested cardiac amyloidosis with interventricular septum diameter (IVSD) 1.6 cm, biventricular and bi-atrial hypertrophy, posterior wall thickness 0.6cm, right atrial wall thickness 0.7 cm, right ventricular free wall (basal) 1.5 cm, ejection fraction was 57%. Cardiac catheterization showed pulmonary hypertension and increased pulmonary vascular resistance Cardiac biopsy following cardiac catheterization was consistent with cardiac amyloidosis AL type.
Treatment for congestive heart failure, with diuretics anti-platelet and anti-hypertensive drugs was initiated. Pleural fluid continued to reaccumulate and was drained as necessary. Patient was rehospitalised several times for heart failure.
Transthoracic ECHO in Feb 2017 revealed further reduction of left ventricular ejection fraction to 47%, sparkling features of interventricular septum, concentric left ventricular hypertrophy without systolic anterior wall motion or Left Ventricular Outflow Tract Obstruction sparkling features of IVS normal left ventricular size with mildly depressed systolic function with regional wall abnormalities. Other findings on transthoracic ECHO were similar to those of 2D ECHO.
In February 2017 bone marrow examination with immunophenotyping, serum and urine electrophoresis, urine and serum free light chains were performed. Bone marrow showed quantitatively, normal maturation red cell series, slightly reduced by normal maturing granulocytic series, adequate megakaryocytes and lymphopoiesis, and an infiltration of 30% plasma cells, mainly mature appearing. Immunophenotyping showed presence of 2.5% to 3.5% clonal plasma cells with lambda light chain expression, highly suggestive of plasma cell myeloma.
Serum protein electrophoresis (SPE) showed a monoclonal band, IgG Lambda in the gamma region measuring 22.1g/L. Urine free light chains revealed kappa 26.10 mg/L (0.00- 25.82), lambda 7.80 mg/L (0.00-11.29), ratio 0.30. Blood (plain) kappa was 22.1 mg/L (6.7-22.4) and lambda was 56.0 (8.30-27.00) and ratio was 0.39. Serum kappa was 36.29mg/L (3.3-19.4) lambda 95.43mg/L (5.71-26.3) ratio 0.38 (0.26-1.65). Skeletal survey was not requested because of normal renal function, calcium level and no bone rib erosions on chest X-ray. The diagnosis was AL cardiac amyloidosis with plasma dyscrasia compatible with smoldering myeloma. Chemotherapy for myeloma was planned but patient developed left sided embolic stroke, septicemia and shock and succumbed.
Discussion
The patient presented with central chest pain mimicking ischemic heart disease in Oct 2016 and diagnosis of cardiac amyloidosis by cardiac biopsy was documented followed by diagnosis of AL type associated with plasma cell dyscrasia in Feb. 2017 based on bone marrow aspiration, immunophenotying and SPE. The average time to death of patients with cardiac amyloidosis presenting with overt heart failure quoted in the literature is six months [10] and such presentation is the most important predictor of survival [11-12].
The patient had many typical ECG features of cardiac amyloidosis including peudoinfarct, low voltage in limb leads, poor R wave progression (Figure 3) and multiple blocks [12] Chest pain in cardiac amyloidosis may be due to pulmonary hypertension, with microvascular disease such as epicardial coronary arteries, normal on coronary angiography. [13] Chest pain may be the first cardiac manifestation of systemic amyloidosis [14-15]. Typical ECHO features of cardiac amyloidosis with left ventricular hypertrophy, bi-atrial enlargement, thickened atrial walls and interatrial septum and poor R wave progression in ECG (Figure 3) [6,7] were present in our patient, MRI of heart (Figure 1 and 2) in Oct 2016 had typical features of cardiac amyloidosis as outlined in case presentation.
Although granular or sparkling appearance is a hallmark of LV amyloidosis with reported specificity of 71-81% [6,16] its sensitivity is low, 26-36%. However, diagnosis of cardiac amyloidosis based on such studies is usually when disease is relatively advanced, and irreversible functional and structural myocardial changes have occurred. [6,16,17]
Recently Tissue Doppler Imaging (TDI) emerged as a valuable technique in assessment of LV function, and several studies suggest that both the systolic and diastolic TDI-derived parameters may be sensitive indicators of cardiac involvement, and very helpful in making an early diagnosis. [2,18]. Pulsed TDI has limitations of translation artefacts [20], but TDI-derived strain and strain rate imaging demonstrate a significant difference in systolic function in patients with cardiac amyloidosis and is able to detect an early and subclinical impairment in systolic function when traditional systole is still normal [18]. 2D speckle-tracking echocardiography may show a particular twisting/untwisting motion of LV useful in early diagnosis [21].
Most typical cardiac support measures, with the exception of diuretics, are ineffective and may even worsen clinical symptoms [22], emphasizing the need for early and accurate diagnosis. Patients with severe cardiac involvement have poor outcomes; heart transplantation is rarely an option because of multiorgan involvement and rapid clinical deterioration. Hence, early screening and awareness might be helpful and all unexplained low voltage in limb leads with slow R wave progression on ECG with left ventricular hypertrophy on echocardiogram should be screened for cardiac amyloidosis.
Advances in the non-invasive diagnostic detection and improvements in early disease recognition will undoubtedly allow a greater proportion of patients to receive early therapy when it is most effective. For AL due to light chain (LC) disease, early diagnosis and prompt treatment that limit the production of amyloidogenic LC are associated with better survival and improvement in organ function after a median of 2.4 months following hematological complete response [23].
Macroglossia present in our patient, a hallmark of AL, is seen in a minority of patents. AL also may present with cutaneous ecchymosis around the eyes (so-called raccoon eyes sign) almost pathognomonic of AL but found only in 10% of patients. [11]
Bilateral cervical lymphadenopathy present in our case, is not a poor prognostic sign absent hematological malignancies or rapid increases in size of lymph nodes [24]. In cases of systemic type amyloidosis, intensive chemotherapy and possibly autologous stem cell transplant should be considered to avoid possible involvement of visceral organs.
Our patient’s plasma cell dyscrasia associated with cardiac amyloidosis was that of smoldering myeloma since he did not meet the definition of multiple myeloma given the absence of CRAB features (normal calcium and renal function, no anemia no bone erosions), and plasma cells in the bone marrow 30% with free light chain < 30g/dL) [25,26]
Conclusion
A patient with advanced cardiac amyloidosis and heart failure secondary to smoldering myeloma presented with chest pain mimicking ischemic heart disease, and died within 6 months, despite prompt diagnosis and treatment. Cardiac amyloidosis is rare and ECG evaluation is important in suspecting the disease so timely referral, diagnosis and therapy may be started to prolong survival of such patients.
References
- Siddiqi OK and Ruberg FL. “Cardiac amyloidosis: An update on pathophysiology, diagnosis, and treatment”. Trends in Cardiovascular Medicine 28.1 (2017): 10-21.
- Dispenzieri A., et al. “Survival in patients with primary systemic amyloidosis and raised serum cardiac troponins”. Lancet 361.9371 (2003): 1787-1789.
- Palladini G., et al. “Circulating amyloidogenic free light chains and serum N-terminal natriuretic peptide type B decrease simultaneously in association with improvement of survival in AL”. Blood 107.10 (2006): 3854-3858.
- Kristen AV., et al. “Assessment of disease severity and outcome in patients with systemic light-chain amyloidosis by the high-sensitivity troponin-T assay”. Blood 116.14 (2010): 2455-2461.
- Palladini G., et al. “The combination of high-sensitivity cardiac troponin T (hs-cTnT) at presentation and changes in N-terminal natriuretic peptide type B (NT-proBNP) after chemotherapy best predicts survival in AL amyloidosis”. Blood 116.18 (2010): 3426-3430.
- Rahman JE.,et al. “Noninvasive diagnosis of biopsy-proven cardiac amyloidosis”. Journal of the American College of Cardiology 43.3 (2004): 410-415.
- Bhandari AK and Nanda NC. “Myocardial texture characterization by two-dimensional echocardiography”. American Journal of Cardiology 51.5 (1983): 817-825.
- Perugini E., et al. “Noninvasive etiologic diagnosis of cardiac amyloidosis using 99mTc-3, 3-diphosphono-1, 2-propanodicarboxylic acid scintigraphy”. Journal of the American College of Cardiology 46.6 (2005): 1076-1084.
- Koyama J., et al. “Usefulness of pulsed tissue Doppler imaging for evaluating systolic and diastolic left ventricular function in patients with AL (primary) amyloidosis”. American Journal of Cardiology 89.9 (2002): 1067-1071.
- Perfetto F.,et al. “Cardiac amyloidosis: the heart of the matter”. Internal and Emergency Medicine 8.3 (2013): 191-203.
- Kyle RA and Gertz MA. “Primary systemic amyloidosis: Clinical and laboratory features in 474 cases”. Seminars in Hematology 32.1 (1995): 45-59.
- Gertz MA and Kyle RA. “Secondary systemic amyloidosis: response and survival in 64 patients”. Medicine 70.4 (1991): 246-256.
- Mueller PS., et al.“Symptomatic ischemic heart disease resulting from obstructive intramural coronary amyloidosis”. The American Journal of Medicine109.3 (2000): 181-188.
- Al Suwaidi J., et al. “Systemic amyloidosis presenting with angina pectoris”. The American Journal of Medicine 131.11 (1999): 838-841.
- Hongo M., et al. “Comparison of electrocardiographic findings in patients with AL (primary) amyloidosis and in familial amyloid polyneuropathy and angina pain and their relation to histopathologic findings”. American Journal of Cardiology 85.7 (2000): 845-853.
- Simons M and Isner JM. “Assessment of relative sensitivities of noninvasive tests for cardiac amyloidosis in documented cardiac amyloidosis”. American Journal of Cardiology 69.4 (1992): 425-427.
- Hongo M., et al. “Doppler echocardiographic assessments of left ventricular diastolic filling in patients with amyloid heart disease”. Journal of Cardiology 21.2 (1991): 391-401.
- Koyama J., et al. “Usefulness of pulsed tissue Doppler imaging for evaluating systolic and diastolic left ventricular function in patients with AL (primary) amyloidosis”.American Journal of Cardiology 89.9 (2002): 1067-1071.
- Porciani MC., et al. “Tissue Doppler and strain imaging: a new tool for early detection of cardiac amyloidosis”. Amyloid 16.2 (2009): 63-70.
- Koyama J., et al. “Longitudinal myocardial function assessed by tissue velocity, strain and strain rate tissue Doppler Echocardiography in patients with AL (primary cardiac amyloidosis)”. Circulation107.19 (2003): 2446-2452.
- Notomi Y., et al. “Enhanced ventricular untwisting during exercise: a mechanistic manifestation of elastic recoil described by Doppler tissue imaging”. Circulation 113.21 (2006): 2524-2533.
- Grogan M., et al.“Light-chain cardiac amyloidosis: strategies to promote early diagnosis and cardiac response”. Heart 103.14 (2017): 1065-1072.
- Tuzovic M., et al. “Cardiac Amyloidosis: Diagnosis and Treatment Strategies”. Current Oncology Reports 19.7 (2017): 46.
- Matsuda M., et al. “AL amyloidosis manifesting as systemic lymphadenopathy”. The Journal of Protein Folding Disorders 15.2 (2008): 117-124.
- Kyle RA and Greipp PR. “Smoldering multiple myeloma”. The New England Journal of Medicine 302.24 (1980): 1347-1349.
- Rajkumar SV., et al. “Smoldering multiple myeloma”. Blood 125.20 (2015): 3069-3075.
Citation:
Mra Aye., et al. “Cardiac Amyloidosis: Case Report and Short Literature Review”. Therapeutic Advances in Cardiology 2.2
(2018): 244-250.
Copyright: © 2018 Mra Aye., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.





























 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.