Case Report
Volume 3 Issue 1 - 2020
Aortocoronary Saphenous Vein Graft Aneurysm, Closure with Amplatzer Device, First Case Reported in the Literature with Subsequent Dilatation of
Bilateral Renal Artery with Stent
Bilateral Renal Artery with Stent
1Head of the Cardiac Catheterization Unit of the Colllet Foundation
2Catheterization Laboratory Assistant
3Clinical Pharmacology Unit, Vargas Medical School, Central University of Venezuela, Caracas, Venezuela
2Catheterization Laboratory Assistant
3Clinical Pharmacology Unit, Vargas Medical School, Central University of Venezuela, Caracas, Venezuela
*Corresponding Author: Manuel Velasco MD, Professor, Director, Clinical Pharmacology Unit, Vargas Medical School, Central University of Venezuela, Caracas, Venezuela.
Saphenous vein graft aortocoronary aneurysms are an unfrequent complication of myocardial revascularization surgery. A 63 -year-old female patient with a history of hyperlipidemia, arterial hypertension, three-vessel coronary artery bypass graft, who is admitted for severe arterial hypertension, and unstable angina. Electrogram, Cardiac Doppler and chest X-ray were observed, showing a right paracardiac mass.
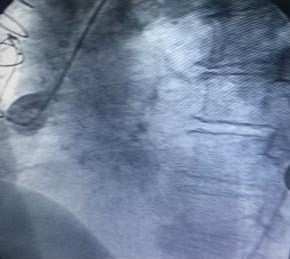
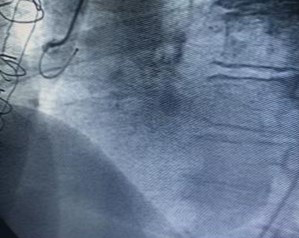
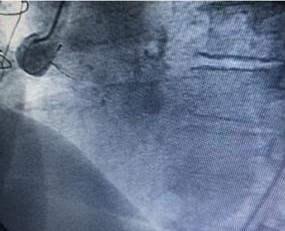
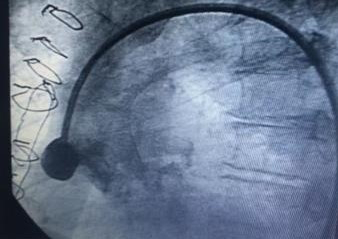
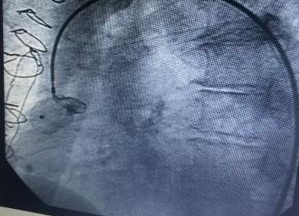
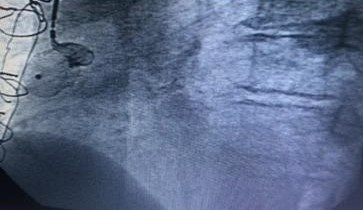
Cardiac catheterization was shown to have coronary artery disease of three vessels, ada, cx, cd aortocoronary bypass to ada and cx occluded, coronary artery aneurysm of the saphein vein graft is shown to right coronary artery. Stenosis of the bilateral renal artery.
Guide 9f and the aneurysm is cannulated with guide 0035, and the amplatzer occluder device is placed with total closure of the aneurysm, fig 1,2,3,4,5. After 15 days, bilateral renal angioplasty with stent is performed.
Discussion
Aortocoronary vein graft aneurysms are rare. Up to 2004, 60 cases with diagnosis and treatment with cardiac surgery have been described. These can be located in the proximal third with the distal coronary artery. Symptoms are nonspecific such dyspnea, angina, nausea, and vomiting, hemoptysis but the patients are generally asymptomatic. Surgical treatment has been documented as well as Coils embolization. Arteriovenous graft aneurysm closure with an amplatzer device has not been described to date with an 18-year follow-up. We have reviewed that the closures with this device have been performed in aneurysms of the broken valsalva sinuses to the right atrium and the right ventricle. We report closure of the vein graft aneurysm to the right coronary artery with an Amplatzer occluder device with a successful result and follow-up until July 2020 with definitive closure and asymptomatic patient. The treatment of this entity is surgical, at present this technique can be used without danger to the patient through the right Valsalva sinus, and the graft aneurysm is successfully excluded in 18-year follow-up. This is the first case in the literature described total exclusion of the aneurism graft with this device.
References
- S Gupta., et al. “Clinical Conference on Management Dilemmas: A Growing Vascular Mass in the Chest”. Chest 118.6 (2000): 1769-1775.
- D Shuttherland and Block PC. “Images in Clinical Medicine. Aneurysm of a Saphenous-Vein Bypass Graft”. The New England Journal of Medicine 344.15 (2001): 1139.
- Pascal Lacombe., et al. “Aneurysms of saphenous vein grafts as late complication of coronary artery bypass surgery: successful exclusion by percutaneous transcatheter embolization” European Radiology 12 (2002): 915-919.
- Hitoshi Kusagawa., et al. Surgical Repair of a Pseudoaneurysm Derived From a Nine-Year-Old Saphenous Vein Graft After Coronary Artery Bypass. The Japanese Society for Cardiovascular Surgery 50.3 (2002): 129-132.
Citation:
Manuel Velasco MD., et al. “Aortocoronary Saphenous Vein Graft Aneurysm, Closure with Amplatzer Device, First Case Reported in the Literature with Subsequent Dilatation of Bilateral Renal Artery with Stent”. Therapeutic Advances in Cardiology 3.1 (2020): 08-10.
Copyright: © 2020 Manuel Velasco MD., et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted
use, distribution, and reproduction in any medium, provided the original author and source are credited.






























 Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.
Scientia Ricerca is licensed and content of this site is available under a Creative Commons Attribution 4.0 International License.